Pharmacological Evaluation of a Traditional Thai Polyherbal Formula for Alzheimer’s Disease: Evidence from In Vitro and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. In Vitro Experimental Validation Results
2.1.1. Preparation of the PC Formula and Each Herbal Plant Extract
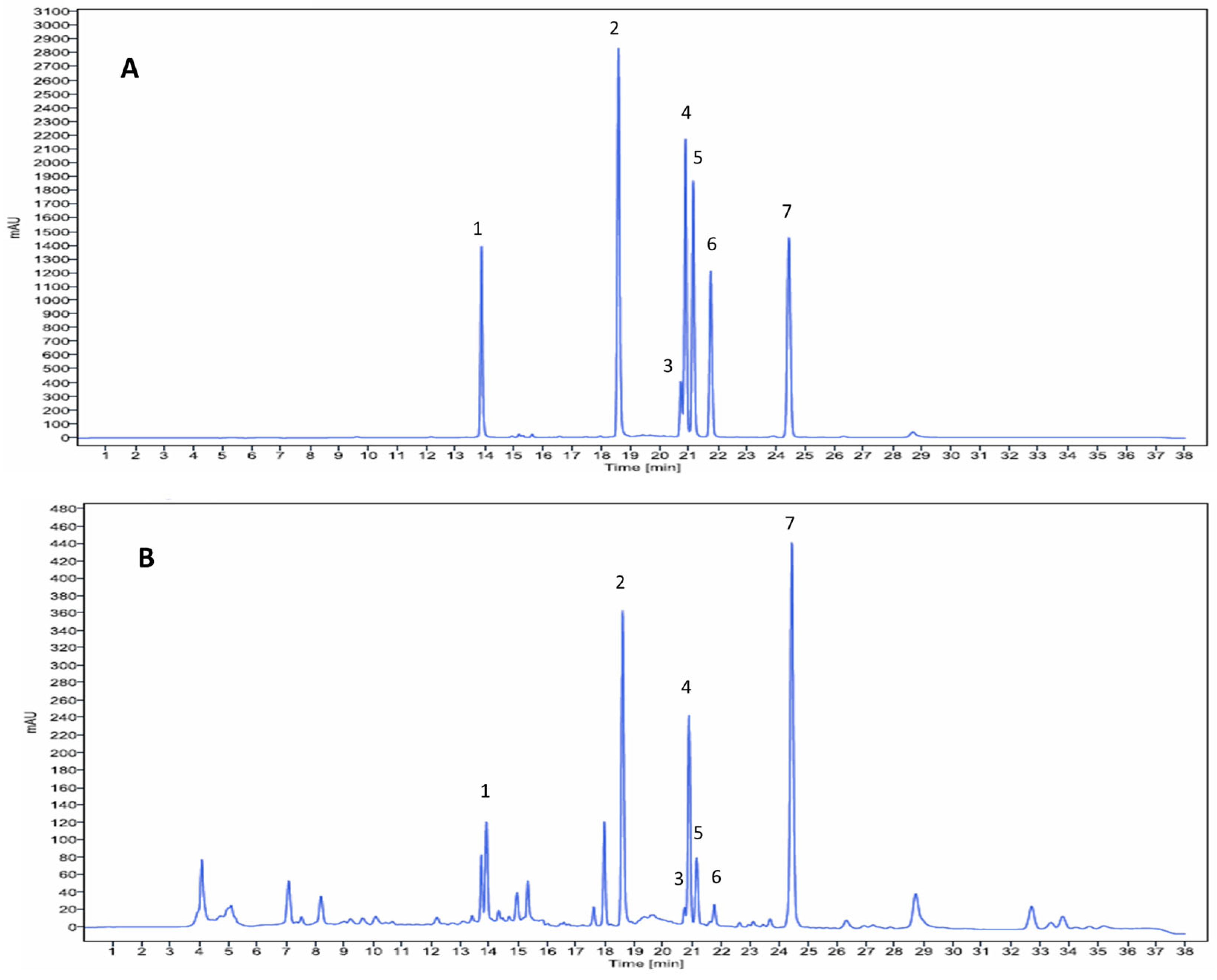
2.1.2. Identification of PC Formula Components by High-Performance Liquid Chromatography (HPLC)
