Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
2.1. BM-MSC Phenotyping Characterization

2.2. Cytotoxic Effect of GEM + FLU on BM-MSCs and PCCs
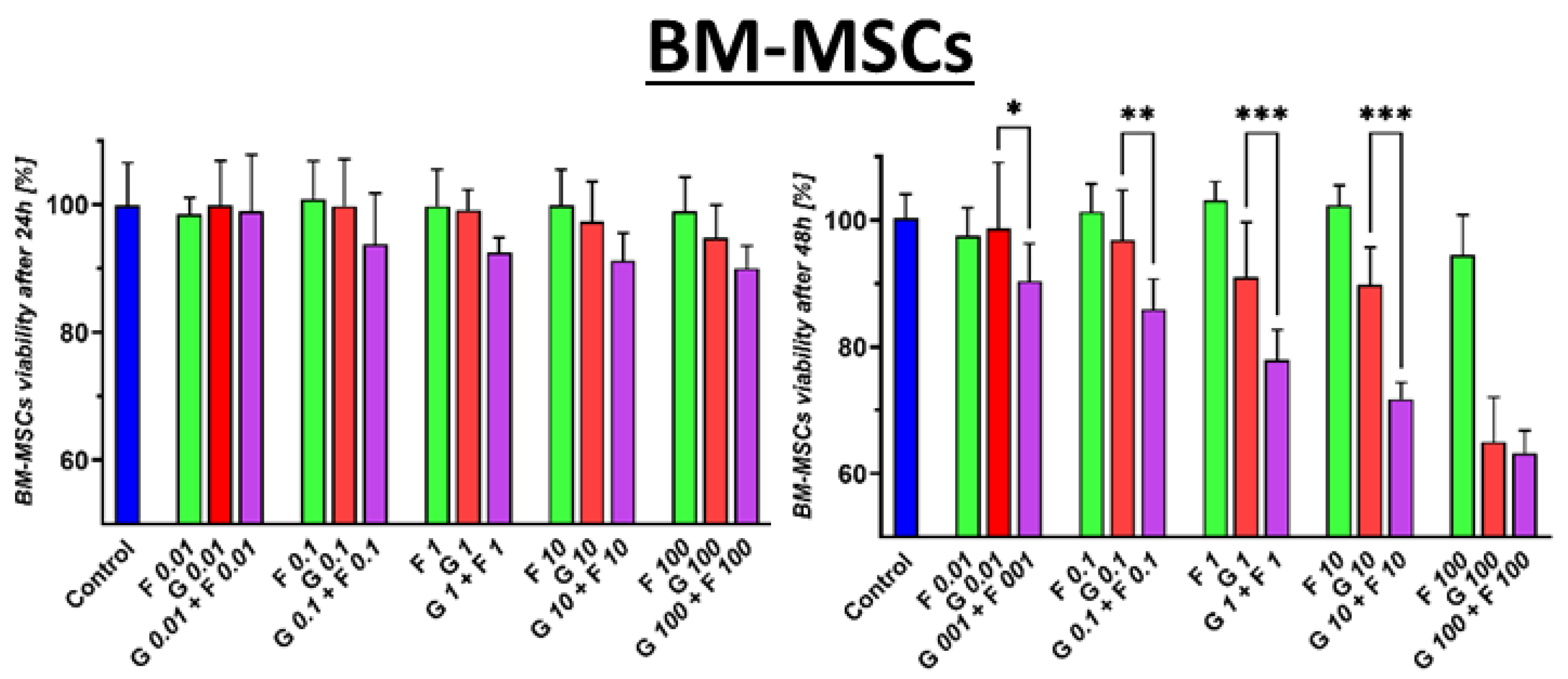
