Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications
Abstract
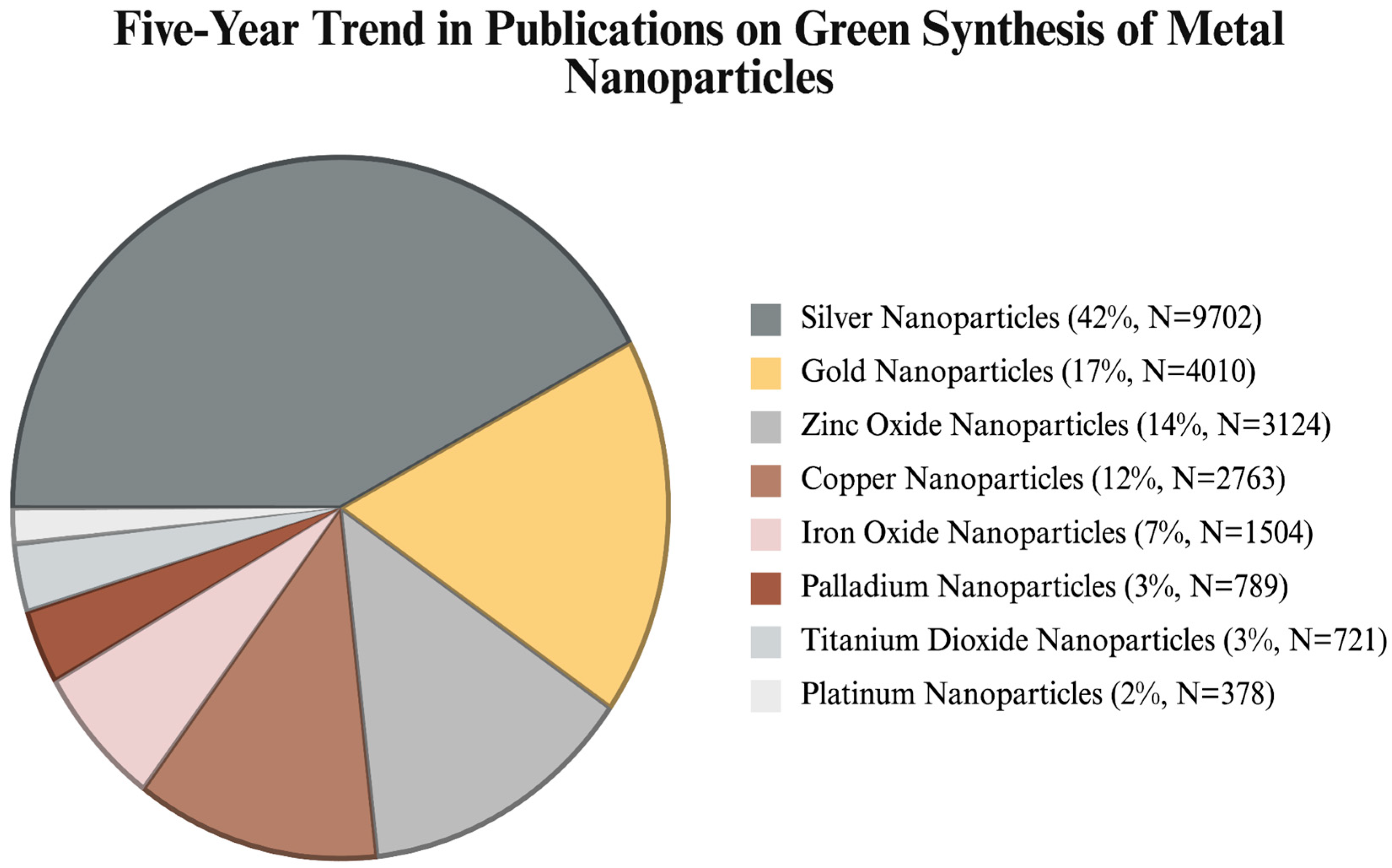
1. Introduction
2. Properties of AgNPs
2.1. Physical Properties
2.2. Chemical Properties
2.3. Optical Properties
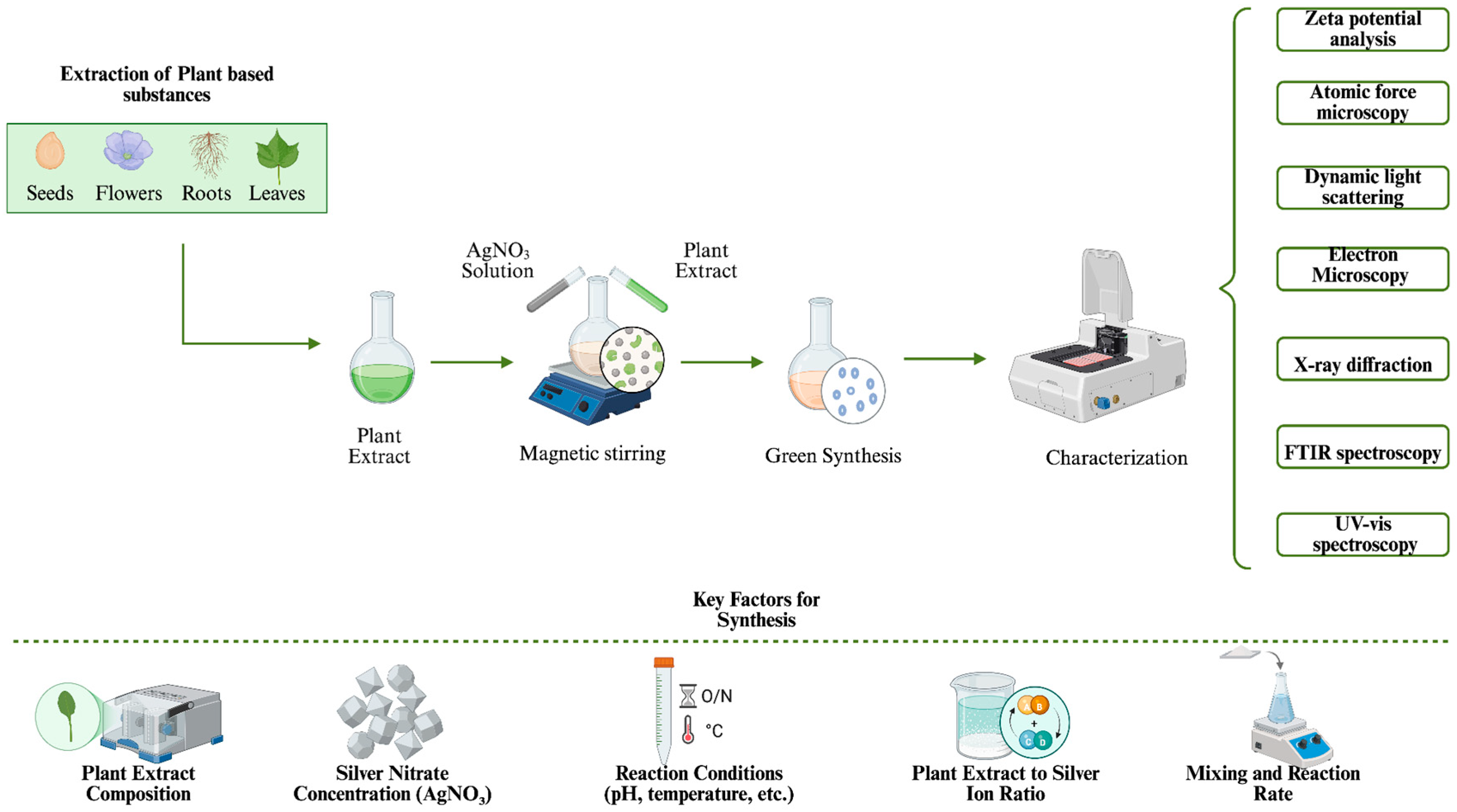
3. Mechanism and Optimization of Plant-Based Green Synthesis of AgNPs
| Plant Specie | Plant Part | Synthesis Conditions | Nanoparticle Property | Reference |
|---|---|---|---|---|
| Cakile maritima |
|
|
| [61] |
| Cassia occidentalis L. |
|
|
| [62] |
| Ziziphora clinopodioides |
|
|
| [63] |
| Cucurbita spp. |
|
|
| [64] |
| Aloe fleurentinorum |
|
|
| [65] |
| Thymus Vulgaris |
|
|
| [66] |
| Trillium govanianum |
|
|
| [67] |
| Glycyrrhiza glabra Linn |
|
|
| [68] |
| Tagetes erecta |
|
|
| [69] |
| Martynia annua |
|
|
| [70] |
| Aerva lanata |
|
|
| [71] |
| Morus nigra L. |
|
|
| [72] |
| Paullinia cupana |
|
|
| [73] |
| Clerodendrum serratum |
|
|
| [74] |
| Pandanus tectorius |
|
|
| [75] |
| Moringa oleifera |
|
|
| [76] |
| Neurada procumbens |
|
|
| [77] |
| Kalanchoe fedtschenkoi |
|
|
| [78] |
| Hibiscus tiliaceus L. |
|
|
| [79] |
| Sambucus ebulus |
|
|
| [80] |
| Lycium shawii |
|
|
| [81] |
| Acacia raddiana |
|
|
| [82] |
| Lallemantia royleana |
|
|
| [83] |
| Ocimum americanum Azadirachta indica Allium sativum Eucalyptus hybrida Zingiber officinale |
|
|
| [84] |
| Ginkgo biloba Cichorium Intybus Adiantum Capillus-Veneris Rosmarinus Officinalis |
|
|
| [85] |
| Anchusa Officinalis |
|
|
| [86] |
| Lagerstroemia speciosa |
|
|
| [87] |
| Parthenium hysterophorus |
|
|
| [88] |
| Pongamia pinnata L. |
|
|
| [89] |
| Cichorium intybus |
|
|
| [90] |
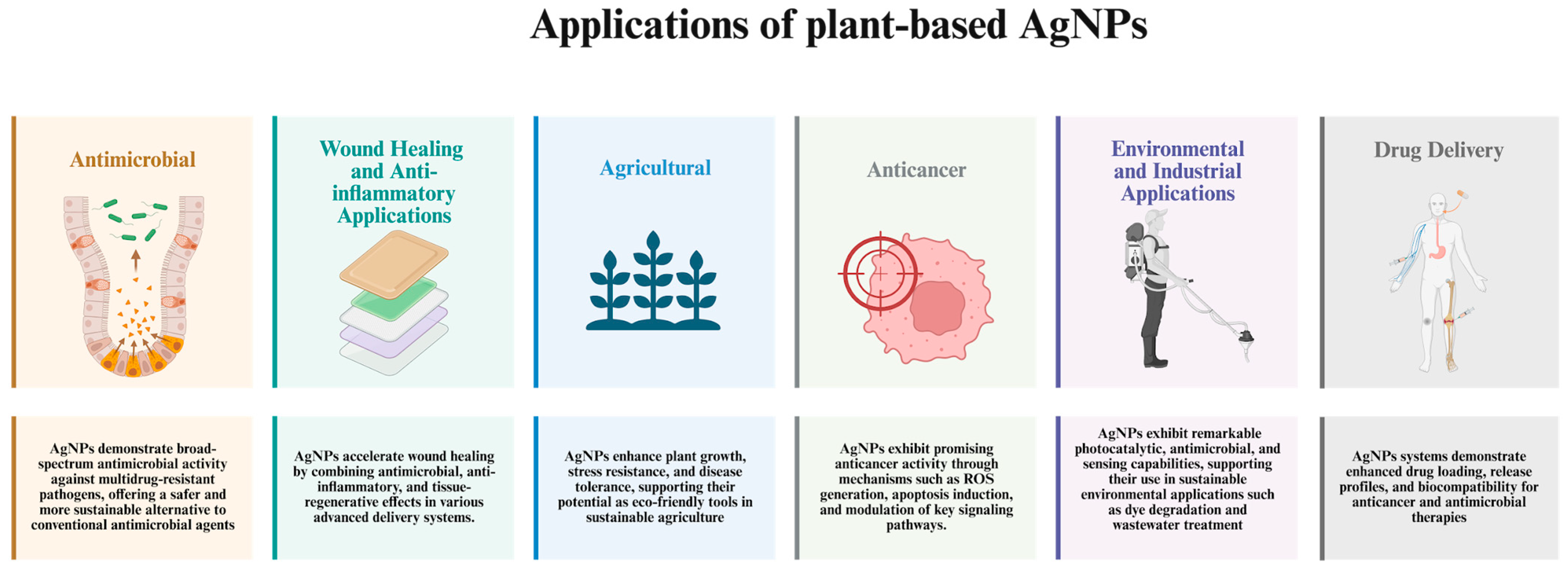
4. Application of Plant-Based Synthesized AgNPs
4.1. Antimicrobial Applications
4.2. Agricultural Applications
4.3. Anticancer Applications
4.4. Wound Healing and Anti-İnflammatory Applications
4.5. Environmental and Industrial Applications
4.6. Drug Delivery-Based Applications
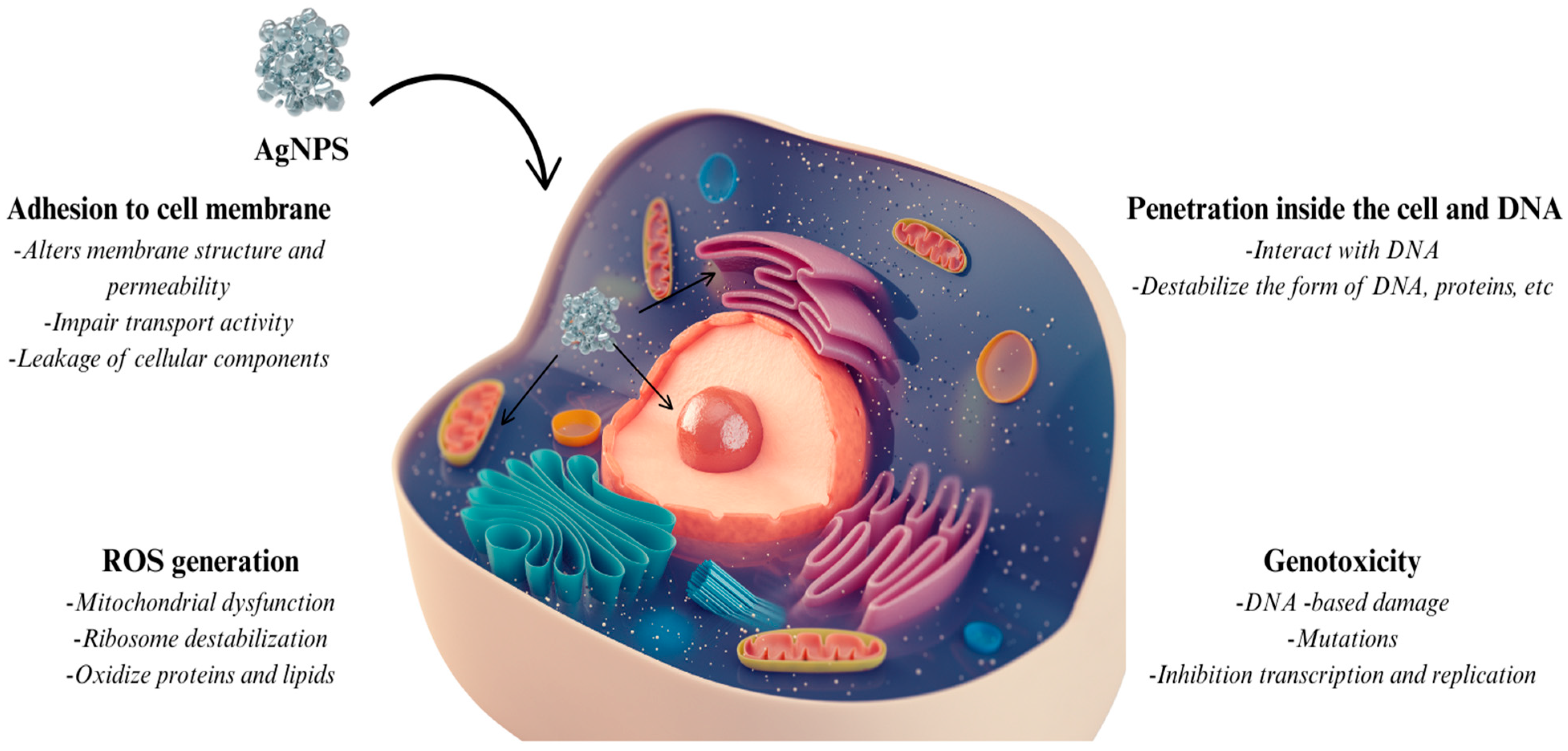
5. Future Perspective and Toxicity
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green Synthesis of Silver Nanoparticles Via Plant Extracts: Beginning a New Era in Cancer Theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Omara, M.S.; Omar, A.H.; Elakshar, M.M.; Shoukhba, Y.M.; Duman, H.; Karav, S.; Rashwan, A.K.; El-Seedi, A.H.; Altaleb, H.A.; et al. Updated Review of Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. Bioengineering 2024, 11, 1095. [Google Scholar] [CrossRef]
- Thomas, S.; Gonsalves, R.A.; Jose, J.; Zyoud, S.H.; Prasad, A.R.; Garvasis, J. Plant-Based Synthesis, Characterization Approaches, Applications and Toxicity of Silver Nanoparticles: A Comprehensive Review. J. Biotechnol. 2024, 394, 135–149. [Google Scholar] [CrossRef]
- Mishra, V.K.; Husen, A.; Rahman, Q.I.; Iqbal, M.; Sohrab, S.S.; Yassin, M.O. Plant-Based Fabrication of Silver Nanoparticles and Their Application. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 135–175. ISBN 978-3-030-05568-4. [Google Scholar]
- Aggarwal, R.; Sheikh, A.; Akhtar, M.; Ghazwani, M.; Hani, U.; Sahebkar, A.; Kesharwani, P. Understanding Gold Nanoparticles and Their Attributes in Ovarian Cancer Therapy. Mol. Cancer 2025, 24, 88. [Google Scholar] [CrossRef]
- Rupanshi; Kumar, V.; Yadav, N.; Singh, D.; Beniwal, V.; Chhabra, J.; Singh, B. Biogenic Silver Nanoparticles as Next-Generation Green Catalysts for Multifaceted Applications. Trans. Tianjin Univ. 2025, 31, 145–178. [Google Scholar] [CrossRef]
- Ramesh, N.; Lai, C.W.; Johan, M.R.B.; Mousavi, S.M.; Badruddin, I.A.; Kumar, A.; Sharma, G.; Gapsari, F. Progress in Photocatalytic Degradation of Industrial Organic Dye by Utilising the Silver Doped Titanium Dioxide Nanocomposite. Heliyon 2024, 10, e40998. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.V.; Elumalai, K. Comprehensive Review of Platinum Nanoparticles: Properties, Applications, and Toxicological Considerations. Biomed. Mater. Devices 2025, 3, 1–23. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Gold Nanoparticles in Nanomedicine: Unique Properties and Therapeutic Potential. Nanomaterials 2024, 14, 1854. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Applications 2015, 4, 325. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Habouti, S.; Solterbeck, C.-H.; Es-Souni, M. Synthesis of Silver Nano-Fir-Twigs and Application to Single Molecules Detection. J. Mater. Chem. 2010, 20, 5215. [Google Scholar] [CrossRef]
- Hu, X.; Chan, C.T. Photonic Crystals with Silver Nanowires as a Near-Infrared Superlens. Appl. Phys. Lett. 2004, 85, 1520–1522. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Chen, G.; Lu, J.; Lam, C.; Yu, Y. A Novel Green Synthesis Approach for Polymer Nanocomposites Decorated with Silver Nanoparticles and Their Antibacterial Activity. Analyst 2014, 139, 5793–5799. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and Its Nanoparticles: A Multifaceted Approach to Antibacterial Applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Berger, M. The Future is Flat—Two-Dimensional Nanomaterials. In Nanotechnology; Royal Society of Chemistry: Cambridge, UK, 2016; Chapter 4; pp. 85–114. ISBN 978-1-78262-526-1. [Google Scholar]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions onEscherichia Coli andStaphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering Nanosilver as an Antibacterial, Biosensor and Bioimaging Material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmadi, M.; Fargas, G.; Perinka, N.; Reguera, J.; Lanceros-Méndez, S.; Llanes, L.; Jiménez-Piqué, E. Silver Nanoparticles for Conductive Inks: From Synthesis and Ink Formulation to Their Use in Printing Technologies. Metals 2022, 12, 234. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical Synthesis and Antibacterial Activity of Novel-Shaped Silver Nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Huang, W. Optimized Biosynthesis and Antifungal Activity of Osmanthus Fragrans Leaf Extract-Mediated Silver Nanoparticles. Int. J. Agric. Biol. 2017, 19, 668–672. [Google Scholar] [CrossRef]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-Dependent Catalytic Activity of Silver Nanoparticles for the Oxidation of Styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A Review on the Green Synthesis of Nanoparticles, Their Biological Applications, and Photocatalytic Efficiency against Environmental Toxins. Environ. Sci. Pollut. Res. 2023, 30, 69796–69823. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Eker, F.; Duman, H.; Singh, P.; Bechelany, M.; Karav, S. Lactoferrin as a Versatile Agent in Nanoparticle Applications: From Therapeutics to Agriculture. Nanomaterials 2024, 14, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kockert, M.; Kojda, D.; Mitdank, R.; Mogilatenko, A.; Wang, Z.; Ruhhammer, J.; Kroener, M.; Woias, P.; Fischer, S.F. Nanometrology: Absolute Seebeck Coefficient of Individual Silver Nanowires. Sci. Rep. 2019, 9, 20265. [Google Scholar] [CrossRef]
- Bogle, K.A.; Dhole, S.D.; Bhoraskar, V.N. Silver Nanoparticles: Synthesis and Size Control by Electron Irradiation. Nanotechnology 2006, 17, 3204–3208. [Google Scholar] [CrossRef]
- Iyahraja, S.; Rajadurai, J.S. Study of Thermal Conductivity Enhancement of Aqueous Suspensions Containing Silver Nanoparticles. AIP Adv. 2015, 5, 057103. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, X.; Wu, F.; Liu, F. High-Performance Thermal Management Nanocomposites: Silver Functionalized Graphene Nanosheets and Multiwalled Carbon Nanotube. Crystals 2018, 8, 398. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Yu, M.; Kathaperumal, M.; Wong, C.-P. A Review of the Thermal Conductivity of Silver-Epoxy Nanocomposites as Encapsulation Material for Packaging Applications. Chem. Eng. J. 2022, 446, 137319. [Google Scholar] [CrossRef]
- Wu, H.; Carrete, J.; Zhang, Z.; Qu, Y.; Shen, X.; Wang, Z.; Zhao, L.-D.; He, J. Strong Enhancement of Phonon Scattering Through Nanoscale Grains in Lead Sulfide Thermoelectrics. NPG Asia Mater. 2014, 6, e108. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Corrigendum: Silver Nanoparticles: Synthesis, Properties, Toxicology, Applications and Perspectives. Adv. Nat. Sci Nanosci. Nanotechnol. 2018, 9, 049501. [Google Scholar] [CrossRef]