2.1.3. Determination of Total Phenolic (TPC) and Flavonoid Contents (TFC)
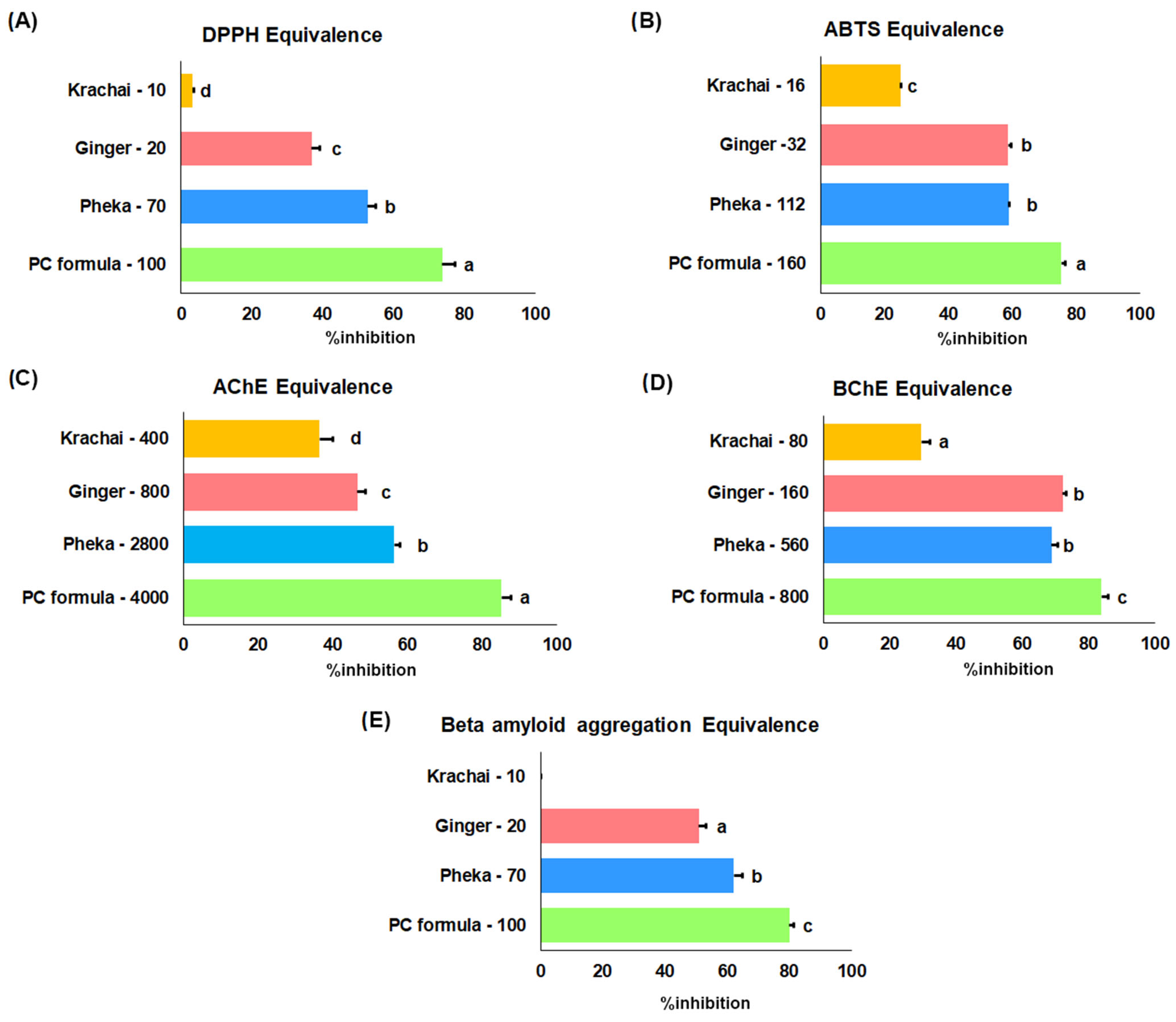
2.1.4. Antioxidant Effect by DPPH and ABTS Assays
2.1.5. Cholinesterase Inhibition
2.1.6. Aβ Aggregation
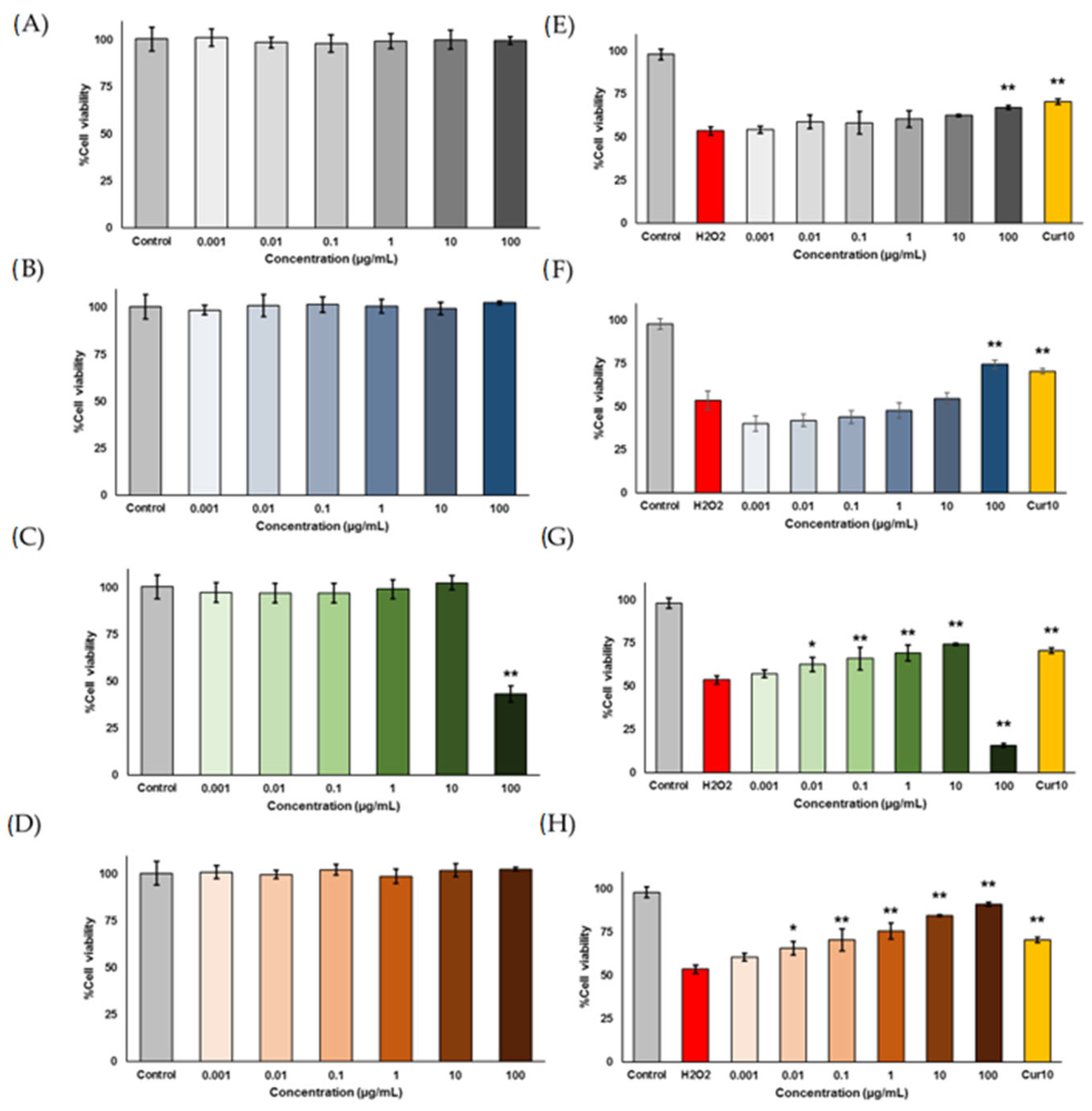
2.1.7. Neuroprotective Effects
2.2. Pharmacology Network Analysis
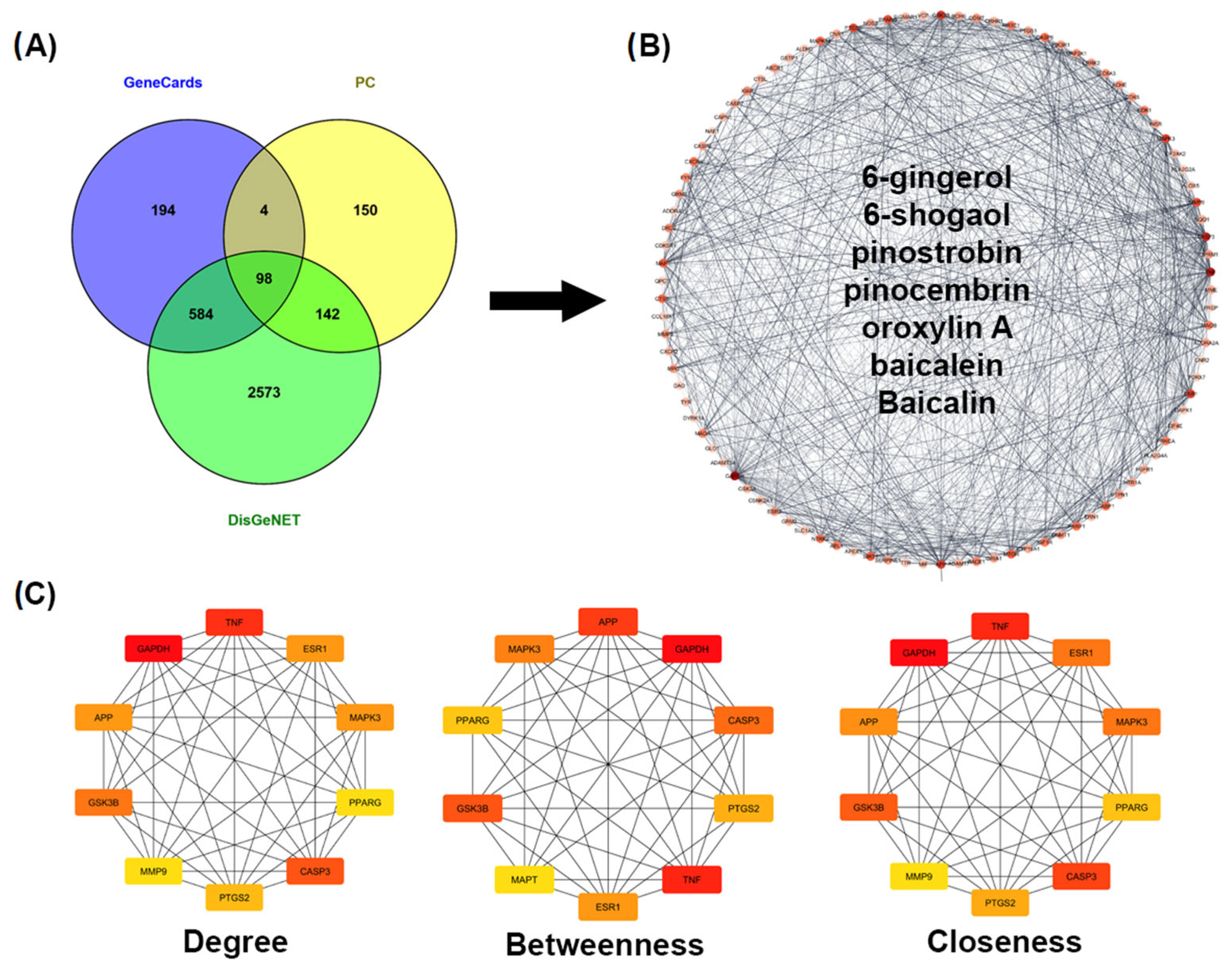
2.2.1. Predicted Targets for AD
2.2.2. Construction and Analysis of the Target PPI Network
