Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site
Abstract
1. Introduction
2. Results
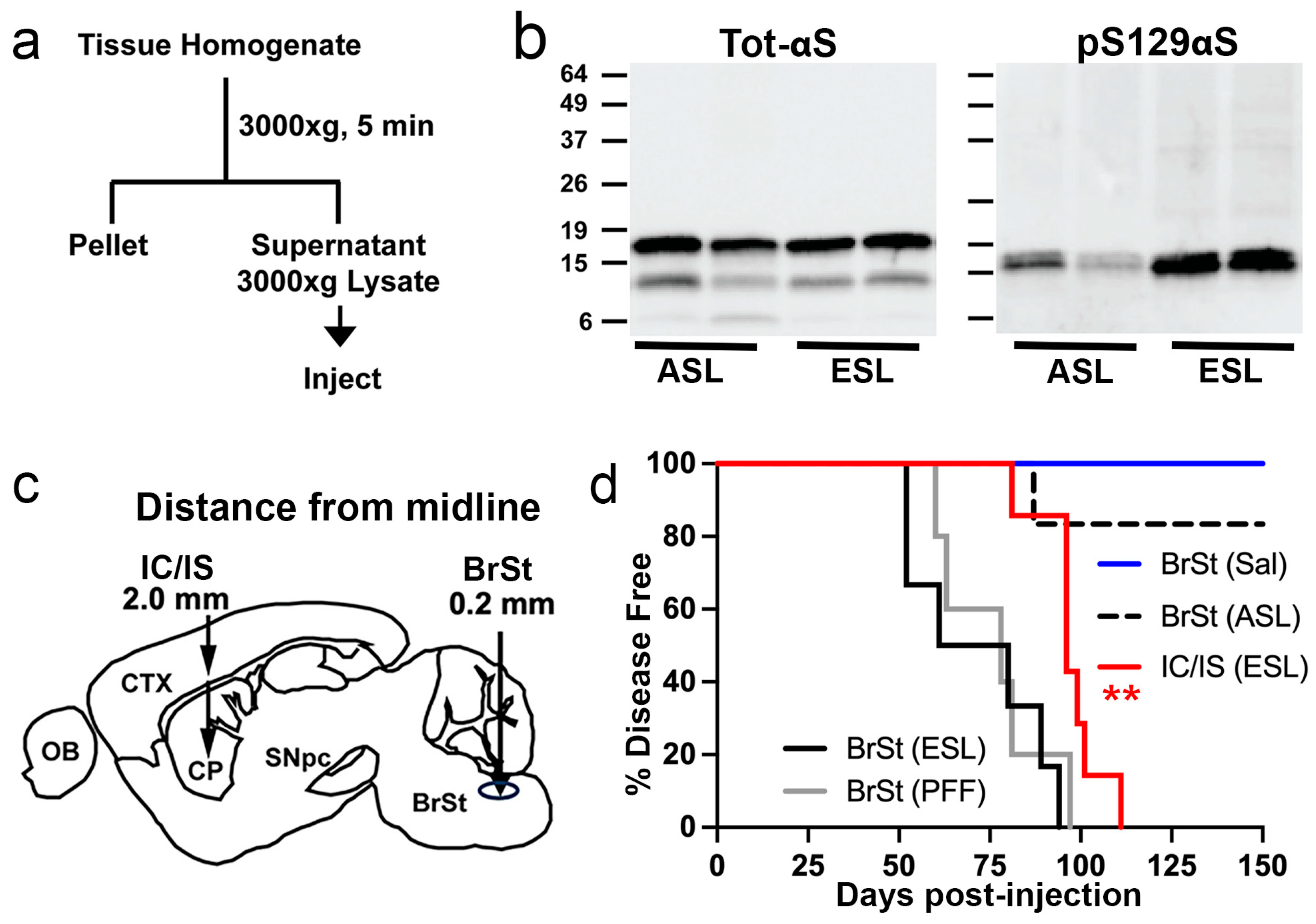
2.1. Lysates from Symptomatic TgA53T Mice and αS PFF Induce α-Synucleinopathy with Similar End-Stage Distribution
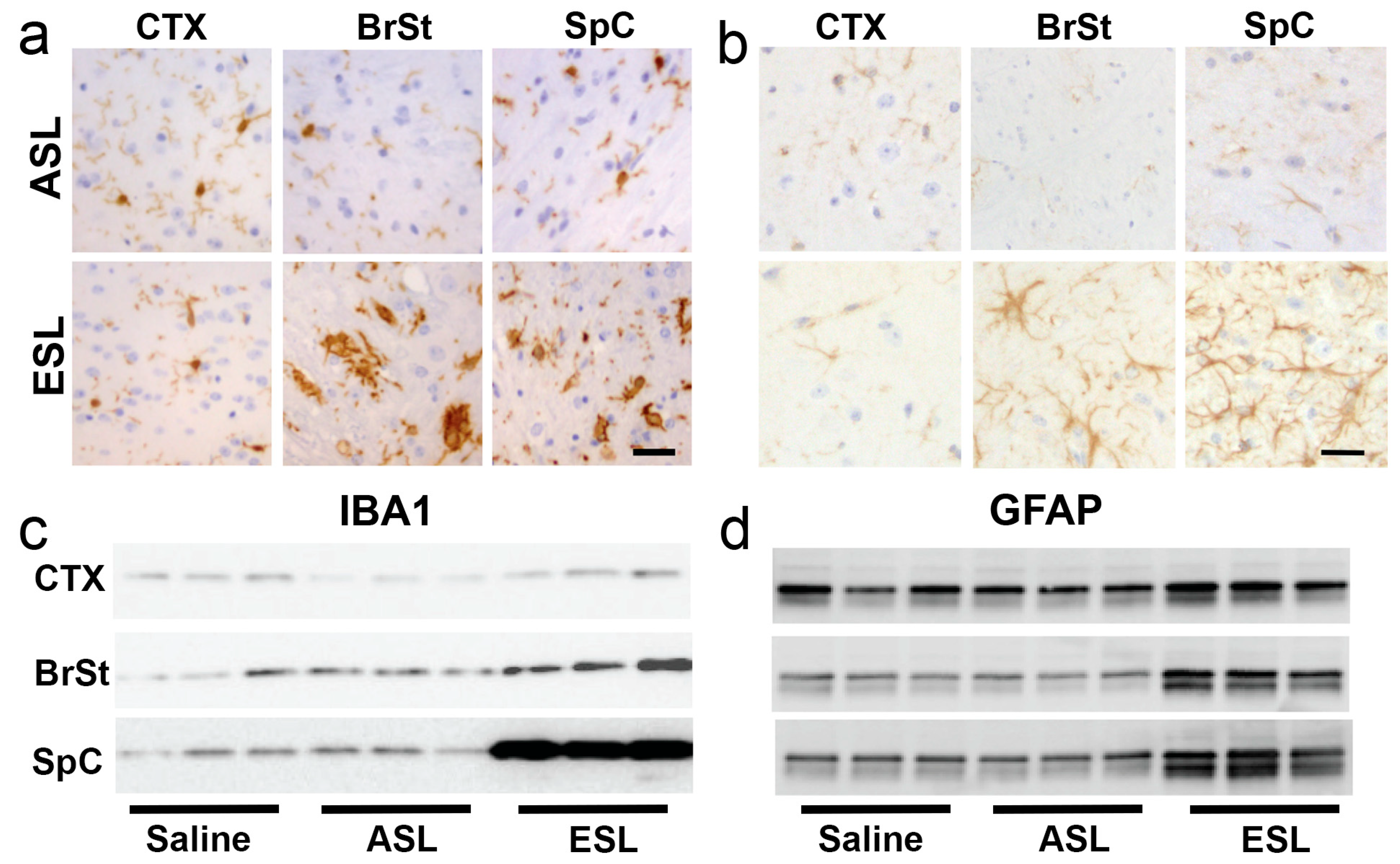
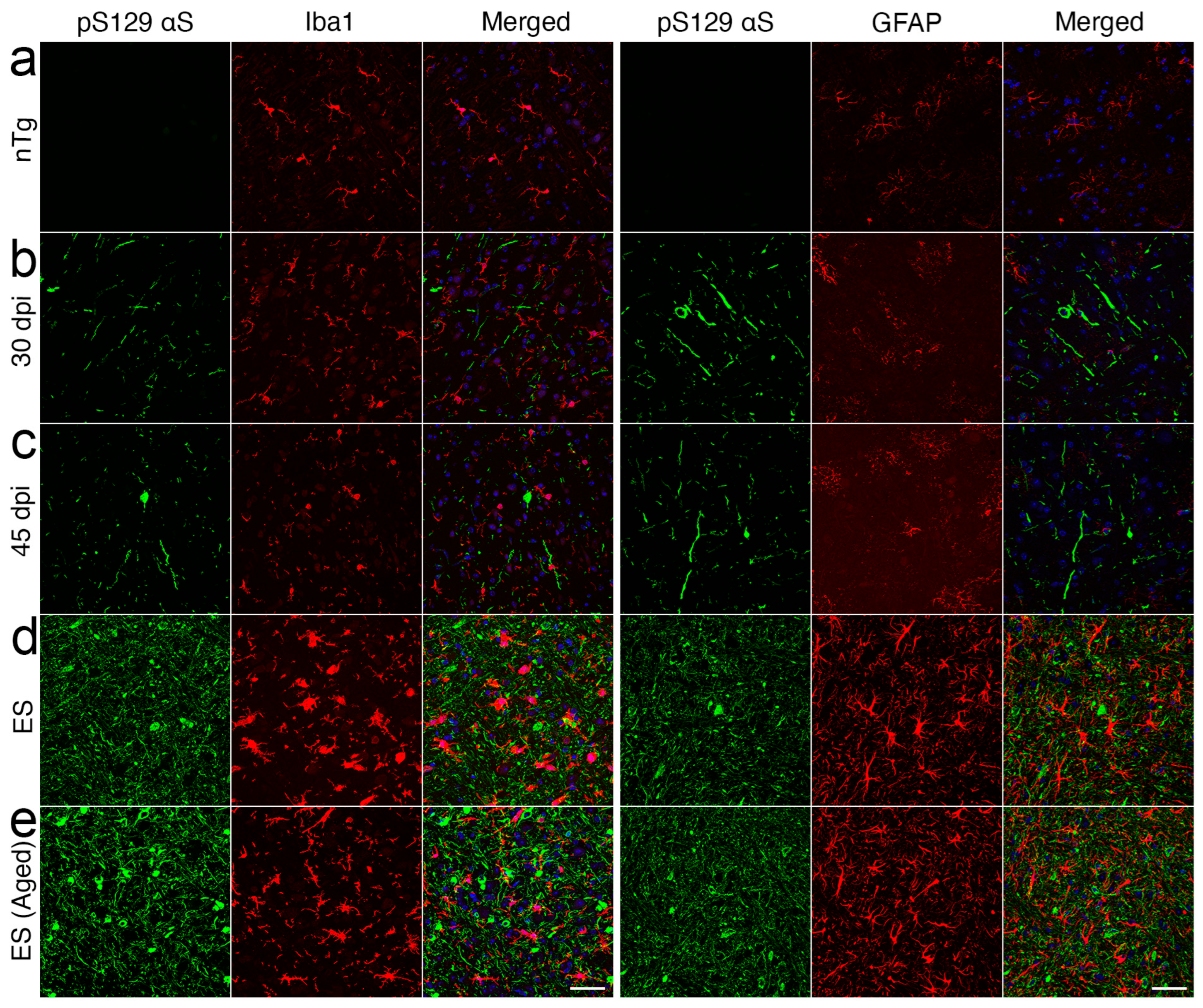
2.2. Neuroinflammation Follows Onset of α-Synucleinopathy
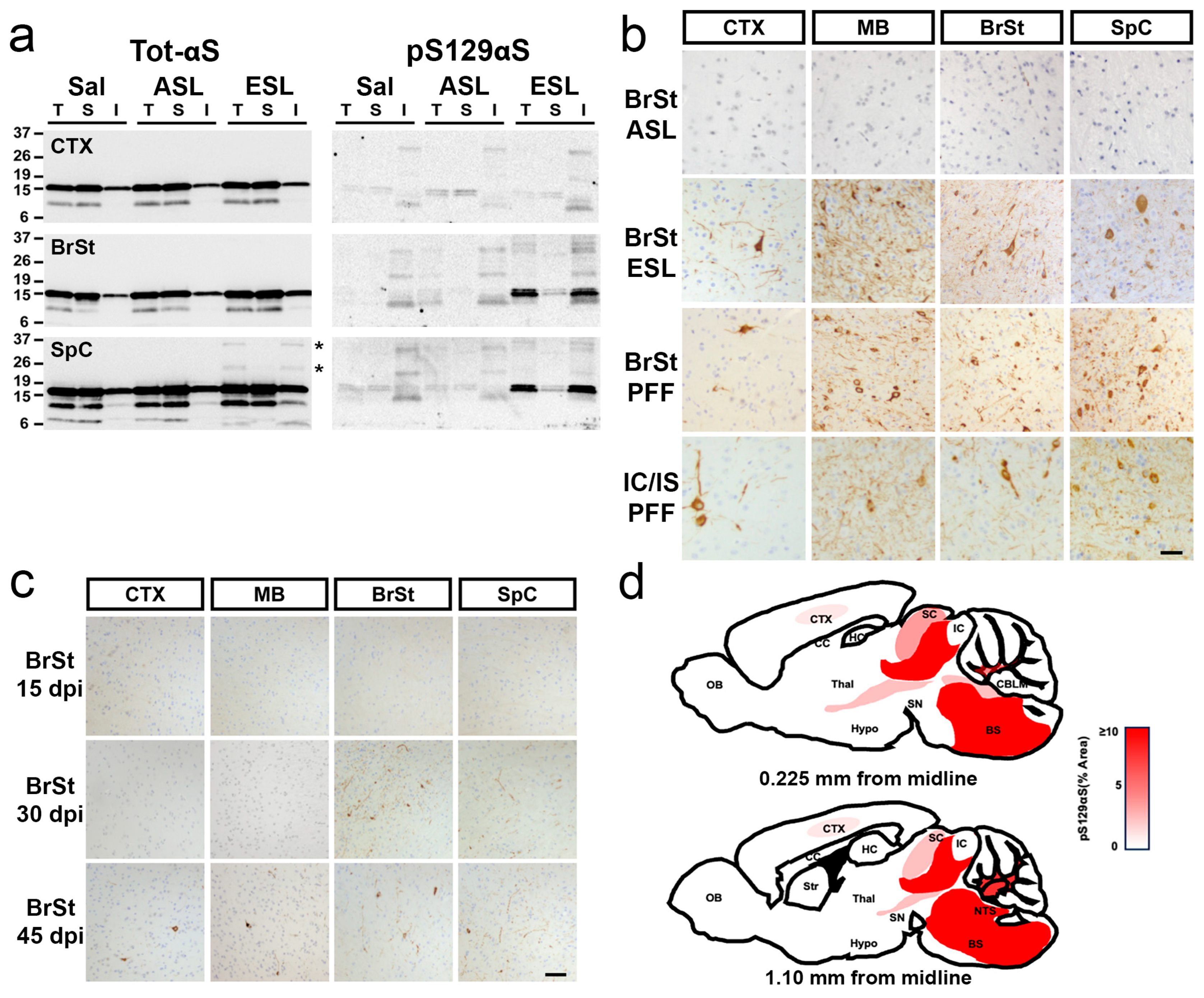
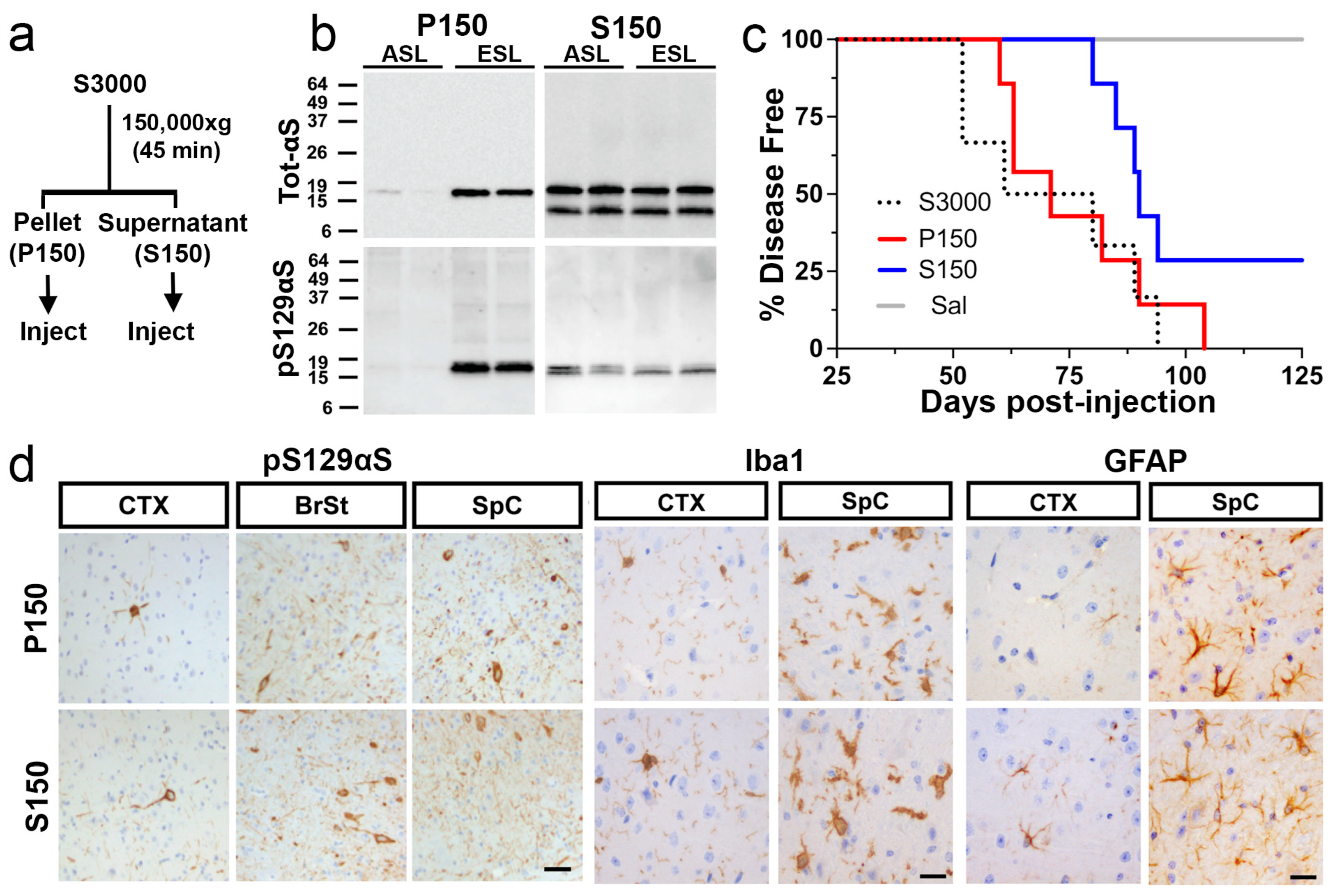
2.3. Both Highly Soluble and Insoluble Fractions Induce αS Pathology
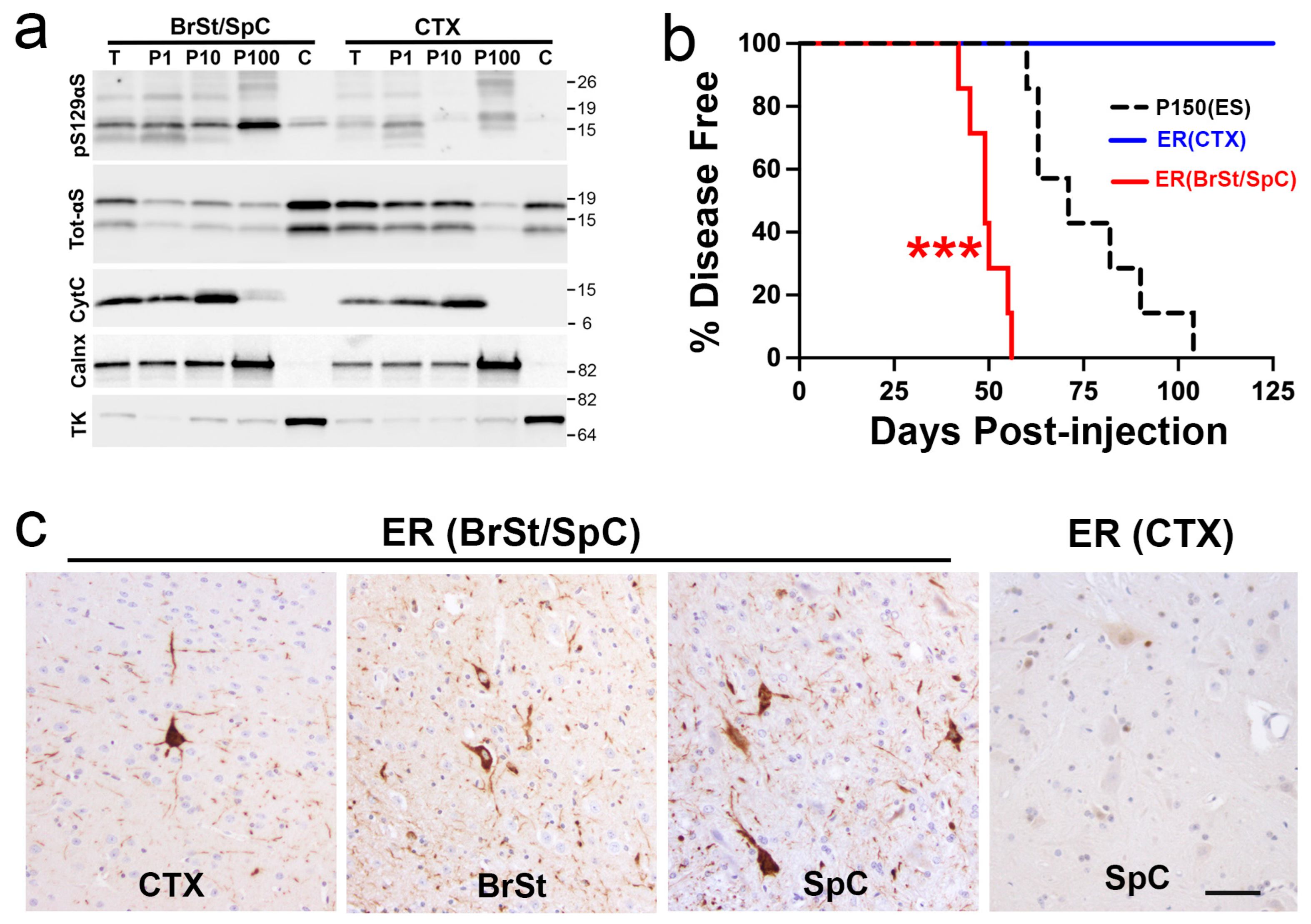
2.4. Microsomes from Symptomatic TgA53T Mice Induce Rapid α-Synucleinopathy
3. Discussion
4. Materials and Methods
4.1. Transgenic Mouse Lines
4.2. Inoculation Material
4.3. Inoculation of TgA53T Mice
4.4. Immunohistochemistry and Mapping of αS Pathology
4.5. Immunofluorescence Staining
4.6. Western Blot (Immunoblot) Analysis
4.7. Antibodies
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Vila, M.; Przedborski, S. Genetic clues to the pathogenesis of Parkinson’s disease. Nat. Med. 2004, 10 (Suppl. S7), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.; Halliday, G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Killinger, B.A.; Kordower, J.H. Spreading of alpha-synuclein—Relevant or epiphenomenon? J. Neurochem. 2019, 150, 605–611. [Google Scholar] [CrossRef]