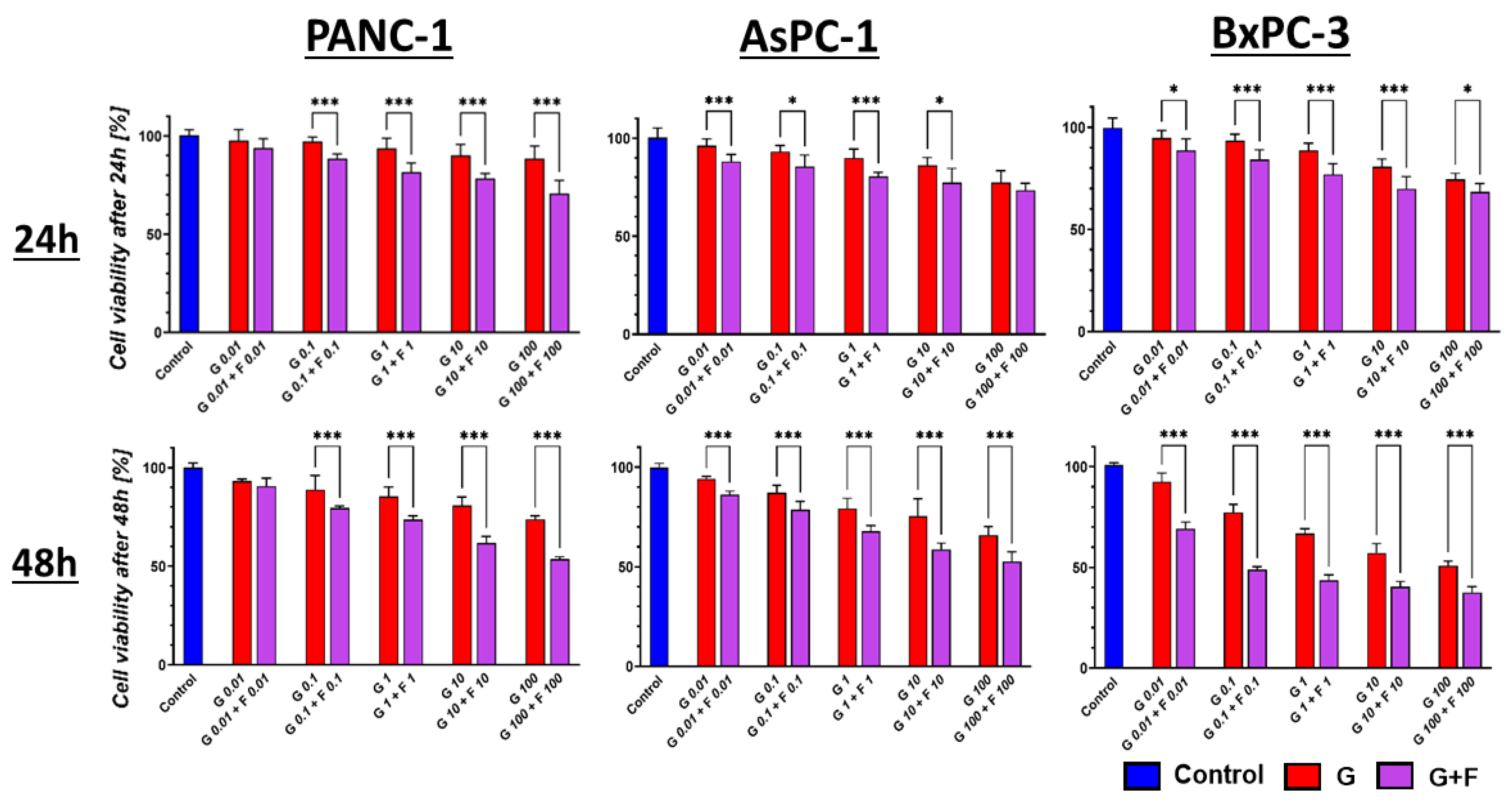
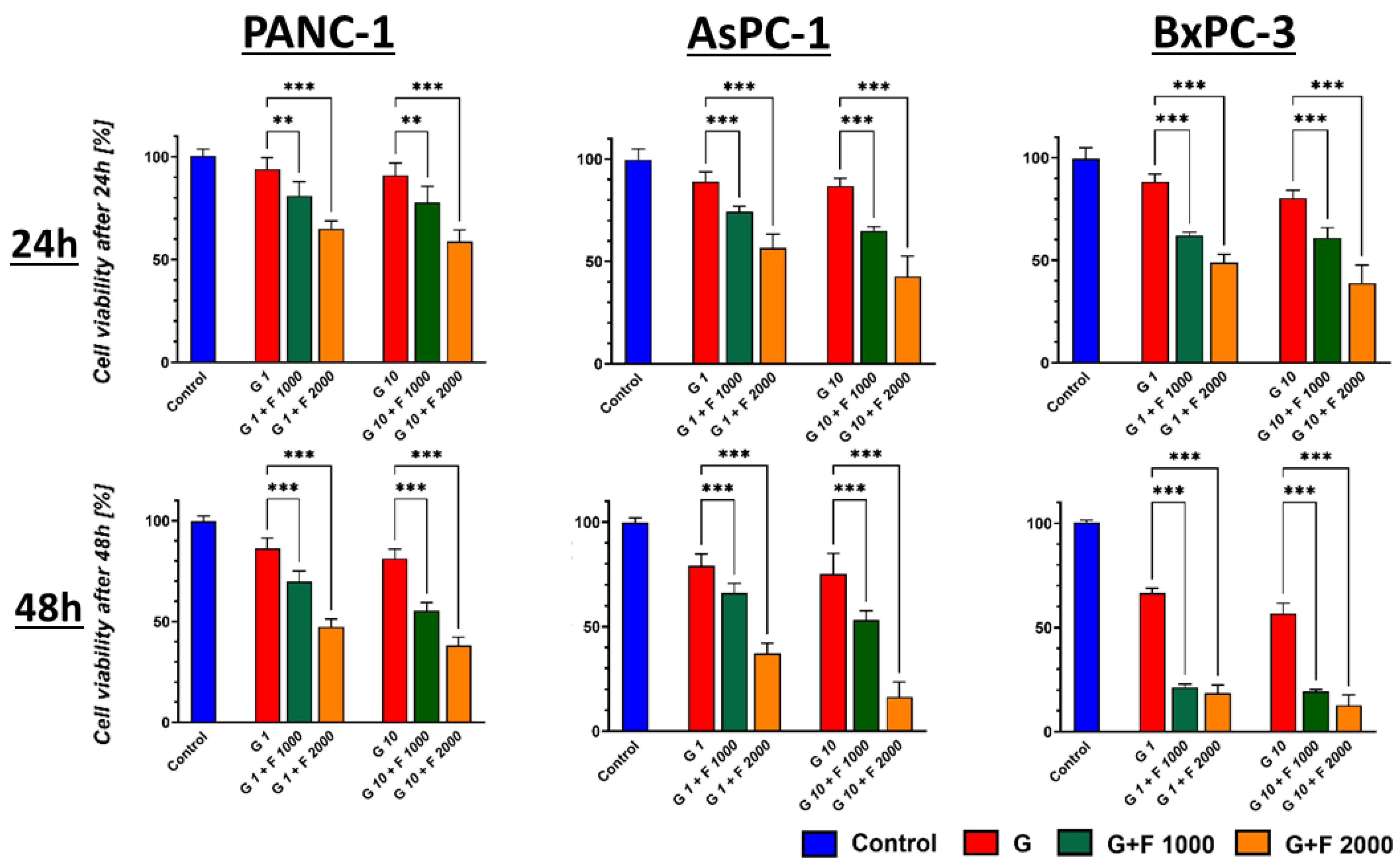
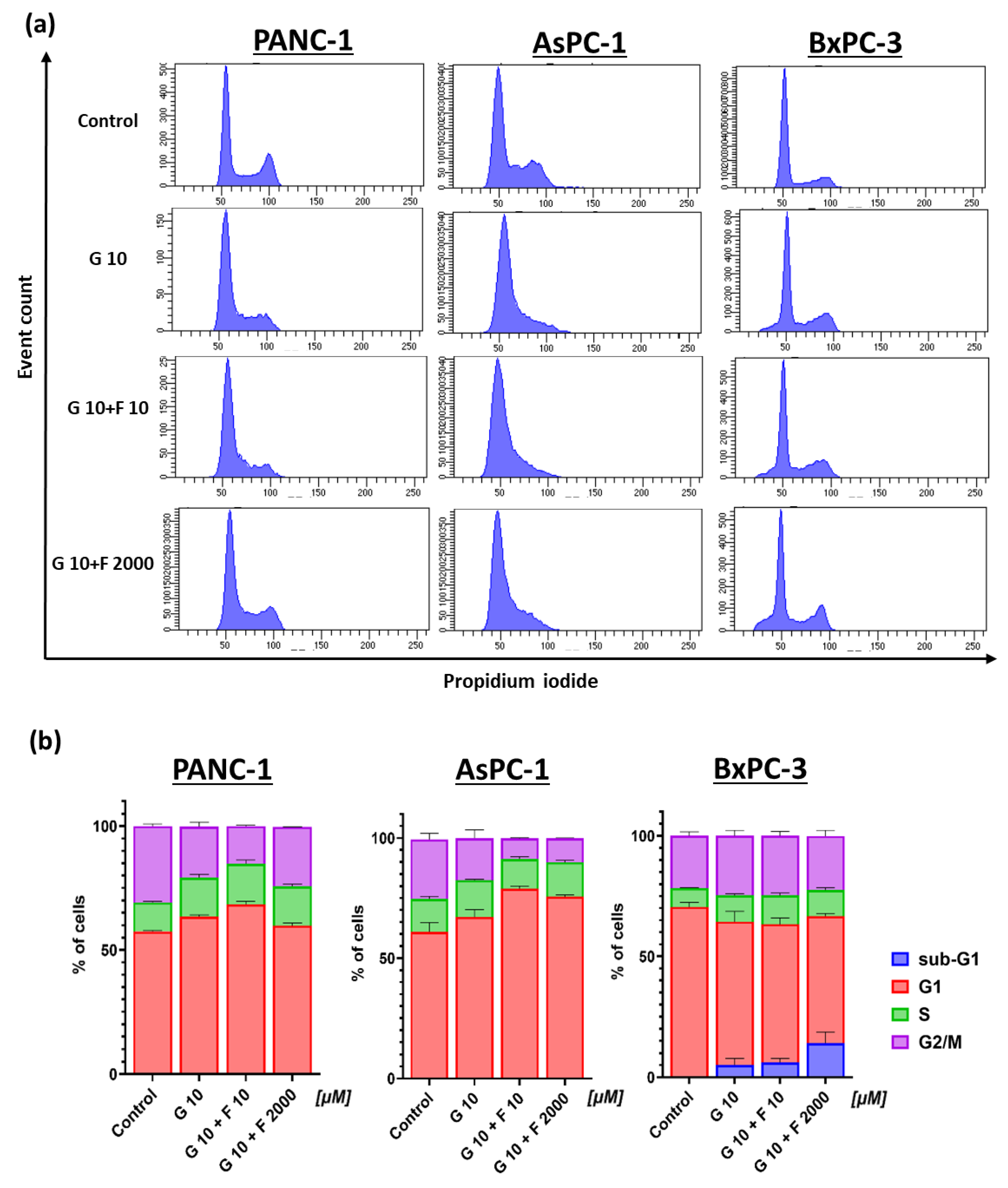