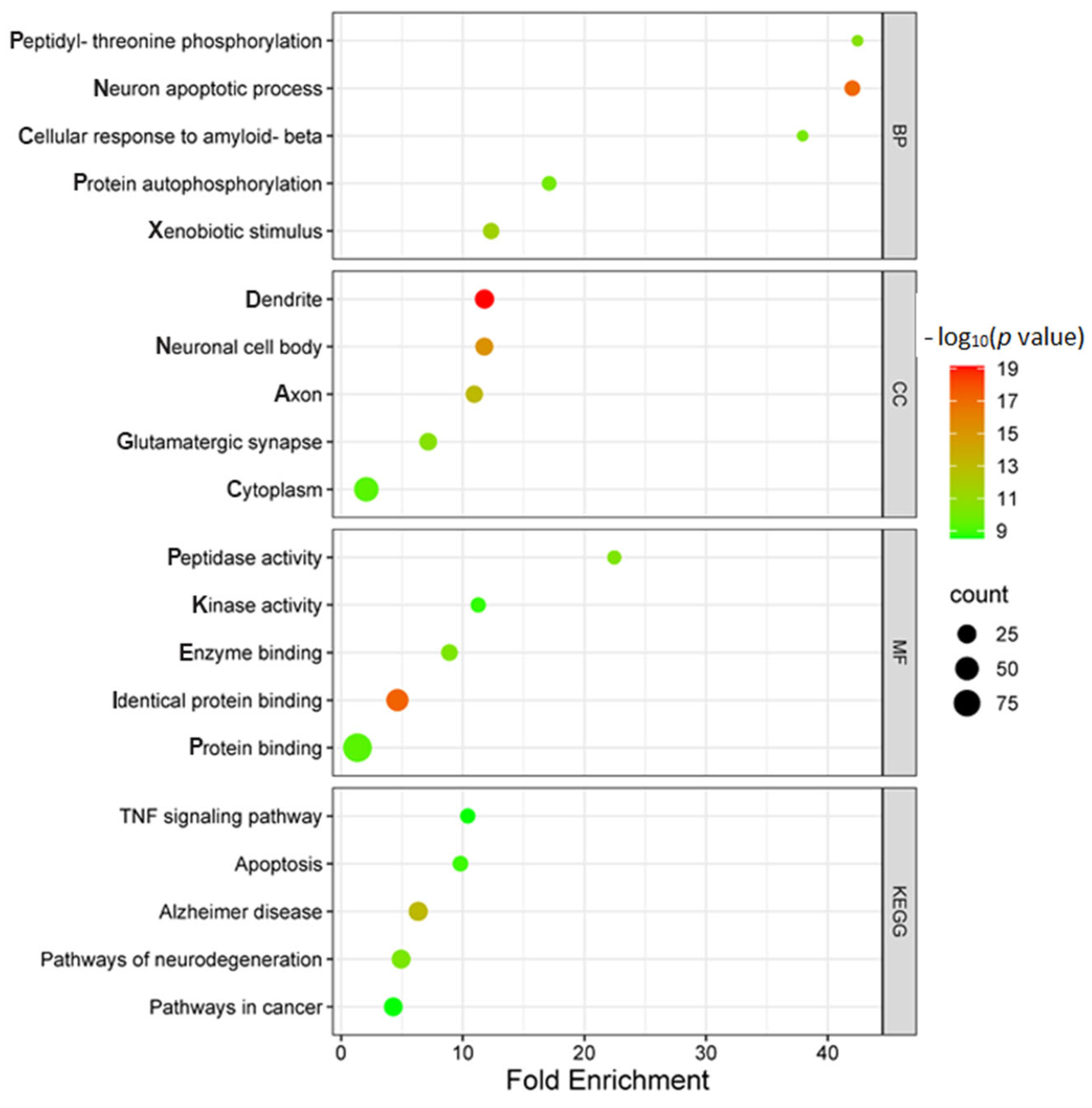
2.2.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
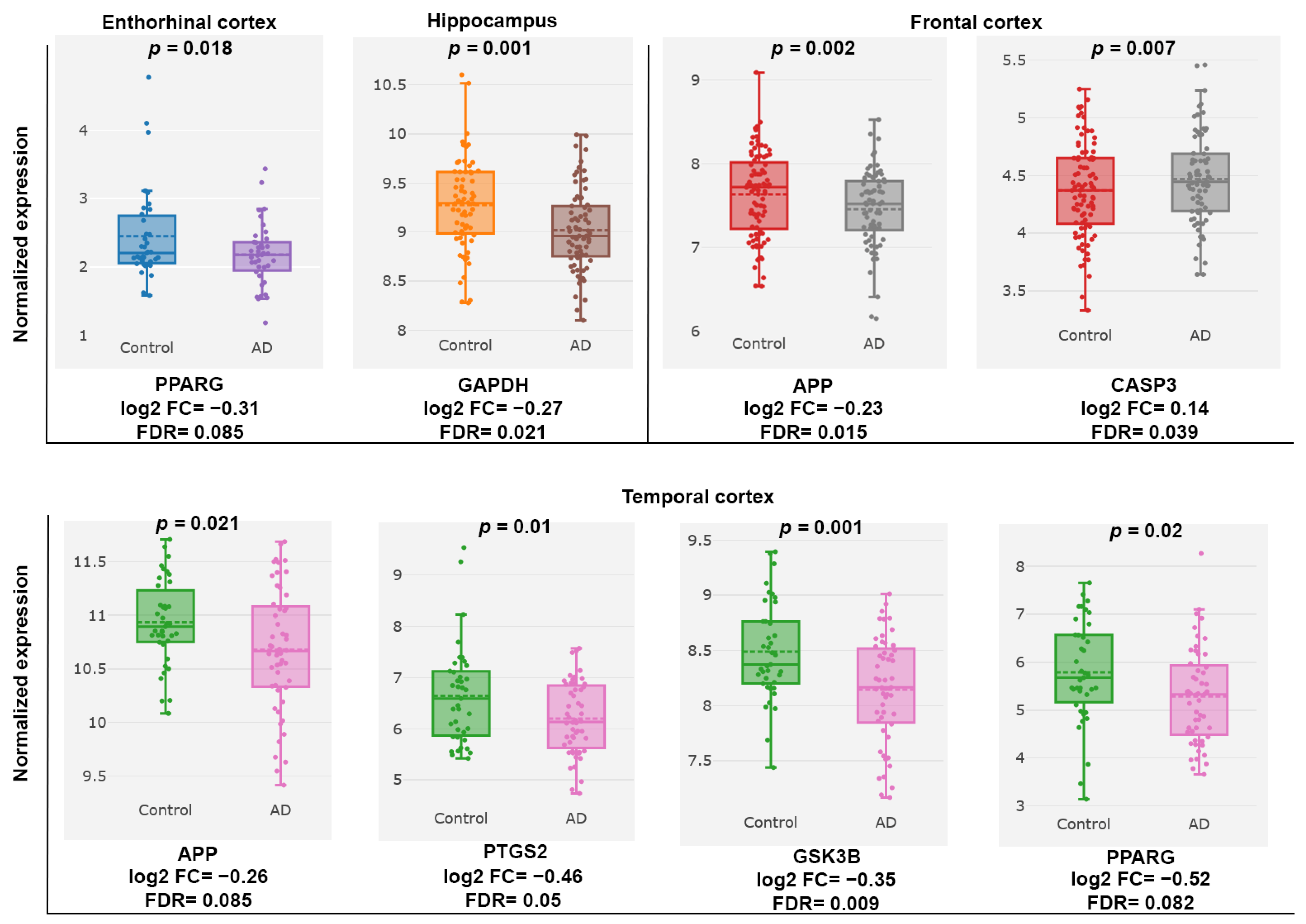
2.2.4. Gene Expression Omnibus (GEO) Dataset Analysis of AD-Associated PC Targets
3. Discussion
4. Materials and Methods
4.1. In Vitro Experimental Validation
4.1.1. Formula Preparation
4.1.2. Identification of PC Formula Components by HPLC
4.1.3. Investigation of Total Phenolic Content (TPC) and Total Flavonoid Contents (TFC)
4.1.4. Investigation of Antioxidant Activities Using Diphenyl-Picrylhydrazyl (DPPH), and 2, 2’-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
4.1.5. Investigation of Cholinesterase Inhibitory Activity
4.1.6. Investigation of Amyloid Beta (Aβ) Aggregation Inhibition
4.1.7. Cytotoxicity and Neuroprotective Effect on Hydrogen Peroxide (H2O2)-Induced Cell Damage
4.2. Pharmacology Network Analysis
4.2.1. Collection of PC Formula Targets and the Identification of AD Potential Targets
4.2.2. Protein–Protein Interaction Network Construction and Screening of Core Targets
4.2.3. Enrichment Analysis of Gene Ontology, and the Kyoto Encyclopedia of Genes
4.2.4. Validation Analysis of Target Genes Correlated with AD
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association Report. Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 20, 3708–3821.