- Del Tredici, K.; Rub, U.; De Vos, R.A.; Bohl, J.R.; Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002, 61, 413–426. [Google Scholar] [CrossRef]
- Li, J.Y.; Englund, E.; Holton, J.L.; Soulet, D.; Hagell, P.; Lees, A.J.; Lashley, T.; Quinn, N.P.; Rehncrona, S.; Bjorklund, A.; et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008, 14, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.M.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Henderson, M.X.; Cornblath, E.J.; Darwich, A.; Zhang, B.; Brown, H.; Gathagan, R.J.; Sandler, R.M.; Bassett, D.S.; Trojanowski, J.Q.; Lee, V.M.Y. Spread of alpha-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat. Neurosci. 2019, 22, 1248–1257. [Google Scholar] [CrossRef]
- Chung, H.K.; Ho, H.A.; Perez-Acuna, D.; Lee, S.J. Modeling alpha-Synuclein Propagation with Preformed Fibril Injections. J. Mov. Disord. 2019, 12, 139–151. [Google Scholar] [CrossRef]
- Sacino, A.N.; Brooks, M.; Thomas, M.A.; McKinney, A.B.; Lee, S.; Regenhardt, R.W.; McGarvey, N.H.; Ayers, J.I.; Notterpek, L.; Borchelt, D.R.; et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Bratzke, H.; Hamm-Clement, J.; Sandmann-Keil, D.; Rub, U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J. Neurol. 2002, 249 (Suppl. 