- De Silva, C.; Nawawi, N.M.; Abd Karim, M.M.; Abd Gani, S.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries. Animals 2021, 11, 2097. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Chen, Y.; McLellan, J.M.; Xiong, Y.; Li, Z.-Y.; Ginger, D.; Xia, Y. Synthesis and Optical Properties of Silver Nanobars and Nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef]
- Duman, H.; Akdaşçi, E.; Eker, F.; Bechelany, M.; Karav, S. Gold Nanoparticles: Multifunctional Properties, Synthesis, and Future Prospects. Nanomaterials 2024, 14, 1805. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Nosheen, E.; Shah, A.; Iftikhar, F.J.; Aftab, S.; Bakirhan, N.K.; Ozkan, S.A. Optical Nanosensors for Pharmaceutical Detection. In New Developments in Nanosensors for Pharmaceutical Analysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–140. ISBN 978-0-12-816144-9. [Google Scholar]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized Surface Plasmon Resonance Sensing of Trenbolone Acetate Dopant Using Silver Nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M. Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective. Appl. Biochem. Biotechnol. 2024, 196, 3636–3669. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green Synthesis of Silver Nanoparticles Using Medicinal Plants: Characterization and Application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Tasca, F.; Antiochia, R. Biocide Activity of Green Quercetin-Mediated Synthesized Silver Nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef]
- Ahani, M.; Khatibzadeh, M. Green Synthesis of Silver Nanoparticles Using Gallic Acid as Reducing and Capping Agent: Effect of pH and Gallic Acid Concentration on Average Particle Size and Stability. Inorg. Nano-Metal. Chem. 2022, 52, 234–240. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.-L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant-Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-Puma, R.M.; Vega-Chacón, J.; Raúl Jauja-Ccana, V.; Villa, J.E.L.; La Rosa-Toro, A. Green Synthesis and Characterization of Silver Nanoparticles Using Grape Stalk Extract. J. Mol. Liq. 2024, 403, 124927. [Google Scholar] [CrossRef]
- Liu, L.; Yu, C.; Ahmad, S.; Ri, C.; Tang, J. Preferential Role of Distinct Phytochemicals in Biosynthesis and Antibacterial Activity of Silver Nanoparticles. J. Environ. Manag. 2023, 344, 118546. [Google Scholar] [CrossRef]
- Yadav, R.; Preet, S. Comparative Assessment of Green and Chemically Synthesized Glutathione Capped Silver Nanoparticles for Antioxidant, Mosquito Larvicidal and Eco-Toxicological Activities. Sci. Rep. 2023, 13, 8152. [Google Scholar] [CrossRef] [PubMed]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H.; Dashtbani-Roozbehani, A. Comparative Assessment of the Biological Activity of the Green Synthesized Silver Nanoparticles and Aqueous Leaf Extract of Perilla frutescens (L.). Sci. Rep. 2023, 13, 6391. [Google Scholar] [CrossRef] [PubMed]
- Elazab, N.T.; Baka, Z.A.M.; Saleh, H.H.; El-Zahed, M.M. Green Synthesis of Silver Nanoparticles Using Cakile Maritima Seed Extract: Molecular, Antifungal and Physiological Studies. Physiol. Mol. Plant Pathol. 2024, 129, 102183. [Google Scholar] [CrossRef]
- Arya, A.; Tyagi, P.K.; Bhatnagar, S.; Bachheti, R.K.; Bachheti, A.; Ghorbanpour, M. Biosynthesis and Assessment of Antibacterial and Antioxidant Activities of Silver Nanoparticles Utilizing Cassia occidentalis L. Seed. Sci. Rep. 2024, 14, 7243. [Google Scholar] [CrossRef]
- Pirabbasi, E.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R.; Kalantar, M. Chemical Characterization and Effect of Ziziphora clinopodioides Green-Synthesized Silver Nanoparticles on Cytotoxicity, Antioxidant, and Antidiabetic Activities in Streptozotocin-Induced Hepatotoxicity in Wistar Diabetic Male Rats. Food Sci. Nutr. 2024, 12, 3443–3451. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Eftekhari, A.; Shafaroodi, A.; Tavakoli, S.; Jafari, S.; Baran, A.; Baran, M.F.; Jafari, S.; Ahmadian, E. Green-Synthesized Silver Nanoparticles from Peel Extract of Pumpkin as a Potent Radiosensitizer against Triple-Negative Breast Cancer (TNBC). Cancer Nanotechnol. 2024, 15, 47. [Google Scholar] [CrossRef]
- Jamil, Y.M.S.; Al-Hakimi, A.N.; Al-Maydama, H.M.A.; Almahwiti, G.Y.; Qasem, A.; Saleh, S.M. Optimum Green Synthesis, Characterization, and Antibacterial Activity of Silver Nanoparticles Prepared from an Extract of Aloe fleurentinorum. Int. J. Chem. Eng. 2024, 2024, 2804165. [Google Scholar] [CrossRef]
- Ejaz, U.; Afzal, M.; Mazhar, M.; Riaz, M.; Ahmed, N.; Rizg, W.Y.; Alahmadi, A.A.; Badr, M.Y.; Mushtaq, R.Y.; Yean, C.Y. Characterization, Synthesis, and Biological Activities of Silver Nanoparticles Produced via Green Synthesis Method Using Thymus Vulgaris Aqueous Extract. Int. J. Nanomed. 2024, 19, 453–469. [Google Scholar] [CrossRef]
- Manzoor, S.I.; Jabeen, F.; Patel, R.; Alam Rizvi, M.M.; Imtiyaz, K.; Malik, M.A.; Dar, T.A. Green Synthesis of Biocompatible Silver Nanoparticles Using Trillium govanianum Rhizome Extract: Comprehensive Biological Evaluation and in Silico Analysis. Mater. Adv. 2025, 6, 682–702. [Google Scholar] [CrossRef]
- Vijapur, L.S.; Shalavadi, M.; Desai, A.R.; Hiremath, J.N.; Gudigennavar, A.S.; Shidramshettar, S.L.; Hiremath, S.R.; Peram, M.R.; Kittur, B.S. Wound Healing Potential of Green Synthesized Silver Nanoparticles of Glycyrrhiza Glabra Linn Root Extract: A Preclinical Study. J. Trace Elem. Miner. 2025, 11, 100214. [Google Scholar] [CrossRef]
- Deepika; Singh, R.K.; Priyadarshini, A.; Yeam, D.A.; Anjali; Bhattacharya, A.; Vaishnav, R.; Kumar, A. Green Synthesis of Silver Nanoparticles Using Marigold Flowers Extract for Eco-Friendly Management of Root Knot Nematode. Plant Nano Biol. 2025, 12, 100150. [Google Scholar] [CrossRef]
- Abbigeri, M.B.; Thokchom, B.; Singh, S.R.; Bhavi, S.M.; Harini, B.P.; Yarajarla, R.B. Antioxidant and Anti-Diabetic Potential of the Green Synthesized Silver Nanoparticles Using Martynia annua L. Root Extract. Nano TransMed 2025, 4, 100070. [Google Scholar] [CrossRef]
- Ganesan, T.; Muthukrishnan, S.; Albeshr, M.F.; Selvankumar, T.; Pradeepkumar, S.; Indumathi, K.P.; Anto, B. Aerva lanata Flower Extract Mediated Green Synthesis of Silver Nanoparticles: Their Characterization, In vitro Antioxidants and Antimicrobial Investigations. Polym. Adv. Technol. 2024, 35, e6354. [Google Scholar] [CrossRef]
- Jeon, Y.-N.; Ryu, S.-J.; Lee, H.-Y.; Kim, J.-O.; Baek, J.-S. Green Synthesis of Silver Nanoparticle Using Black Mulberry and Characterization, Phytochemical, and Bioactivity. Antibiotics 2024, 13, 686. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Vieira, Í.R.S.; Souza, L.M.D.S.; Florêncio, I.; Silva, I.G.M.D.; Tavares Junior, A.G.; Machado, Y.A.A.; Santos, L.C.D.; Taube, P.S.; Nakazato, G.; et al. Green Synthesis of Silver Nanoparticles Using Paullinia Cupana Kunth Leaf Extract Collected in Different Seasons: Biological Studies and Catalytic Properties. Pharmaceutics 2025, 17, 356. [Google Scholar] [CrossRef]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Dehari, D.; Nath, G.; Singh, M. Facile Green Synthesis of Silver Nanoparticles Derived from the Medicinal Plant Clerodendrum Serratum and Its Biological Activity against Mycobacterium Species. Heliyon 2024, 10, e31116. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Ragavendran, C.; Arulbalachandran, D.; Fahad Alrefaei, A.; Rajendran, R. Green Synthesis of Silver Nanoparticles Using Pandanus Tectorius Aerial Root Extract: Characterization, Antibacterial, Cytotoxic, and Photocatalytic Properties, and Ecotoxicological Assessment. Inorg. Chem. Commun. 2024, 168, 112882. [Google Scholar] [CrossRef]
- Haris, Z.; Ahmad, I. Green Synthesis of Silver Nanoparticles Using Moringa Oleifera and Its Efficacy against Gram-Negative Bacteria Targeting Quorum Sensing and Biofilms. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 156–167. [Google Scholar] [CrossRef]