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26 (Suppl. 8), S177–S183. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Peng, Y.; Jin, H.; Xue, Y.; Chen, Q.; Yao, S.; Du, M.; Liu, S. Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Front. Aging Neurosci. 2023, 15, 6572. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Chheng, C.; Waiwut, P.; Plekratoke, K.; Chulikhit, Y.; Daodee, S.; Monthakantirat, O.; Pitiporn, S.; Musigavong, N.; Kwankhao, P.; Boonyarat, C. Multitarget Activities of Kleeb Bua Daeng, a Thai Traditional Herbal Formula, Against Alzheimers Disease. Pharmaceuticals 2020, 13, 79. [Google Scholar] [CrossRef]
- Boonyarat, C.; Yenjai, C.; Monthakantirat, O.; Kaewamatawong, R.; Poonsawas, P.; Wangboonskul, J.; Chaiwiwatrakul, S.; Waiwut, P. Multifunctionality of Clausena harmandiana Extract and Its Active Constituents against Alzheimer’s Disease. Curr. Issues Mol. Biolology 2022, 44, 3681–3694. [Google Scholar] [CrossRef]
- Summat, R.; Waiwut, P.; Daodee, S.; Nualkaew, N.; Phemphunananchai, K.; Arsito, P.N.; Chulikhit, Y.; Montakantirat, O.; Khamphukdee, C.; Boonyarat, C. Phytomedicine Potential of Oroxylum indicum Root and Its Constituents: Targeting Alzheimer’s Disease. Plants 2025, 14, 223. [Google Scholar] [CrossRef]
- Arsito, N.V.; Waiwut, P.; Yenjai, C.; Arthan, S.; Monthakantirat, O.; Nualkaew, N.; Takomthong, P.; Boonyarat, C. Multifunctional effect of flavonoids from Millettia brandisiana against Alzheimer’s disease pathogenesis. Heliyon 2023, 9, e21894. [Google Scholar]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Wangboonskul, J.; Plekratoke, K.; Arsito, N.V.; Ballatore, C.; Boonyarat, C. Kaempferia parviflora extract and its methoxyflavones as potential anti-Alzheimer assessing In Vitro, Integrated Computational Approach, and In Vivo Impact on behaviour in Scopolamine-Induced Amnesic Mice. PLoS ONE 2025, 20, e0316888. [Google Scholar]
- Yoo, K.; Park, S. Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef] [PubMed]
- He, K. Traditional Chinese and Thai medicine in a comparative perspective. Complement. Ther. Med. 2015, 23, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Pimpa, R. Health Promotion in Thai Traditional Medicine Practice: Related Theories and Strategies. J. Thai Tradit. Altern. Med. 2022, 20, 605–618. [Google Scholar]
- Vadhnapijyakul, A.; Suttipanta, N. The Promotion of Thai Traditional Medicine Policy in Government Hospitals: Myth or Reality. Isan J. Pharm. Sci. IJPS 2014, 9, 149–154. [Google Scholar]
- Che, C.-T.; Wang, Z.J.; Chow, M.S.S.; Lam, C.W.K. Herb-Herb Combination for Therapeutic Enhancement and Advancement: Theory, Practice and Future Perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential Therapeutic Applications of Plant-Derived Alkaloids against Inflammatory and Neurodegenerative Diseases. Evid. -Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Einstein, G.P.; Tulp, O.L.; Sainvil, F.; Branly, R. Introduction to Traditional Medicine and Their Role in Prevention and Treatment of Emerging and Re-Emerging Diseases. Biomolecules 2022, 12, 1442. [Google Scholar] [CrossRef]
- Pitiporn, S.; Tengtermwong, N. List of Herbal Medicine Products, Chao Phya Abhaibhubejhr Hospital, 1st ed.; Poramut Printing: Bangkok, Thailand, 2016. [Google Scholar]
- Sinphurmsukskul, S.; Sangwatanaroj, S. The effect of Paeka capsules on LDL cholesterol levels in patients with hypercholesterolemia. Chula J. Intern. Med. 2019, 32, 150–159. [Google Scholar]
- Mairuae, N.; Connor, J.R.; Buranrat, B.; Lee, S.Y. Oroxylum indicum (L.) extract protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2019, 20, 1933–1942. [Google Scholar] [CrossRef]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Antioxidant and anti-inflammatory activities of Oroxylum indicum Kurz (L.) fruit extract in lipopolysaccharide-stimulated BV2 microglial cells. Trop. J. Pharm. Res. 2022, 20, 833–838. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B 2016, 1011, 223–232. [Google Scholar] [CrossRef]
- Talebi, M.; İlgün, S.; Ebrahimi, V.; Talebi, M.; Farkhondeh, T.; Ebrahimi, H.; Samarghandian, S. Zingiber officinale ameliorates Alzheimer’s disease and Cognitive Impairments: Lessons from preclinical studies. Biomed. Pharmacother. 2021, 133, 111088. [Google Scholar] [CrossRef]
- Tung, B.T.; Thu, D.K.; Thu, N.T.K.; Hai, N.T. Antioxidant and acetylcholinesterase inhibitory activities of ginger root (Zingiber officinale Roscoe) extract. J. Complement. Integr. Med. 2017, 14, 20160116. [Google Scholar] [CrossRef]