3), iii1–iii5. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, S.C.; Christensen, A.; Meints, J.; Singh, B.; Martell-Martinez, H.; Karim, M.R.; Lee, M.K. Loss of tau expression attenuates neurodegeneration associated with alpha-synucleinopathy. Transl. Neurodegener. 2022, 11, 34. [Google Scholar] [CrossRef]
- Karim, M.R.; Liao, E.E.; Kim, J.; Meints, J.; Martinez, H.M.; Pletnikova, O.; Troncoso, J.C.; Lee, M.K. alpha-Synucleinopathy associated c-Abl activation causes p53-dependent autophagy impairment. Mol. Neurodegener. 2020, 15, 27. [Google Scholar] [CrossRef]
- Teravskis, P.J.; Covelo, A.; Miller, E.C.; Singh, B.; Martell-Martinez, H.A.; Benneyworth, M.A.; Gallardo, C.; Oxnard, B.R.; Araque, A.; Lee, M.K.; et al. A53T mutant alpha-synuclein induces tau dependent postsynaptic impairment independent of neurodegenerative changes. J. Neurosci. 2018, 38, 9754–9767. [Google Scholar] [CrossRef]
- Luk, K.C.; Covell, D.J.; Kehm, V.M.; Zhang, B.; Song, I.Y.; Byrne, M.D.; Pitkin, R.M.; Decker, S.C.; Trojanowski, J.Q.; Lee, V.M. Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity. Cell Rep. 2016, 16, 3373–3387. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Mante, M.; Mallory, M.; Masliah, E. beta-Synuclein inhibits alpha-synuclein aggregation: A possible role as an anti-parkinsonian factor. Neuron 2001, 32, 213–223. [Google Scholar] [CrossRef]