- Alomar, T.; AlMasoud, N.; Awad, M.; AlOmar, R.; Merghani, N.; El-Zaidy, M.; Bhattarai, A. Designing Green Synthesis-Based Silver Nanoparticles for Antimicrobial Theranostics and Cancer Invasion Prevention. Int. J. Nanomed. 2024, 19, 4451–4464. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Sánchez-Ante, G.; Cerro-López, M.; Minutti-Calva, Y.; Navarro-López, D.E.; Lozada-Ramírez, J.D.; Bach, H.; López-Mena, E.R.; Sánchez-Arreola, E. Green Synthesis of Silver Nanoparticles with Extracts from Kalanchoe fedtschenkoi: Characterization and Bioactivities. Biomolecules 2024, 14, 782. [Google Scholar] [CrossRef]
- Konduri, V.V.; Kalagatur, N.K.; Gunti, L.; Mangamuri, U.K.; Kalagadda, V.R.; Poda, S.; Krishna, S.B.N. Green Synthesis of Silver Nanoparticles from Hibiscus tiliaceus L. Leaves and Their Applications in Dye Degradation, Antioxidant, Antimicrobial, and Anticancer Activities. S. Afr. J. Bot. 2024, 168, 476–487. [Google Scholar] [CrossRef]
- Karan, T.; Gonulalan, Z.; Erenler, R.; Kolemen, U.; Eminagaoglu, O. Green Synthesis of Silver Nanoparticles Using Sambucus Ebulus Leaves Extract: Characterization, Quantitative Analysis of Bioactive Molecules, Antioxidant and Antibacterial Activities. J. Mol. Struct. 2024, 1296, 136836. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, R.; Alhan, S.; Sharma, H.; Singh, N.; Yogi, R.; Chhokar, V.; Beniwal, V.; Kumar Ghosh, M.; Kumar Chandraker, S.; et al. Lycium Shawii Mediated Green Synthesis of Silver Nanoparticles, Characterization and Assessments of Their Phytochemical, Antioxidant, Antimicrobial Properties. Inorg. Chem. Commun. 2024, 159, 111735. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Taha, G.M.; Hagaggi, N.S.A.; Moghazy, M.A. Green Synthesis of Silver Nanoparticles and Its Environmental Sensor Ability to Some Heavy Metals. BMC Chem. 2024, 18, 7. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Elshafie, H.S.; Pohl, P. Green Synthesis of Silver Nanoparticles (AgNPs) by Lallemantia Royleana Leaf Extract: Their Bio-Pharmaceutical and Catalytic Properties. J. Photochem. Photobiol. A Chem. 2024, 448, 115318. [Google Scholar] [CrossRef]
- Sharma, K.; Raj, H.; Sharma, A.; Sharma, M.; Sharma, A.; Thakur, A. Green Silver Nanoparticles and Their Activity as Antifungal Agents Against Penicillium Digitatum Causing Green Mould of Citrus. ChemistrySelect 2024, 9, e202402180. [Google Scholar] [CrossRef]
- Mohamed, A.; Dayo, M.; Alahmadi, S.; Ali, S. Anti-Inflammatory and Antimicrobial Activity of Silver Nanoparticles Green-Synthesized Using Extracts of Different Plants. Nanomaterials 2024, 14, 1383. [Google Scholar] [CrossRef]
- Keskin, C.; Aslan, S.; Baran, M.F.; Baran, A.; Eftekhari, A.; Adıcan, M.T.; Ahmadian, E.; Arslan, S.; Mohamed, A.J. Green Synthesis and Characterization of Silver Nanoparticles Using Anchusa Officinalis: Antimicrobial and Cytotoxic Potential. Int. J. Nanomed. 2025, 20, 4481–4502. [Google Scholar] [CrossRef] [PubMed]
- Varadavenkatesan, T.; Nagendran, V.; Vinayagam, R.; Goveas, L.C.; Selvaraj, R. Green Synthesis of Silver Nanoparticles Using Lagerstroemia speciosa Fruit Extract: Catalytic Efficiency in Dye Degradation. Mater. Technol. 2025, 40, 2463955. [Google Scholar] [CrossRef]
- Gunasekar, B.; Pal, D.B.; Kumar, S.; Kapoor, A. Biogenic Synthesis of Silver Nanoparticles Using Parthenium hysterophorus Floral Extract and Their Multifaceted Biomedical Applications. Plant Nano Biol. 2025, 12, 100148. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Ahmad, I.S.; Ahmed, R.; Aftab, Z.-H.; Rizwana, H. Phytofabricated Silver Nanoparticles Unlock New Potential in Tomato Plants by Combating Wilt Infection and Enhancing Plant Growth. Sci. Rep. 2025, 15, 10527. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Jimenez, L.E.; Álvarez, R.M.S.; Dalmasso, P.R.; Hollmann, A. Physicochemical and Biological Characterization and Applications of Silver Nanoparticles Obtained by Green Synthesis Using Cichorium Intybus. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136075. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of Plant-Mediated Synthesis of Silver Nanoparticles—A Review on Biomolecules Involved, Characterisation and Antibacterial Activity. Chem.-Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green Synthesized Plant-Based Silver Nanoparticles: Therapeutic Prospective for Anticancer and Antiviral Activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ulbrich, P.; Ruml, T. Effect of the Physicochemical Changes in the Antimicrobial Durability of Green Synthesized Silver Nanoparticles During Their Long-Term Storage. RSC Adv. 2022, 12, 30386–30403. [Google Scholar] [CrossRef]
- Zulfiqar, Z.; Khan, R.R.M.; Summer, M.; Saeed, Z.; Pervaiz, M.; Rasheed, S.; Shehzad, B.; Kabir, F.; Ishaq, S. Plant-Mediated Green Synthesis of Silver Nanoparticles: Synthesis, Characterization, Biological Applications, and Toxicological Considerations: A Review. Biocatal. Agric. Biotechnol. 2024, 57, 103121. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of Silver Nanoparticles Using Plants Extract and Analysis of Their Antimicrobial Property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green Synthesis of Silver Nanoparticles Using Hagenia Abyssinica (Bruce) J.F. Gmel Plant Leaf Extract and Their Antibacterial and Anti-Oxidant Activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green Synthesis of Silver Nanoparticles: Effect of Synthesis Reaction Parameters on Antimicrobial Activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Rahman, K.U.; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Ni, Q.; Zhu, T.; Wang, W.; Guo, D.; Li, Y.; Chen, T.; Zhang, X. Green Synthesis of Narrow-Size Silver Nanoparticles Using Ginkgo Biloba Leaves: Condition Optimization, Characterization, and Antibacterial and Cytotoxic Activities. Int. J. Mol. Sci. 2024, 25, 1913. [Google Scholar] [CrossRef]
- Felimban, A.I.; Alharbi, N.S.; Alsubhi, N.S. Optimization, Characterization, and Anticancer Potential of Silver Nanoparticles Biosynthesized Using Olea Europaea. Int. J. Biomater. 2022, 2022, 6859637. [Google Scholar] [CrossRef]
- Geetha, A.R.; George, E.; Srinivasan, A.; Shaik, J. Optimization of Green Synthesis of Silver Nanoparticles from Leaf Extracts of Pimenta dioica (Allspice). Sci. World J. 2013, 2013, 362890. [Google Scholar] [CrossRef]
- Subha, T.; Srilatha, M.; Naveen, P.; Thirumalaisamy, R. Green Synthesis, Characterization and Optimization of Silver Nanoparticles from Carica Papaya Using Box Behnken Design and Its Activity Against Dental Caries Causing Streptococcus sp. Chem. Data Collect. 2024, 51, 101139. [Google Scholar] [CrossRef]
- Fazil, M.M.; Gul, A.; Jawed, H. Optimization of Silver Nanoparticles Synthesis via Plackett–Burman Experimental Design: In vitro Assessment of Their Efficacy against Oxidative Stress-Induced Disorders. RSC Adv. 2024, 14, 20809–20823. [Google Scholar] [CrossRef] [PubMed]
- Laime-Oviedo, L.A.; Soncco-Ccahui, A.A.; Peralta-Alarcon, G.; Arenas-Chávez, C.A.; Pineda-Tapia, J.L.; Díaz-Rosado, J.C.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Davies, N.M.; Yáñez, J.A.; et al. Optimization of Synthesis of Silver Nanoparticles Conjugated with Lepechinia meyenii (Salvia) Using Plackett-Burman Design and Response Surface Methodology—Preliminary Antibacterial Activity. Processes 2022, 10, 1727. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Ghosh, M.K.; Lal, M.; Shukla, R. A Review on Plant-Mediated Synthesis of Silver Nanoparticles, Their Characterization and Applications. Nano Express 2021, 2, 022008. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications. Nanomaterials 2024, 14, 1618. [Google Scholar] [CrossRef]
- Aryan; Ruby; Mehata, M.S. Green Synthesis of Silver Nanoparticles Using Kalanchoe pinnata Leaves (Life Plant) and Their Antibacterial and Photocatalytic Activities. Chem. Phys. Lett. 2021, 778, 138760. [Google Scholar] [CrossRef]