2.3. Cell Cycle Analysis and Morphology of PCCs After GEM + FLU Treatment
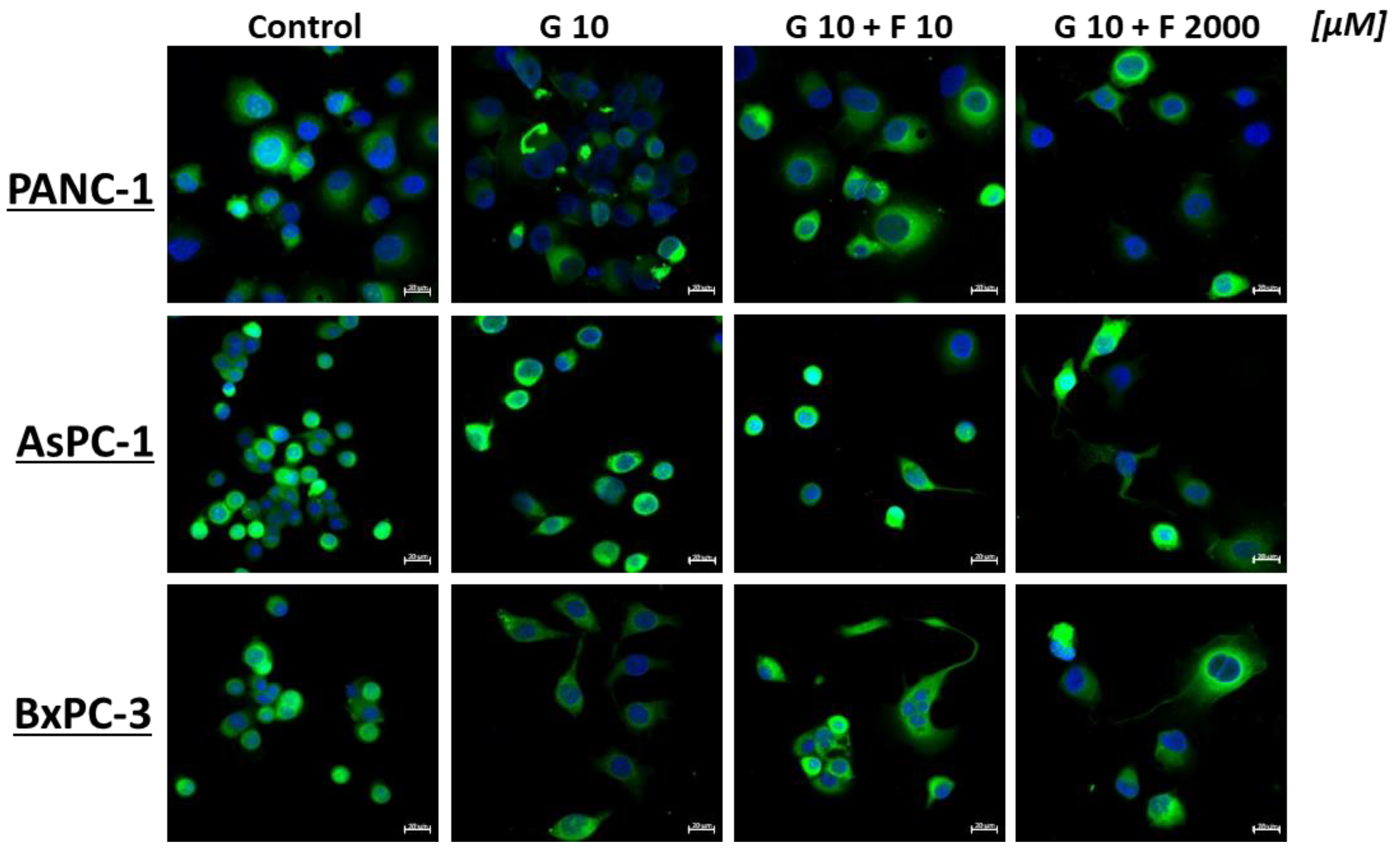