- Brown, J.W.; Buell, A.K.; Michaels, T.C.; Meisl, G.; Carozza, J.; Flagmeier, P.; Vendruscolo, M.; Knowles, T.P.; Dobson, C.M.; Galvagnion, C. beta-Synuclein suppresses both the initiation and amplification steps of alpha-synuclein aggregation via competitive binding to surfaces. Sci. Rep. 2016, 6, 36010. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, L.; Liu, X.; Shi, T.; Zhang, Y.; Xiao, Y.; Wang, C.; Song, L.; Li, N.; Liu, X.; et al. beta-synuclein regulates the phase transitions and amyloid conversion of alpha-synuclein. Nat. Commun. 2024, 15, 8748. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; He, W.; Fan, F.; Chen, S.; Zhu, M.; Hou, Y.; Zheng, L.; Yu, H.; Liu, Y. beta-synuclein blocks alpha-synuclein condensate fusion to disrupt the maturation of phase separation. Cell Rep. 2025, 44, 115761. [Google Scholar] [CrossRef]
- George, S.; Rey, N.L.; Tyson, T.; Esquibel, C.; Meyerdirk, L.; Schulz, E.; Pierce, S.; Burmeister, A.R.; Madaj, Z.; Steiner, J.A.; et al. Microglia affect alpha-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol. Neurodegener. 2019, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, Z.A.; Brooks, M.M.T.; Hudson, V., 3rd; Rutherford, N.J.; Golde, T.E.; Giasson, B.I.; Chakrabarty, P. Intrastriatal injection of alpha-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol. Neurodegener. 