- Hashemi, Z.; Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Sustainable Biosynthesis of Metallic Silver Nanoparticles Using Barberry Phenolic Extract: Optimization and Evaluation of Photocatalytic, In vitro Cytotoxicity, and Antibacterial Activities against Multidrug-Resistant Bacteria. Inorg. Chem. Commun. 2022, 139, 109320. [Google Scholar] [CrossRef]
- Tesfaye, M.; Gonfa, Y.; Tadesse, G.; Temesgen, T.; Periyasamy, S. Green Synthesis of Silver Nanoparticles Using Vernonia Amygdalina Plant Extract and Its Antimicrobial Activities. Heliyon 2023, 9, e17356. [Google Scholar] [CrossRef]
- Ghasemi, S.; Dabirian, S.; Kariminejad, F.; Koohi, D.E.; Nemattalab, M.; Majidimoghadam, S.; Zamani, E.; Yousefbeyk, F. Process Optimization for Green Synthesis of Silver Nanoparticles Using Rubus Discolor Leaves Extract and Its Biological Activities against Multi-Drug Resistant Bacteria and Cancer Cells. Sci. Rep. 2024, 14, 4130. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Díaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado-Soto, D.I.; Treviño-González, F.M.; et al. In vivo Antimicrobial Activity of Silver Nanoparticles Produced via a Green Chemistry Synthesis Using Acacia Rigidula as a Reducing and Capping Agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Ahsan Bajwa, A.; Parveen, A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In vitro Therapeutic Evaluation. Molecules 2020, 25, 3324. [Google Scholar] [CrossRef]
- Hawar, S.N.; Al-Shmgani, H.S.; Al-Kubaisi, Z.A.; Sulaiman, G.M.; Dewir, Y.H.; Rikisahedew, J.J. Green Synthesis of Silver Nanoparticles from Alhagi Graecorum Leaf Extract and Evaluation of Their Cytotoxicity and Antifungal Activity. J. Nanomater. 2022, 2022, 1058119. [Google Scholar] [CrossRef]
- Jebril, S.; Ben Jenana, R.K.; Dridi, C. Green Synthesis of Silver Nanoparticles Using Melia azedarach Leaf Extract and Their Antifungal Activities: In vitro and In vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm Activity of Green Synthesized Silver Nanoparticles Against Biofilm Associated Enterococcal Urinary Pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Nabil, A.; El-Hosainy, H.; Tahway, R.; Taha, M.S. Green Synthesis of Silver Nanoparticles Using Curcumin: A Comparative Study of Antimicrobial and Antibiofilm Effects on Acinetobacter baumannii against Chemical Conventional Methods. Results Chem. 2024, 7, 101274. [Google Scholar] [CrossRef]
- Secario, M.K.; Truong, T.T.V.; Chen, C.-C.; Lai, J.-Y.; Lue, S.J. Size-Dependent Antibacterial Efficacy of Silver Nanoparticles from a Green Synthesis Method: Effects of Extract Quantity and Origin. J. Taiwan Inst. Chem. Eng. 2024, 161, 105511. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainable–Green Synthesis of Silver Nanoparticles Using Aqueous Hyssopus officinalis and Calendula officinalis Extracts and Their Antioxidant and Antibacterial Activities. Molecules 2022, 27, 7700. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the Effect of the Different Parts of Morinda citrifolia L. (Noni) on the Green Synthesis of Silver Nanoparticles and Their Antibacterial Activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- Shafiq, A.; Deshmukh, A.R.; AbouAitah, K.; Kim, B.-S. Green Synthesis of Controlled Shape Silver Nanostructures and Their Peroxidase, Catalytic Degradation, and Antibacterial Activity. J. Funct. Biomater. 2023, 14, 325. [Google Scholar] [CrossRef]
- Saadh, M. Potent Antiviral Effect of Green Synthesis Silver Nanoparticles on Newcastle Disease Virus. Arab. J. Chem. 2022, 15, 103899. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Aseel, D.G.; El-Gendi, H.; Sobhy, S.; Samy, M.A.; Hamdy, E.; El-Messeiry, S.; Behiry, S.I.; Elbeaino, T.; Abdelkhalek, A. Antiviral Activity of Biosynthesized Silver Nanoparticles from Pomegranate (Punica granatum L.) Peel Extract Against Tobacco Mosaic Virus. Plants 2023, 12, 2103. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Mosidze, E.; Folliero, V.; Lamparelli, E.P.; Lopardo, V.; Pagliano, P.; Porta, G.D.; Galdiero, M.; Bakuridze, A.D.; Franci, G. Eco-Friendly Synthesis of Silver Nanoparticles from Peel and Juice C. Limon and Their Antiviral Efficacy against HSV-1 and SARS-CoV-2. Virus Res. 2024, 349, 199455. [Google Scholar] [CrossRef] [PubMed]
- Abo-El-Yazid, Z.H.; Ahmed, O.K.; El-Tholoth, M.; Ali, M.A.-S. Green Synthesized Silver Nanoparticles Using Cyperus rotundus L. Extract as a Potential Antiviral Agent against Infectious Laryngotracheitis and Infectious Bronchitis Viruses in Chickens. Chem. Biol. Technol. Agric. 2022, 9, 55. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Yilmaz, A.; Karaman, A.; Aysin, F.; Aksakal, O. Monitoring Chemically and Green-Synthesized Silver Nanoparticles in Maize Seedlings via Surface-Enhanced Raman Spectroscopy (SERS) and Their Phytotoxicity Evaluation. Talanta 2021, 225, 121952. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, Translocation, and Transformation of Silver Nanoparticles in Plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, J.; Yin, L.; Liu, J.; Chen, C. Silver Nanoparticles: From In vitro Green Synthesis to In vivo Biological Effects in Plants. Adv. Agrochem. 2023, 2, 313–323. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Hamad, N.A.; Ali, H.M.; Khan, M.T.; Haq, I.U.; Zaman, Q.U. Green Synthesized Silver Nanoparticles: A Novel Approach for the Enhanced Growth and Yield of Tomato Against Early Blight Disease. Microorganisms 2023, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant Mediated Fabrication of Silver Nanoparticles, Process Optimization, and Impact on Tomato Plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Ilyas, N.; Naz, F.; Amjad, M.S.; Malik, N.Z.; Chaudhari, S.K. Protective Role of Foliar Application of Green-Synthesized Silver Nanoparticles Against Wheat Stripe Rust Disease Caused by Puccinia striiformis. Green Process. Synth. 2022, 11, 29–43. [Google Scholar] [CrossRef]
- Haq, S.I.U.; Wali, S.; Sama, N.U.; Kamran, K.; Ullah, Z.; Mohamed, H.I. Environmentally Friendly Synthesis of Silver Nanoparticles (AgNPs) Using Mentha Arvensis Plants Modulates Physiological and Biochemical Attributes and Yield of Sunflower (Helianthus annuus L.). J. Soil Sci. Plant Nutr. 2024, 24, 3610–3630. [Google Scholar] [CrossRef]
- Paul, A.; Roychoudhury, A. Go Green to Protect Plants: Repurposing the Antimicrobial Activity of Biosynthesized Silver Nanoparticles to Combat Phytopathogens. Nanotechnol. Environ. Eng. 2021, 6, 10. [Google Scholar] [CrossRef]
- Al-khattaf, F.S. Gold and Silver Nanoparticles: Green Synthesis, Microbes, Mechanism, Factors, Plant Disease Management and Environmental Risks. Saudi J. Biol. Sci. 2021, 28, 3624–3631. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Kumari, A.; Chaudhary, A.K.; Srivastava, R.; Kamil, D.; Vashishtha, P.; Sharma, S.N. An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica. Physchem 2023, 3, 125–146. [Google Scholar] [CrossRef]
- El-Ashmouny, R.S.; Rady, M.H.; Merdan, B.A.; El-Sheikh, T.A.A.; Hassan, R.E.; El Gohary, E.G.E. Larvicidal and Pathological Effects of Green Synthesized Silver Nanoparticles from Artemisia herba-alba Against Spodoptera littoralis Through Feeding and Contact Application. Egypt. J. Basic Appl. Sci. 2022, 9, 239–253. [Google Scholar] [CrossRef]
- Elkobrosy, D.; Al-Askar, A.A.; El-Gendi, H.; Su, Y.; Nabil, R.; Abdelkhalek, A.; Behiry, S. Nematocidal and Bactericidal Activities of Green Synthesized Silver Nanoparticles Mediated by Ficus sycomorus Leaf Extract. Life 2023, 13, 1083. [Google Scholar] [CrossRef]
- Tian, Y.; Luo, J.; Wang, H.; Zaki, H.E.M.; Yu, S.; Wang, X.; Ahmed, T.; Shahid, M.S.; Yan, C.; Chen, J.; et al. Bioinspired Green Synthesis of Silver Nanoparticles Using Three Plant Extracts and Their Antibacterial Activity against Rice Bacterial Leaf Blight Pathogen Xanthomonas oryzae pv. oryzae Plants 2022, 11, 2892. [Google Scholar] [CrossRef]