- Zhang, S.; Mak, K.-K.; Marappan, P.; Balijepalli, M.K.; Chong, G.-H.; Rao Pichika, M. Simple HPLC Method for the Quantification of Gingerols (4-, 6-, 8-, and 10-) and Shogaols (6-, 8-, AND 10-) in Zingiber officinale var. Rubrum Supercritical Carbon Dioxide (SC-CO2) Extract. Rasayan J. Chem. 2022, 15, 65–71. [Google Scholar] [CrossRef]
- Ho, S.-C.; Chang, K.-S.; Lin, C.-C. Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chem. 2013, 141, 3183–3191. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.E.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Panomai, P.; Thapphasaraphong, S.; Nualkaew, N. A Comparative Study of Two Oroxylum indicum (L.) Kurz. Phenotypes Based on Phytochemicals and Antioxidant Effects, and the Anti-Inflammatory Activity of Leaf and Pod Extracts. Plants 2024, 13, 2110. [Google Scholar] [CrossRef]
- Pattamadilok, D.; Sakpetch, A. Isolation of Pinostrobin, a Chemical Marker from Fingerroots for Quality Control Purposes. J. Thai Tradit. Altern. Med. 2021, 19, 424–434. [Google Scholar]
- Rojsanga, P.; Schwaiger, S.; Stuppner, H.; Sithisarn, P. Determination of Phytochemical Contents in Extracts from Different Growth Stages of Oroxylum indicum Fruits Using HPLC-DAD and QAMS Methods. Molecules 2023, 28, 6837. [Google Scholar] [CrossRef]
- Tabboon, P.; Tantiyasawasdikul, S.; Sripanidkulchai, B. Development of simultaneous determination of five active compounds of ginger by high performance liquid chromatographic method. Isan J. Pharm. Sci. 2021, 17, 39–48. [Google Scholar] [CrossRef]
- Tan, B.C.; Tan, S.K.; Wong, S.M.; Ata, N.; Rahman, N.A.; Khalid, N. Distribution of Flavonoids and Cyclohexenyl Chalcone Derivatives in Conventional Propagated and In Vitro-Derived Field-Grown Boesenbergia rotunda (L.) Mansf. Evid.-Based Complement. Altern. Med. Ecam 2015, 2015, 451870. [Google Scholar] [CrossRef]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Orhan, I.; Şener, B.; Choudhary, M.I.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef]
- Zhao, T.; Ding, K.; Zhang, L.; Cheng, X.; Wang, C.; Wang, Z. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Žužek, M.C. Advances in cholinesterase inhibitor research—An overview of preclinical studies of selected organoruthenium(II) complexes. Int. J. Mol. Sci. 2024, 25, 9049. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Yang, S.; Hong, F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [PubMed]
- Košak, U.; Brus, B.; Knez, D.; Šink, R.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šlenc, J.; Gobec, M.; Živin, M.; et al. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef]
- Moon, H.; Yun, J. Neuroprotective effects of hesperetin on H2O2-induced damage in neuroblastoma SH-SY5Y cells. Nutr. Res. Pract. 2023, 17, 899–916. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Aβ: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Simone, A.D.; Tumiatti, V.; Andrisano, V.; Milelli, A. Glycogen Synthase Kinase 3β: A new gold rush in anti-Alzheimer’s disease multitarget drug discovery? J. Med. Chem. 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Hardas, S.S.; Bader Lange, M.L. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer disease: Many pathways to neurodegeneration. J. Alzheimer’s Dis.: JAD 2010, 20, 369–393. [Google Scholar] [CrossRef]
- Itakura, M.; Nakajima, H.; Kubo, T.; Semi, Y.; Kume, S.; Higashida, S.; Kaneshige, A.; Kuwamura, M.; Harada, N.; Kita, A.; et al. Glyceraldehyde-3-phosphate Dehydrogenase Aggregates Accelerate Amyloid-β Amyloidogenesis in Alzheimer Disease. J. Biol. Chem. 2015, 290, 26072–26087. [Google Scholar] [CrossRef]
- Cornett, K.; Puderbaugh, A.; Back, O.; Craven, R. GAPDH in neuroblastoma: Functions in metabolism and survival. Front. Oncol. 2022, 12, 979683. [Google Scholar] [CrossRef]
- Tsai, C.W.; Tsai, C.F.; Lin, K.H.; Chen, W.J.; Lin, M.S.; Hsieh, C.C.; Lin, C.C. An investigation of the correlation between the S-glutathionylated GAPDH levels in blood and Alzheimer’s disease progression. PLoS ONE 2020, 15, e0233289. [Google Scholar] [CrossRef]
- Sobue, A.; Komine, O.; Yamanaka, K. Neuroinflammation in Alzheimer’s disease: Microglial signature and their relevance to disease. Inflamm. Regen. 2023, 43, 26. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory processes in Alzheimer’s disease—Pathomechanism, diagnosis and treatment: A review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, B.; Wang, Z.; Cheng, X.; Huang, Y.; Liu, Y.; Huang, Z. Influence of four polymorphisms in ABCA1 and PTGS2 genes on risk of Alzheimer’s disease: A meta-analysis. Neurol. Sci. 2016, 37, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Moussa, N.; Dayoub, N. Exploring the role of COX-2 in Alzheimer’s disease: Potential therapeutic implications of COX-2 inhibitors. Saudi Pharm. J. SPJ 2023, 31, 101729. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Wang, P. Integrated communications between cyclooxygenase-2 and Alzheimer’s disease. FASEB J. 2019, 33, 13–33. [Google Scholar] [CrossRef]