2017, 12, 40. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Giasson, B.I.; Chakrabarty, P. alpha-Synuclein and astrocytes: Tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol. 2019, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, Z.A.; Xia, Y.; Funk, C.; Riffe, C.J.; Rutherford, N.J.; Ceballos Diaz, C.; Sacino, A.N.; Price, N.D.; Golde, T.E.; Giasson, B.I.; et al. Motor neuron loss and neuroinflammation in a model of alpha-synuclein-induced neurodegeneration. Neurobiol. Dis. 2018, 120, 98–106. [Google Scholar] [CrossRef]
- Lindersson, E.; Beedholm, R.; Hojrup, P.; Moos, T.; Gai, W.; Hendil, K.B.; Jensen, P.H. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J. Biol. Chem. 2004, 279, 12924–12934. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007, 2, 18. [Google Scholar] [CrossRef]
- Colla, E.; Jensen, P.H.; Pletnikova, O.; Troncoso, J.C.; Glabe, C.; Lee, M.K. Accumulation of Toxic alpha-Synuclein Oligomer within Endoplasmic Reticulum Occurs in alpha-Synucleinopathy In Vivo. J. Neurosci. 2012, 32, 3301–3305. [Google Scholar] [CrossRef]
- Colla, E.; Coune, P.; Liu, Y.; Pletnikova, O.; Troncoso, J.C.; Iwatsubo, T.; Schneider, B.L.; Lee, M.K. Endoplasmic Reticulum Stress Is Important for the Manifestations of alpha-Synucleinopathy In Vivo. J. Neurosci. 2012, 32, 3306–3320. [Google Scholar] [CrossRef] [PubMed]
- Colla, E.; Panattoni, G.; Ricci, A.; Rizzi, C.; Rota, L.; Carucci, N.; Valvano, V.; Gobbo, F.; Capsoni, S.; Lee, M.K.; et al. Toxic properties of microsome-associated alpha-synuclein species in mouse primary neurons. Neurobiol. Dis. 2018, 111, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Thi Lai, T.; Kim, Y.E.; Nguyen, L.T.N.; Thi Nguyen, T.; Kwak, I.H.; Richter, F.; Kim, Y.J.; Ma, H.I. Microglial inhibition alleviates alpha-synuclein propagation and neurodegeneration in Parkinson’s disease mouse model. NPJ Park. Dis. 2024, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; West, N.; Colla, E.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, T.M.; Jakala, P.; Hartmann, T.; Price, D.L.; et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 2162–2167. [Google Scholar] [CrossRef]
- Sonustun, B.; Altay, M.F.; Strand, C.; Ebanks, K.; Hondhamuni, G.; Warner, T.T.; Lashuel, H.A.; Bandopadhyay, R. Pathological Relevance of Post-Translationally Modified Alpha-Synuclein (pSer87, pSer129, nTyr39) in Idiopathic Parkinson’s Disease and Multiple System Atrophy. Cells 