- Nayab, D.-E.; Akhtar, S. Green Synthesized Silver Nanoparticles from Eucalyptus Leaves Can Enhance Shelf Life of Banana Without Penetrating in Pulp. PLoS ONE 2023, 18, e0281675. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-Negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Chandel, M.; Gupta, V.; Kaur, K.; Patel, A.; Kaur, K.; Kishore, A.; Prabhakar, P.K.; Singh, A.; Prasad, J.S.; et al. Valorisation of Fruit Peel Bioactive into Green Synthesized Silver Nanoparticles to Modify Cellulose Wrapper for Shelf-Life Extension of Packaged Bread. Food Res. Int. 2023, 164, 112321. [Google Scholar] [CrossRef] [PubMed]
- Pandian, H.; Senthilkumar, K.; Ratnam, M.V.; Naveenkumar, M.; Samraj, S. Azadirachta Indica Leaf Extract Mediated Silver Nanoparticles Impregnated Nano Composite Film (AgNP/MCC/Starch/Whey Protein) for Food Packaging Applications. Environ. Res. 2023, 216, 114641. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green Synthesis of Silver Nanoparticles by Conocarpus Lancifolius Plant Extract and Their Antimicrobial and Anticancer Activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef]
- Jang, S.J.; Yang, I.J.; Tettey, C.O.; Kim, K.M.; Shin, H.M. In-Vitro Anticancer Activity of Green Synthesized Silver Nanoparticles on MCF-7 Human Breast Cancer Cells. Mater. Sci. Eng. C 2016, 68, 430–435. [Google Scholar] [CrossRef]
- Faheem, M.M.; Bhagat, M.; Sharma, P.; Anand, R. Induction of P53 Mediated Mitochondrial Apoptosis and Cell Cycle Arrest in Human Breast Cancer Cells by Plant Mediated Synthesis of Silver Nanoparticles from Bergenia ligulata (Whole Plant). Int. J. Pharm. 2022, 619, 121710. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles with Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 575415. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green Synthesis of Silver Nanoparticles Using Teucrium Polium Leaf Extract and Assessment of Their Antitumor Effects Against MNK45 Human Gastric Cancer Cell Line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S. Green Synthesis and Anticancer Activity of Silver Nanoparticles Prepared Using Fruit Extract of Azadirachta indica. J. Radiat. Res. Appl. Sci. 2022, 15, 335–345. [Google Scholar] [CrossRef]
- Al Baloushi, K.S.Y.; Senthilkumar, A.; Kandhan, K.; Subramanian, R.; Kizhakkayil, J.; Ramachandran, T.; Shehab, S.; Kurup, S.; Alyafei, M.A.M.; Al Dhaheri, A.; et al. Green Synthesis and Characterization of Silver Nanoparticles Using Moringa Peregrina and Their Toxicity on MCF-7 and Caco-2 Human Cancer Cells. Int. J. Nanomed. 2024, 19, 3891–3905. [Google Scholar] [CrossRef]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.R.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green Synthesis of Silver Nanoparticles Using Aqueous Rhizome Extract of Zingiber Officinale and Curcuma Longa: In-Vitro Anti-Cancer Potential on Human Colon Carcinoma HT-29 Cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef]
- Antony, J.J.; Sithika, M.A.A.; Joseph, T.A.; Suriyakalaa, U.; Sankarganesh, A.; Siva, D.; Kalaiselvi, S.; Achiraman, S. In vivo Antitumor Activity of Biosynthesized Silver Nanoparticles Using Ficus Religiosa as a Nanofactory in DAL Induced Mice Model. Colloids Surf. B Biointerfaces 2013, 108, 185–190. [Google Scholar] [CrossRef]
- Amini, S.M.; Samareh Salavati Pour, M.; Vahidi, R.; Kouhbananinejad, S.M.; Sattarzadeh Bardsiri, M.; Farsinejad, A.; MirzaeiParsa, M. Green Synthesis of Stable Silver Nanoparticles Using Teucrium Polium Extract: In-Vitro Anticancer Activity on NALM-6. Nanomed. Res. J. 2021, 6, 170–178. [Google Scholar] [CrossRef]
- Barabadi, H.; Vahidi, H.; Kamali, K.D.; Rashedi, M.; Hosseini, O.; Ghomi, A.R.G.; Saravanan, M. Emerging Theranostic Silver Nanomaterials to Combat Colorectal Cancer: A Systematic Review. J. Clust. Sci. 2020, 31, 311–321. [Google Scholar] [CrossRef]
- Barabadi, H.; Hosseini, O.; Kamali, K.D.; Shoushtari, F.J.; Rashedi, M.; Haghi-Aminjan, H.; Saravanan, M. Emerging Theranostic Silver Nanomaterials to Combat Lung Cancer: A Systematic Review. J. Clust. Sci. 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma Lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; López-Mena, E.R.; Sánchez-Arreola, E. Activities Against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review. Biomedicines 2023, 11, 389. [Google Scholar] [CrossRef]
- Venmani, S.; Kesavan, M.P.; Ayyanaar, S.; Muniyappan, N. Cymodocea Serrulata-Capped Silver Nanoparticles for Battling Human Lung Cancer, Breast Cancer, Hepatic Cancer: Optimization by Full Factorial Design and In vitro Cytotoxicity Evaluation. Heliyon 2023, 9, e20039. [Google Scholar] [CrossRef]
- Meti, R.S.; Neelagund, S.E.; Urs, D.; Dharmappa, K.K.; Kotresh, K.R. Green Synthesis of Silver Nanoparticles from Acacia Sinuata Seed Extract and Evaluation of Their Mosquitocidal and Anticancer (Caco-2 and MG-63 Cell) Activity. Biomass Conv. Bioref. 2025, 15, 175–184. [Google Scholar] [CrossRef]
- Imchen, L.; Manisekaran, R.; Jamir, I.; Rathore, H.S.; Senthilvelan, T. A Review on Plant-Mediated Synthesis of AgNPs and Their Formulations for Wound Healing Application. Mol. Biol. Rep. 2025, 52, 419. [Google Scholar] [CrossRef]
- Oselusi, S.O.; Sibuyi, N.R.S.; Meyer, M.; Madiehe, A.M. Phytonanotherapeutic Applications of Plant Extract-Synthesized Silver Nanoparticles in Wound Healing—A Prospective Overview. BioNanoScience 2024, 14, 3455–3475. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Elizabeth Rani, E.; Karthikha, V.S.; Sakthi Sanjana, D.; Jeevitha, H.; Rajeshkumar, S.; Venugopal, V.; Priyadharshan, A. Advancing Engineered Approaches for Sustainable Wound Regeneration and Repair: Harnessing the Potential of Green Synthesized Silver Nanoparticles. Eng. Regen. 2024, 5, 306–325. [Google Scholar] [CrossRef]
- Lakkim, V.; Reddy, M.C.; Pallavali, R.R.; Reddy, K.R.; Reddy, C.V.; Inamuddin; Bilgrami, A.L.; Lomada, D. Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial Activity Against Multidrug-Resistant Bacteria and Wound Healing Efficacy Using a Murine Model. Antibiotics 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Assad, N.; Naeem-ul-Hassan, M.; Ajaz Hussain, M.; Abbas, A.; Sher, M.; Muhammad, G.; Assad, Y.; Farid-ul-Haq, M. Diffused Sunlight Assisted Green Synthesis of Silver Nanoparticles Using Cotoneaster nummularia Polar Extract for Antimicrobial and Wound Healing Applications. Nat. Prod. Res. 2025, 39, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.; Ramesh, B.; Kumaran, S.; Radhakrishnan, M.; Saravanan, D.; Saravanan, P.; Pugazhvendan, S.R.; Nalinasundari, M.S. Development of Nanobiomaterial for Wound Healing Based on Silver Nanoparticles Loaded on Chitosan Hydrogel. 3 Biotech 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Maghimaa, M.; Alharbi, S.A. Green Synthesis of Silver Nanoparticles from Curcuma longa L. and Coating on the Cotton Fabrics for Antimicrobial Applications and Wound Healing Activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111806. [Google Scholar] [CrossRef]
- Gupta, I.; Kumar, A.; Bhatt, A.N.; Sapra, S.; Gandhi, S. Green Synthesis-Mediated Silver Nanoparticles Based Biocomposite Films for Wound Healing Application. J. Inorg. Organomet. Polym. 2022, 32, 2994–3011. [Google Scholar] [CrossRef]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A Systematic Review on Green Synthesis of Silver Nanoparticles Using Plants Extract and Their Bio-Medical Applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef]
- Bold, B.-E.; Urnukhsaikhan, E.; Mishig-Ochir, T. Biosynthesis of Silver Nanoparticles with Antibacterial, Antioxidant, Anti-Inflammatory Properties and Their Burn Wound Healing Efficacy. Front. Chem. 2022, 10, 972534. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, M.A.; Bae, S.; Kim, S.; Bae, S.; Kausar, F.; Farooqi, H.M.U.; Hyun, C.G.; Kang, C.U. Eco-Friendly Synthesis of Bioactive Silver Nanoparticles from Black Roasted Gram (Cicer arietinum) for Biomedical Applications. Sci. Rep. 2024, 14, 22922. [Google Scholar] [CrossRef]
- Cascione, M.; Rizzello, L.; Manno, D.; Serra, A.; De Matteis, V. Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials 2022, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Urmi, M.A.; Kamaraj, C.; Malafaia, G.; Ragavendran, C.; Rahman, M. Green Synthesis of Silver Nanoparticles with Its Bioactivity, Toxicity and Environmental Applications: A Comprehensive Literature Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100872. [Google Scholar] [CrossRef]