2.4. Apoptosis and Necrosis of BM-MSCs and PCCs After GEM + FLU Treatment
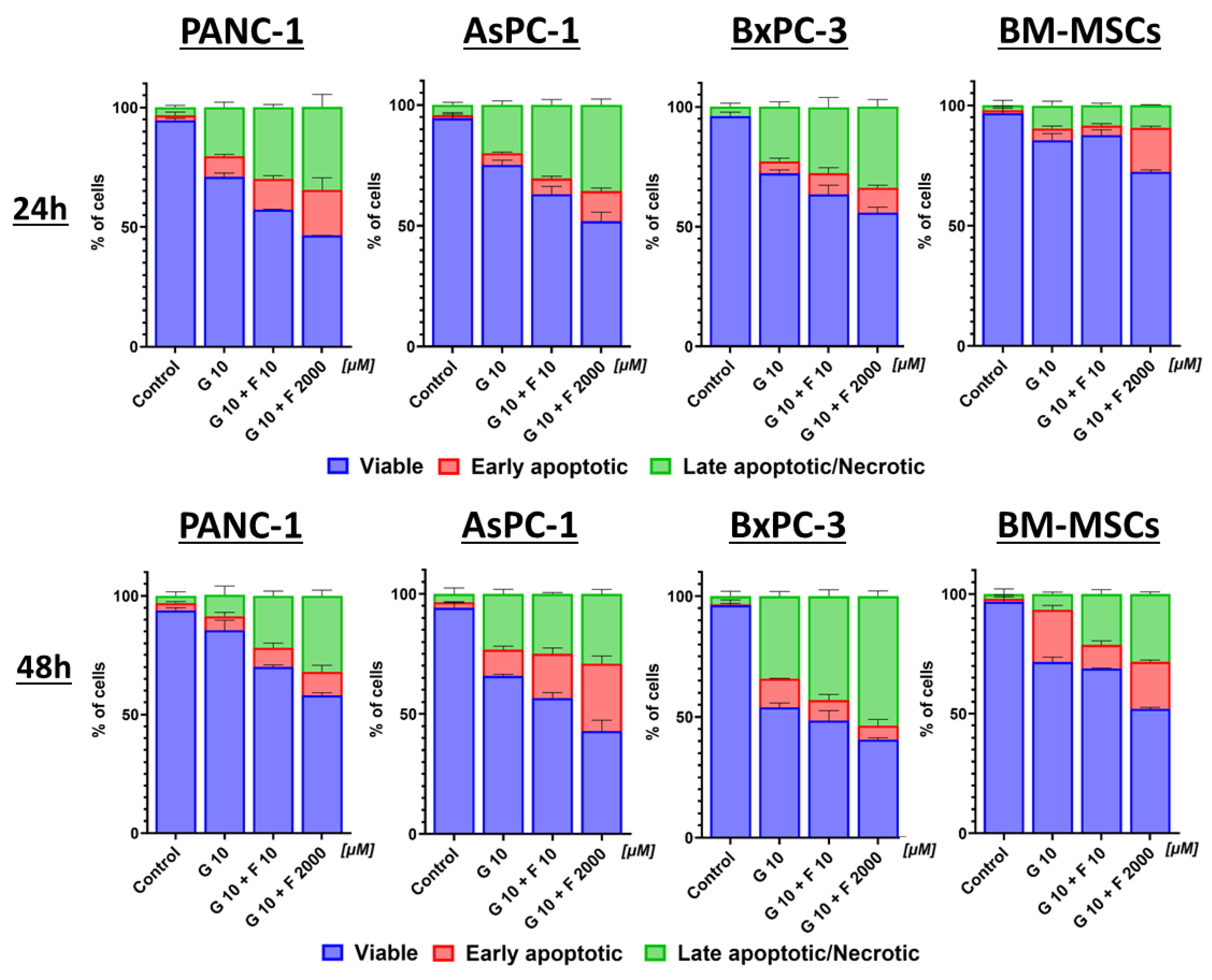
2.5. The Influence of Cytotoxic Activity and the Ability to Activate Apoptosis and