- Michele, S.; Salluzzo, M.G.; Calogero, A.E.; Raffaele, F.; Bosco, P. Association study of COX-2 (PTGS2) –765 G/C promoter polymorphism by pyrosequencing in Sicilian patients with Alzheimer’s disease. Arch. Med. Sci. 2014, 6, 1235–1238. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Bloemer, J.; Ghanei, N.; Suppiramaniam, V.; Amin, R. Signaling mechanisms of selective PPARγ modulators in Alzheimer’s disease. PPAR Res. 2018, 2018, 2010675. [Google Scholar] [CrossRef]
- Heneka, M.T.; Reyes-Irisarri, E.; Hüll, M.; Kummer, M.P. Impact and Therapeutic Potential of PPARs in Alzheimer’s Disease. Curr. Neuropharmacol. 2011, 9, 643–650. [Google Scholar] [CrossRef]
- Strosznajder, A.K.; Wójtowicz, S.; Jeżyna, M.J.; Sun, G.Y.; Strosznajder, J.B. Recent insights on the role of PPAR-β/δ in neuroinflammation and neurodegeneration, and its potential target for therapy. Neuromolecular Med. 2021, 23, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Turgutalp, B.; Kizil, C. Multi-target drugs for Alzheimer’s disease. Trends Pharmacol. Sci. 2024, 45, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, C. Current therapeutics for Alzheimer’s disease and clinical trials. Explor. Neurosci. 2024, 3, 255–271. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Q.; Wang, X.; Jaisi, A.; Olatunji, O.J. Boesenbergia rotunda displayed anti-inflammatory, antioxidant and anti-apoptotic efficacy in doxorubicin-induced cardiotoxicity in rats. Sci. Rep. 2023, 13, 11398. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, D.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.; Bi, R.; Yao, Y. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 215–229. [Google Scholar] [CrossRef]
| Extracts | Total Phenolic Content (mg Gallic Acid Equivalent/g Extract) | Total Flavonoid Content (mg Quercetin Equivalent/g Extract) |
|---|---|---|
| PC formula | 73.65 ± 1.39 b | 31.86 ± 1.13 b |
| OI | 62.58 ± 1.22 a | 14.83 ± 0.65 a |
| ZO | 205.46 ± 7.65 c | 110.48 ± 4.06 c |
| BR | 83.29 ± 5.96 b | 131.81 ± 3.22 d |
| Samples | DPPH (IC50, µg/mL) | ABTS (IC50, µg/mL) |
|---|---|---|
| PC formula | 54.71 ± 4.15 d | 86.67 ± 2.66 d |
| OI | 66.96 ± 3.35 c | 95.14 ± 1.32 e |
| ZO | 27.99 ± 2.79 b | 24.01 ± 1.05 a |
| BR | 180.43 ± 4.16 e | 58.17 ± 0.90 c |
| Trolox (µM) | 19.87 ± 1.53 a | 40.18 ± 1.36 b |
| Samples | IC50 (µg/mL) of ChE | Selectivity | ||
|---|---|---|---|---|
| AChE | BChE | AChE | BChE | |
| PC formula | 2596.94 ± 0.50 d | 105.18 ± 5.43 c | 0.04 | 24.69 |
| OI | 2122.70 ± 0.19 d | 239.49 ± 3.13 e | 0.11 | 8.86 |
| ZO | 1026.59 ± 0.08 b | 59.52 ± 1.13 b | 0.06 | 17.42 |
| BR | 1787.51 ± 0.01 c | 178.47 ± 3.38 d | 0.10 | 10.02 |
| Tacrine (µM) | 0.25 ± 0.02 a | 0.0051 ± 0.0002 a | 0.02 | 48.76 |
| Extracts and Standard | Beta Amyloid Aggregation (IC50, µg/mL) |
|---|---|
| PC formula | 20.60 ± 2.14 b |
| OI | 47.73 ± 1.06 c |
| ZO | 20.71 ± 1.60 b |
| BR | >2000 d |
| Curcumin (µM) | 14.56 ± 0.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waiwut, P.; Takomthong, P.; Anorach, R.; Lomaboot, N.; Daodee, S.; Chulikhit, Y.; Monthakantirat, O.; Khamphukdee, C.; Boonyarat, C. Pharmacological Evaluation of a Traditional Thai Polyherbal Formula for Alzheimer’s Disease: Evidence from In Vitro and In Silico Studies. Int. J. Mol. Sci. 2025, 26, 6287. https://doi.org/10.3390/ijms26136287
Waiwut P, Takomthong P, Anorach R, Lomaboot N, Daodee S, Chulikhit Y, Monthakantirat O, Khamphukdee C, Boonyarat C. Pharmacological Evaluation of a Traditional Thai Polyherbal Formula for Alzheimer’s Disease: Evidence from In Vitro and In Silico Studies. International Journal of Molecular Sciences. 2025; 26(13):6287. https://doi.org/10.3390/ijms26136287
Chicago/Turabian StyleWaiwut, Pornthip, Pitchayakarn Takomthong, Rutchayaporn Anorach, Nattareeyada Lomaboot, Supawadee Daodee, Yaowared Chulikhit, Orawan Monthakantirat, Charinya Khamphukdee, and Chantana Boonyarat. 2025. "Pharmacological Evaluation of a Traditional Thai Polyherbal Formula for Alzheimer’s Disease: Evidence from In Vitro and In Silico Studies" International Journal of Molecular Sciences 26, no. 13: 6287. https://doi.org/10.3390/ijms26136287
APA StyleWaiwut, P., Takomthong, P., Anorach, R., Lomaboot, N., Daodee, S., Chulikhit, Y., Monthakantirat, O., Khamphukdee, C., & Boonyarat, C. (2025). Pharmacological Evaluation of a Traditional Thai Polyherbal Formula for Alzheimer’s Disease: Evidence from In Vitro and In Silico Studies. International Journal of Molecular Sciences, 26(13), 6287. https://doi.org/10.3390/ijms26136287