- Shahzadi, S.; Fatima, S.; Ain, Q.U.; Shafiq, Z.; Janjua, M.R.S.A. A Review on Green Synthesis of Silver Nanoparticles (SNPs) Using Plant Extracts: A Multifaceted Approach in Photocatalysis, Environmental Remediation, and Biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic Degradation of Organic Dyes Using Biosynthesized Silver Nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Dua, T.K.; Giri, S.; Nandi, G.; Sahu, R.; Shaw, T.K.; Paul, P. Green Synthesis of Silver Nanoparticles Using Eupatorium Adenophorum Leaf Extract: Characterizations, Antioxidant, Antibacterial and Photocatalytic Activities. Chem. Pap. 2023, 77, 2947–2956. [Google Scholar] [CrossRef]
- Bustos-Guadarrama, S.; Nieto-Maldonado, A.; Flores-López, L.Z.; Espinoza-Gomez, H.; Alonso-Nuñez, G. Photocatalytic Degradation of Azo Dyes by Ultra-Small Green Synthesized Silver Nanoparticles. J. Taiwan Inst. Chem. Eng. 2023, 142, 104663. [Google Scholar] [CrossRef]
- Muñoz-Carrillo, M.G.; Garcidueñas-Piña, C.; Valerio-García, R.C.; Carrazco-Rosales, J.L.; Morales-Domínguez, J.F. Green Synthesis of Silver Nanoparticles from the Opuntia Ficus-Indica Fruit and Its Activity against Treated Wastewater Microorganisms. J. Nanomater. 2020, 2020, 6908290. [Google Scholar] [CrossRef]
- Raota, C.S.; Cerbaro, A.F.; Salvador, M.; Delamare, A.P.L.; Echeverrigaray, S.; Da Silva Crespo, J.; Da Silva, T.B.; Giovanela, M. Green Synthesis of Silver Nanoparticles Using an Extract of Ives Cultivar (Vitis labrusca) Pomace: Characterization and Application in Wastewater Disinfection. J. Environ. Chem. Eng. 2019, 7, 103383. [Google Scholar] [CrossRef]
- Shittu, K.O.; Ihebunna, O. Purification of Simulated Waste Water Using Green Synthesized Silver Nanoparticles of Piliostigma thonningii Aqueous Leave Extract. Adv. Nat. Sci Nanosci. Nanotechnol. 2017, 8, 045003. [Google Scholar] [CrossRef]
- Aboelghait, K.M.; Abdallah, W.E.; Abdelfattah, I.; El-Shamy, A.M. Green Synthesis of Silver Nanoparticles by Waste of Murcott Mandarin Peel as a Sustainable Approach for Efficient Heavy Metal Removal from Metal Industrial Wastewater. Sep. Purif. Technol. 2024, 347, 127609. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical Sensors Based on Silver Nanoparticles for Determination of Pharmaceuticals: An Overview of Advances in the Last Decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, N.; Habib, A.; Hussain, S.; Sufian, A.; Ahmad, I.; Noreen, F.; Mehmood, A.; Ali, F.; Batoo, K.M.; Ijaz, M.F. Ecofriendly Synthesis of Silver Nanoparticle for Phytochemical Screening, Photocatalytic and Biological Applications. J. Inorg. Organomet. Polym. 2025, 35, 1036–1051. [Google Scholar] [CrossRef]
- Pechyen, C.; Tangnorawich, B.; Toommee, S.; Marks, R.; Parcharoen, Y. Green Synthesis of Metal Nanoparticles, Characterization, and Biosensing Applications. Sens. Int. 2024, 5, 100287. [Google Scholar] [CrossRef]
- Pektaş, S.Ü.; Keskin, M.; Bodur, O.C.; Arslan, F. Green Synthesis of Silver Nanoparticles and Designing a New Amperometric Biosensor to Determine Glucose Levels. J. Food Compos. Anal. 2024, 129, 106133. [Google Scholar] [CrossRef]
- Gangal, A.; Choudhary, K.; Duseja, M.; Shukla, R.K.; Kumar, S. Green Synthesis of Silver Nanoparticles from Plant Oil for Enzyme-Functionalized Optical Fiber Biosensor: Improved Sensitivity and Selectivity in Ascorbic Acid Detection. Opt. Laser Technol. 2025, 186, 112635. [Google Scholar] [CrossRef]
- Rashidi, M.A.; Falahi, S.; Dehghan, S.F.; Ebrahimzadeh, H.; Ghaneialvar, H.; Zendehdel, R. Green Synthesis of Silver Nanoparticles by Smyrnium cordifolium Plant and Its Application for Colorimetric Detection of Ammonia. Sci. Rep. 2024, 14, 24161. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Vahdat-Lasemi, F.; Karav, S.; Kesharwani, P.; Sahebkar, A. Lipid Nanoparticle-Based Delivery of Small Interfering RNAs: New Possibilities in the Treatment of Diverse Diseases. Eur. Polym. J. 2025, 223, 113624. [Google Scholar] [CrossRef]
- Beygi, M.; Oroojalian, F.; Karav, S.; Kesharwani, P.; Sahebkar, A. Nano-Phytoconstituents: Recent Advances, Regulatory Insights, Challenges, and Future Horizons. J. Drug Deliv. Sci. Technol. 2025, 108, 106908. [Google Scholar] [CrossRef]
- Rahiman, N.; Kesharwani, P.; Karav, S.; Sahebkar, A. Curcumin-Based Nanofibers: A Promising Approach for Cancer Therapy. Pathol.-Res. Pract. 2025, 266, 155791. [Google Scholar] [CrossRef]
- Wang, H. A Review of Nanotechnology in microRNA Detection and Drug Delivery. Cells 2024, 13, 1277. [Google Scholar] [CrossRef]
- Hussein, H.A.; Abdullah, M.A. Novel Drug Delivery Systems Based on Silver Nanoparticles, Hyaluronic Acid, Lipid Nanoparticles and Liposomes for Cancer Treatment. Appl. Nanosci. 2022, 12, 3071–3096. [Google Scholar] [CrossRef]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-Mediated Biogenic Synthesis of Silver Nanoparticles and Their Drug Delivery Evaluation: A Biocompatible Anti-Cancer Therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- Ghobadi, M.; Salehi, S.; Ardestani, M.T.S.; Mousavi-Khattat, M.; Shakeran, Z.; Khosravi, A.; Cordani, M.; Zarrabi, A. Amine-Functionalized Mesoporous Silica Nanoparticles Decorated by Silver Nanoparticles for Delivery of Doxorubicin in Breast and Cervical Cancer Cells. Eur. J. Pharm. Biopharm. 2024, 201, 114349. [Google Scholar] [CrossRef] [PubMed]
- Altınay, E.; Köse, F.Z.; Ateş, S.C.; Kızılbey, K. Ibuprofen-Loaded Silver Nanoparticle-Doped PVA Gels: Green Synthesis, In vitro Cytotoxicity, and Antibacterial Analyses. Gels 2024, 10, 143. [Google Scholar] [CrossRef]
- Haripriya, B.S.; Anakha, D.R.; Yamuna, R.; Vinoba, M.; Bhagiyalakshmi, M. Green Synthesized AgNPs Using Clitoria Ternatea Extract and Its Confinement on SBA-15/GPTMS-TAEA for Controlled Drug Release of Ciprofloxacin. J. Porous Mater. 2024, 31, 351–363. [Google Scholar] [CrossRef]
- Alqahtani, Y.S.; Bahafi, A.; Mirajkar, K.K.; Basavaraju, R.R.; Mitra, S.; S, S.; More, S.S.; Muddapur, U.M.; Khan, A.A.; Sudarshan, P.R.; et al. In vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera Indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens. Antibiotics 2022, 11, 1503. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Khan, M.T.; Hamad, N.A.; Ali, H.M.; Abbasi, A.; Mubeen, I.; Intisar, A.; Hasan, M.E.; Jasim, I.K. Evaluation of In vitro and In vivo Antifungal Activity of Green Synthesized Silver Nanoparticles against Early Blight in Tomato. Horticulturae 2023, 9, 369. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Ohiduzzaman, M.; Khan, M.N.I.; Khan, K.A.; Paul, B. Biosynthesis of Silver Nanoparticles by Banana Pulp Extract: Characterizations, Antibacterial Activity, and Bioelectricity Generation. Heliyon 2024, 10, e25520. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Aqueous Extract with the Evaluation of Its Antibacterial Activity against Clinical and Food Pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Govahi, M.; Litkohi, H.R. Green Synthesis of Silver Nanoparticles (AgNPs) and Chitosan-Coated Silver Nanoparticles (CS-AgNPs) Using Ferula Gummosa Boiss. Gum Extract: A Green Nano Drug for Potential Applications in Medicine. Int. J. Biol. Macromol. 2025, 291, 138619. [Google Scholar] [CrossRef]
- Moges, W.; Misskire, Y. Green Synthesis, Characterization and Antibacterial Activities of Silver Nanoparticles Using Sida schimperiana Hochst. ex A. Rich (Chifrig) Leaves Extract. Discov. Mater. 2025, 5, 34. [Google Scholar] [CrossRef]
- Danish, M.; Altaf, M.; Robab, M.I.; Shahid, M.; Manoharadas, S.; Hussain, S.A.; Shaikh, H. Green Synthesized Silver Nanoparticles Mitigate Biotic Stress Induced by Meloidogyne incognita in Trachyspermum ammi (L.) by Improving Growth, Biochemical, and Antioxidant Enzyme Activities. ACS Omega 2021, 6, 11389–11403. [Google Scholar] [CrossRef]