2022, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, R.; Pan, B.; Xu, H.; Olufemi, M.F.; Gathagan, R.J.; Li, Y.; Zhang, L.; Zhang, J.; Xiang, W.; et al. Post-translational modifications of soluble alpha-synuclein regulate the amplification of pathological alpha-synuclein. Nat. Neurosci. 2023, 26, 213–225. [Google Scholar] [CrossRef]
- Lloyd, G.M.; Sorrentino, Z.A.; Quintin, S.; Gorion, K.M.; Bell, B.M.; Paterno, G.; Long, B.; Prokop, S.; Giasson, B.I. Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human alpha-synuclein transgenic mice. Acta Neuropathol. 2022, 143, 663–685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Quittot, N.; Dujardin, S.; Schlaffner, C.N.; Viode, A.; Wiedmer, A.; Beerepoot, P.; Chun, J.E.; Glynn, C.; Fernandes, A.R.; et al. Alzheimer proteopathic tau seeds are biochemically a forme fruste of mature paired helical filaments. Brain 2024, 147, 637–648. [Google Scholar] [CrossRef]
- Mate De Gerando, A.; Welikovitch, L.A.; Khasnavis, A.; Commins, C.; Glynn, C.; Chun, J.E.; Perbet, R.; Hyman, B.T. Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau. Acta Neuropathol. 2023, 146, 191–210. [Google Scholar] [CrossRef]
- Stojkovska, I.; Wani, W.Y.; Zunke, F.; Belur, N.R.; Pavlenko, E.A.; Mwenda, N.; Sharma, K.; Francelle, L.; Mazzulli, J.R. Rescue of alpha-synuclein aggregation in Parkinson’s patient neurons by synergistic enhancement of ER proteostasis and protein trafficking. Neuron 2022, 110, 436–451.e11. [Google Scholar] [CrossRef]
- Makasewicz, K.; Linse, S.; Sparr, E. Interplay of alpha-synuclein with Lipid Membranes: Cooperative Adsorption, Membrane Remodeling and Coaggregation. JACS Au 2024, 4, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, J.; Vermilyea, S.C.; Meints, J.; Martell-Martinez, H.; Lee, M.K. Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site. Int. J. Mol. Sci. 2025, 26, 6254. https://doi.org/10.3390/ijms26136254
Barnes J, Vermilyea SC, Meints J, Martell-Martinez H, Lee MK. Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site. International Journal of Molecular Sciences. 2025; 26(13):6254. https://doi.org/10.3390/ijms26136254
Chicago/Turabian StyleBarnes, Justin, Scott C. Vermilyea, Joyce Meints, Héctor Martell-Martinez, and Michael K. Lee. 2025. "Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site" International Journal of Molecular Sciences 26, no. 13: 6254. https://doi.org/10.3390/ijms26136254
APA StyleBarnes, J., Vermilyea, S. C., Meints, J., Martell-Martinez, H., & Lee, M. K. (2025). Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site. International Journal of Molecular Sciences, 26(13), 6254. https://doi.org/10.3390/ijms26136254