- Kavin, T.; Murugaiyah, V.; Tan, J.K.; Kassim, M.N.I.; Ramakrishna, S.; Vigneswari, S. Eco-Friendly Synthesis of Silver Nanoparticles Using Coffea Arabica Husk for Enhanced Antibacterial and Anti-Cancer Applications. Biomass Bioenergy 2025, 194, 107625. [Google Scholar] [CrossRef]
- Qubtia, M.; Ghumman, S.A.; Noreen, S.; Hameed, H.; Noureen, S.; Kausar, R.; Irfan, A.; Akhtar Shah, P.; Afzal, H.; Hameed, M.; et al. Evaluation of Plant-Based Silver Nanoparticles for Antioxidant Activity and Promising Wound-Healing Applications. ACS Omega 2024, 9, 12146–12157. [Google Scholar] [CrossRef]
- Alsareii, S.A.; Alamri, A.M.; AlAsmari, M.Y.; Bawahab, M.A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J.H.; Kumbar, V. Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity. Molecules 2022, 27, 6306. [Google Scholar] [CrossRef]
- Kim, H.-B.; You, H.-S.; Ryu, S.; Lee, H.-Y.; Baek, J.-S. Green Synthesis of Silver Nanoparticles from Mulberry Leaf through Hot Melt Extrusion: Enhanced Antioxidant, Antibacterial, Anti-Inflammatory, Antidiabetic, and Anticancer Properties. Food Hydrocoll. Health 2024, 6, 100184. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Rehman, S.U.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Lekshmi, A.A.; Sunilkumar, A.; Jayakumar, A.; Lal, S.S.; Sreemayi, M.; Chandran, S.S. Green Synthesis of Silver Nanoparticles: A One-Pot Approach with Emphasis on Antibacterial, Antifungal, and Biosensor Applications. Mater. Today: Proc. 2024, in press. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jawad, K.H.; Çevik, S.; Sulaiman, G.M.; Albukhaty, S.; Sasikumar, P. Investigating the Antimicrobial, Antioxidant, and Anticancer Effects of Elettaria Cardamomum Seed Extract Conjugated to Green Synthesized Silver Nanoparticles by Laser Ablation. Plasmonics 2024, 19, 1187–1200. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Radhakrishnan, S.; Asmare, M.M.; Wahab, S.; Kim, B.-S.; Yun, S.-I. Green Synthesis of Ag and Au NPs Decorated rGO Nanocomposite for High Impedimetric Electrochemical Sensor as Well as Enhanced Antimicrobial Performance against Foodborne Pathogens. Arab. J. Chem. 2024, 17, 105379. [Google Scholar] [CrossRef]
- James, J.M.; Yagoo, A.; Vilvest, J.; Jessie, A.A.A. Green Synthesis of Silver Nanoparticles Using Boerhavia Diffusa Plant and the Potential of Its Antioxidant and Anticancer Efficacy. Pharmacol. Res.-Nat. Prod. 2025, 6, 100142. [Google Scholar] [CrossRef]
- Masood, S.; Haq, I.U.; Khan, N.R.; Fayyaz, M.; Qayum, M.; Khatoon, A.; Jamil, M. Synthesis of Plant-Derived Smoke-Mediated Silver Nanoparticles and Its Stimulatory Effects on Maize Growth Under Wastewater Stress. Arab. J. Sci. Eng. 2025, 50, 65–75. [Google Scholar] [CrossRef]
- Saada, N.S.; Abdel-Maksoud, G.; Abd El-Aziz, M.S.; Youssef, A.M. Green Synthesis of Silver Nanoparticles, Characterization, and Use for Sustainable Preservation of Historical Parchment Against Microbial Biodegradation. Biocatal. Agric. Biotechnol. 2021, 32, 101948. [Google Scholar] [CrossRef]
- Moradi, M.; Hanachi, P.; Bahramikia, S.; Tavan, M. Effect of Green Synthesized Silver Nanoparticles Using the Aqueous Extract of Lavandula angustifolia Mill. Against Ethanol-Induced Gastric Ulcers in Rats. Biocatal. Agric. Biotechnol. 2025, 66, 103584. [Google Scholar] [CrossRef]
- Losetty, V.; Devanesan, S.; AlSalhi, M.S.; Velu, P.P.; Muthupillai, D.; Kumar, K.A.; Lakkaboyana, S.K. Green Synthesis of Silver Nanoparticles Using Malachra Alceifolia (Wild Okra) for Wastewater Treatment and Biomedical Applications with Molecular Docking Approach. Environ. Sci. Pollut. Res. 2024, 31, 55562–55576. [Google Scholar] [CrossRef]
- Awad, M.A.; Virk, P.; Hendi, A.A.; Ortashi, K.M.; AlMasoud, N.; Alomar, T.S. Role of Biosynthesized Silver Nanoparticles with Trigonella Foenum-Graecum Seeds in Wastewater Treatment. Processes 2023, 11, 2394. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Amiri, M.S.; Akbari, S.; Sharifalhoseini, M.; Nourbakhsh, F.; Mashreghi, M.; Yousefi, E.; Abbasi, M.R.; Modarres, M.; Es-Haghi, A. Green Synthesis of Silver Nanoparticles Using Helichrysum Graveolens for Biomedical Applications and Wastewater Treatment. BioNanoScience 2020, 10, 1121–1127. [Google Scholar] [CrossRef]
- Thongwattana, T.; Chaiyo, R.; Ponsanti, K.; Tangnorawich, B.; Pratumpong, P.; Toommee, S.; Jenjob, R.; Yang, S.-G.; Parcharoen, Y.; Natphopsuk, S.; et al. Synthesis of Silver Nanoparticles and Gold Nanoparticles Used as Biosensors for the Detection of Human Serum Albumin-Diagnosed Kidney Disease. Pharmaceuticals 2024, 17, 1421. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and Applications of Silver Nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal Plants Mediated the Green Synthesis of Silver Nanoparticles and Their Biomedical Applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomed. Mater. Devices 2025, 1–26. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Dikshit, P.; Kumar, J.; Das, A.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.; Kim, B. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; El-Monaem, E.M.A.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Aliero, A.S.; Hasmoni, S.H.; Haruna, A.; Isah, M.; Malek, N.A.N.N.; Zawawi, N.A. Bibliometric Exploration of Green Synthesized Silver Nanoparticles for Antibacterial Activity. Emerg. Contam. 2025, 11, 100411. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–A Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Correia, A.C.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Design of Experiment (DoE) as a Quality by Design (QbD) Tool to Optimise Formulations of Lipid Nanoparticles for Nose-to-Brain Drug Delivery. Expert Opin. Drug Deliv. 2023, 20, 1731–1748. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Viegas, J.S.R.; Abriata, J.P.; Viegas, F.; Vicentini, F.T.M.d.C.; Bentley, M.V.L.B.; Chorilli, M.; Marchetti, J.M.; Tapia-Blácido, D.R. Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple Heart Plant Leaves Extract-Mediated Silver Nanoparticle Synthesis: Optimization by Box-Behnken Design. Mater. Sci. Eng. C 2019, 99, 1105–1114. [Google Scholar] [CrossRef]
- Fhionnlaoich, N.M.; Yang, Y.; Qi, R.; Galvanin, F.; Guldin, S. DoE-It-Yourself: A Case Study for Implementing Design of Experiments into Nanoparticle Synthesis. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hao, Z.; Fadare, O.O.; Hanachi, P.; Chen, Y.; Liu, J. Toxicity of Biosynthesized Silver Nanoparticles to Aquatic Organisms of Different Trophic Levels. Chemosphere 2020, 258, 127346. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Tareq, M.; Khadrawy, Y.A.; Rageh, M.M.; Mohammed, H.S. Dose-Dependent Biological Toxicity of Green Synthesized Silver Nanoparticles in Rat’s Brain. Sci. Rep. 2022, 12, 22642. [Google Scholar] [CrossRef] [PubMed]
- Tarbali, S.; Mehrian, S.K.; Khezri, S. Toxicity Effects Evaluation of Green Synthesized Silver Nanoparticles on Intraperitoneally Exposed Male Wistar Rats. Toxicol. Mech. Methods 2022, 32, 488–500. [Google Scholar] [CrossRef] [PubMed]
| Plant Source | Application Type | Biological Activities | Physicochemical Property of the AgNPs | References |
|---|---|---|---|---|
| Lallemantia royleana leaf extract | Antimicrobial Biomedical Photocatalytic | Antibacterial activity against multiple bacteria species Antifungal activity against Candida glabrata and Candida albicans Degradation of methylene blue Anti-inflammatory activity Antioxidant activity | Spherical morphology Average size of 34.5 ± 1.6 nm SPR peak at 425 nm Zeta potential of −24.1 mV | [83] |
| Mangifera indica Aqueous Leaf Extract | Antimicrobial Agricultural | Antibacterial activity against MDR bacteria species Enhanced biochemical constituents of infected wheat crops | Spherical morphology Average size of 52.8 nm SPR peak at 475 nm | [203] |
| Azadirachta indica leaf extract | Antimicrobial Agriculture | Antifungal activity against Alternaria solani Increased resistance of susceptible tomatoes | Spherical morphology Particle size between 22 and 30 nm SPR peak at 424 nm | [204] |
| Aqueous leaf extract of Perilla frutescens L. | Antimicrobial Biomedical | Antibacterial activity against E. coli and S. aureus Antifungal activity against Candida albicans Toxicity against MCF-7 cancer cells Antioxidant activity | Spherical morphology Average size of <61 nm SPR peak at 436 nm Zeta potential of −18.1 ± 0.72 mV | [60] |
| Leaf Extract of Carissa carandas L. | Antimicrobial Biomedical | Antibacterial activity against various human pathogenic bacteria Antioxidant activity | Average size of 35 ± 2 nm SPR peak at 432 nm and 444 nm | [205] |
| Green banana pulp extract | Antimicrobial Industrial | Antibacterial activity against E. coli and S. epidermidis Increased voltage, current and voltage regulation of electrochemical cells | Spherical morphology Average size of 42.97 nm SPR peak at 475 nm | [206] |
| Hypericum perforatum L. aqueous extract | Antimicrobial Agricultural | Antipathogenic activity against several food pathogens Reduction in bacterial growth rate | Spherical and monodisperse morphology Average size between 20 and 40 nm SPR peak at 425 nm Zeta potential of −19 mv | [207] |
| Ferula gummosa Boiss. gum extract | Antimicrobial Biomedical | Antibacterial activity against several bacteria strains Increased antioxidant activity with chitosan coating Notable anticancer activity against cell lines | Spherical morphology Average particle size of 5.63 nm Zeta potential of 52.2 mV SPR peak at 420 nm | [208] |
| Sida schimperiana Hochst. ex A. Rich leaves extract. | Antimicrobial | Antibacterial activity against several bacteria strains | Face-centered cubic morphology Average size of 26.27 nm | [209] |
| Senna siamea | Agricultural | Increased shoot and root length, seed oil content, seed yield, number of branches, photosynthetic pigments and biochemical features of Trachyspermum ammi (L.) inoculated with Meloidogyne incognita | Round and polydiverse morphology Size ranging from 5 to 60 nm SPR peak at 430 nm | [210] |
| Coffea arabica husk extract | Antimicrobial Biomedical | Antibacterial activity against several bacteria strains (E. coli, S. aureus, P. aeruginosa and B. subtilis) Selective cytotoxicity towards MCF-7 cancer cells | Spherical morphology Average size of 147 nm Zeta potential of −27.8 mV | [211] |
| Taro corm extract | Biomedical Antimicrobial | Antibacterial activity against various pathogenic bacteria Antioxidant activity In vivo (rabbits) wound healing activity | Spherical morphology Average size of 244.9–272.2 nm SPR peaks at a range of 438–445 nm Zeta potential of −18.8 mV | [212] |
| Rhodiola rosea | Antimicrobial Biomedical | Antibacterial activity against S. aureus and P. aeruginosa Antioxidant activity Increased IL-10 levels and decreased pro-inflammatory cytokine levels in murine model burn wound healing | Spherical morphology Size of 20 nm SPR peak at 430 nm Zeta potential of −68.38 ± 3.4 mV | [173] |
| Rhizophora apiculata | Antimicrobial Biomedical | In vitro anti-inflammatory activity by 71.65 ± 0.88% Notable anticancer activity In vitro wound healing activity by 82.79% Antioxidant activity | Irregular morphology Size between 35 and 100 SPR peak at 459 nm Zeta potential of −6 mV | [213] |
| Mulberry leaf | Antimicrobial Biomedical | Antioxidant activity Antibacterial activity against E. coli and S. aureus In vitro anti-inflammatory activity In vitro antidiabetic activity by 47.03% amylase inhibition Anticancer activity with cell viability of 17.28%. | Spherical morphology Average size of 100 nm SPR peaks in the range of 420–450 nm Absolute zeta potential of >30 mV | [214] |
| Brachychiton populneus Leaf Extract | Antimicrobial Biomedical | Antioxidant activity In vitro antidiabetic activity by 80% amylase inhibition In vitro anti-inflammatory activity by protein denaturation inhibition | Cubical morphology Average size of 15 nm SPR peak at 453 nm | [215] |
| Euphorbia hirta | Antimicrobial Industrial | Antibacterial activity against E. coli and S. aureus Antifungal activity against Candida albicans and Aspergillus niger Observable color change with heavy metal solutions | Hexagonal morphology Average size of 30 nm SPR peak at 470 nm | [216] |
| Elettaria cardamomum Seed Extract | Antimicrobial Biomedical | Antibacterial activity against E. coli and S. aureus Antioxidant activity Anticancer activity with cytotoxicity of 83.66% | Spherical morphology Average size of 19 nm SPR peak at 412 nm | [217] |
| Allium cepa L. leaves extract | Antimicrobial Environmental | Antibacterial activity against several bacteria strains Antifungal activity against several fungal strains Electrochemical nitrite detection | Spherical morphology Size ranging 1 to 30 nm SPR peak at 432 nm | [218] |
| Ocimum sanctum (tulsi) | Biomedical | Antibacterial activity against E. coli In vitro scratch wound closure rate by 45% Improved mechanical properties of film | Spherical morphology Average size of 28.95 ± 7.74 nm Zeta potential of −17.8 mV | [171] |
| Boerhavia diffusa extract | Biomedical | Antioxidant activity Concentration-dependent anticancer activity by apoptosis induction | Spherical morphology Average size of 34 nm SPR peak at 427 nm | [219] |
| Cymbopogon jwarancusa | Agricultural Environmental | Increased seed germination of wastewater treated maize seedlings up to 60% Increased root and shoot lengths | Spherical and square-to-rectangular morphology Average size of 31 nm SPR peak at 433 nm | [220] |
| Tea tree leaves extract | Agricultural | Antibacterial activity against several bacteria strains Antifungal activity against several fungal strains Microbicidal activity on parchment samples Increased mechanical property of parchment samples | Spherical, oval, and hexagonal morphology Size ranging 20 to 50 nm SPR peak at 468 nm | [221] |
| Lavandula angustifolia Mill | Biomedical | Antiulcerogenic activity against ethanol-induced gastric ulcers in rats Antioxidant activity | Spherical and cubical morphology Average size of 69.35 nm SPR peak at 420 nm | [222] |
| Malachra alceifolia leaf extract | Environmental Biomedical | Antibacterial activity against S. aureus and P. aeruginosa Antioxidant activity up to 53% scavenging ability Photocatalytic activity against methylene blue under direct solar radiation | Spherical morphology Average size of 28 nm SPR peak at 455 nm | [223] |
| Trigonella foenum-graecum Seeds | Environmental | %94.5 degradation of crystal violet dye under UV irradiation Increased water quality parameters of wastewater Antibacterial activity against E. coli and S. aureus | Spherical morphology Average size of 20–50 nm SPR peak at 439.29 nm | [224] |
| Helichrysum graveolens | Environmental | Antibacterial activity against several bacteria strains Photocatalytic activity against methyl orange | Spherical morphology Average size of 11 nm SPR peak at 439 nm | [225] |
| Acacia raddiana leaves | Industrial | Observable detection of multiple heavy metals Heavy metal sensing (Pb, Cu and Co) within real wastewater samples ranging 42.33% to 100.72% | Spherical morphology Average size of 77.35 ± 50.4 nm SPR peak at 423 nm Zeta potential of − 32.2 mV | [82] |
| Phulae pineapple peel extract | Industrial | Detection of serum albumin concentrations from 10 to 400 μg/mL | Spherical morphology Size ranging 10 to 30 nm SPR peaks ranging from 440 to 460 nm | [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications. Int. J. Mol. Sci. 2025, 26, 6222. https://doi.org/10.3390/ijms26136222
Eker F, Akdaşçi E, Duman H, Bechelany M, Karav S. Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications. International Journal of Molecular Sciences. 2025; 26(13):6222. https://doi.org/10.3390/ijms26136222
Chicago/Turabian StyleEker, Furkan, Emir Akdaşçi, Hatice Duman, Mikhael Bechelany, and Sercan Karav. 2025. "Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications" International Journal of Molecular Sciences 26, no. 13: 6222. https://doi.org/10.3390/ijms26136222
APA StyleEker, F., Akdaşçi, E., Duman, H., Bechelany, M., & Karav, S. (2025). Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications. International Journal of Molecular Sciences, 26(13), 6222. https://doi.org/10.3390/ijms26136222