Necrosis After Treatment with BM-MSC-Conditioned Media in PCCs
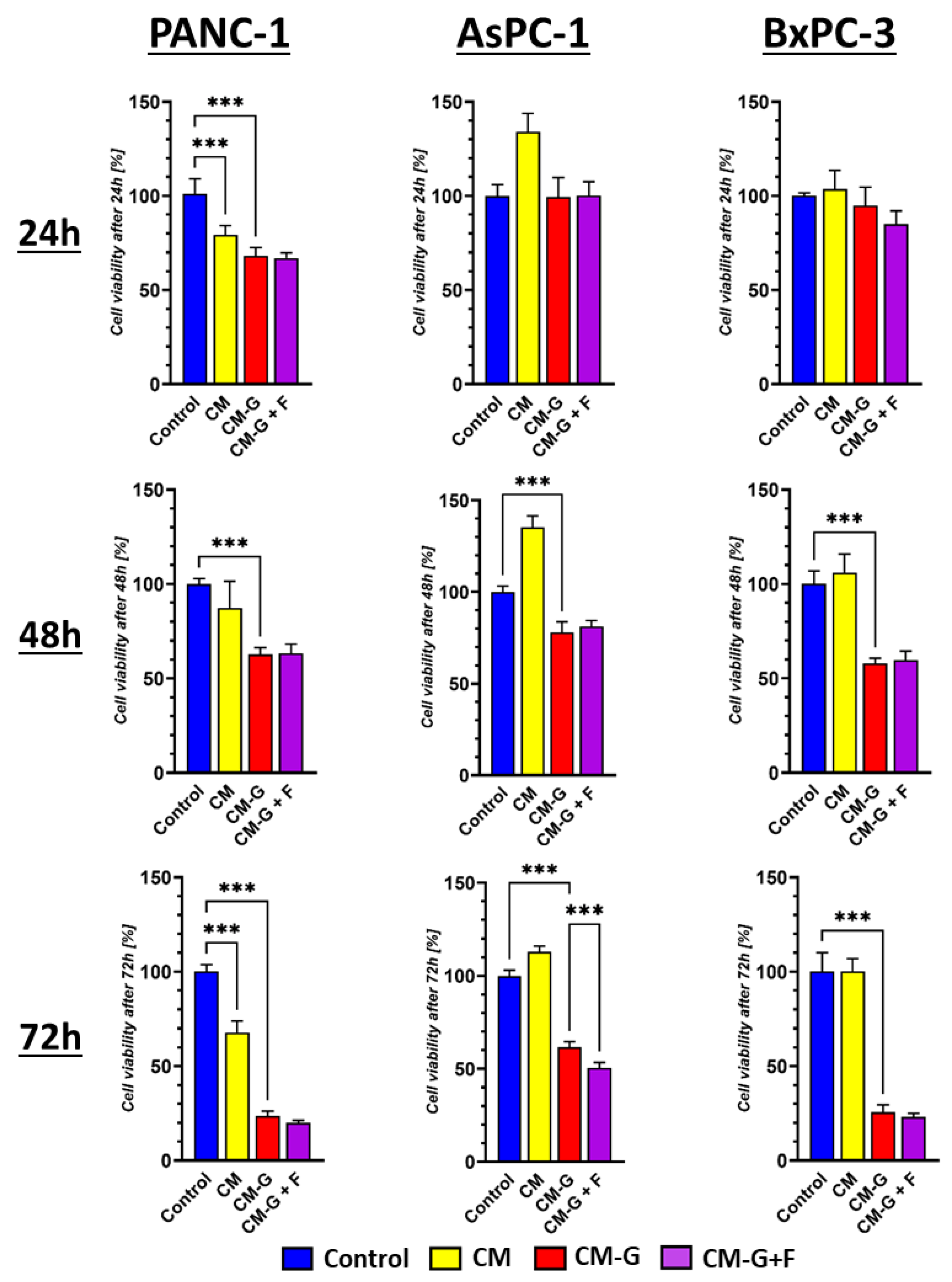
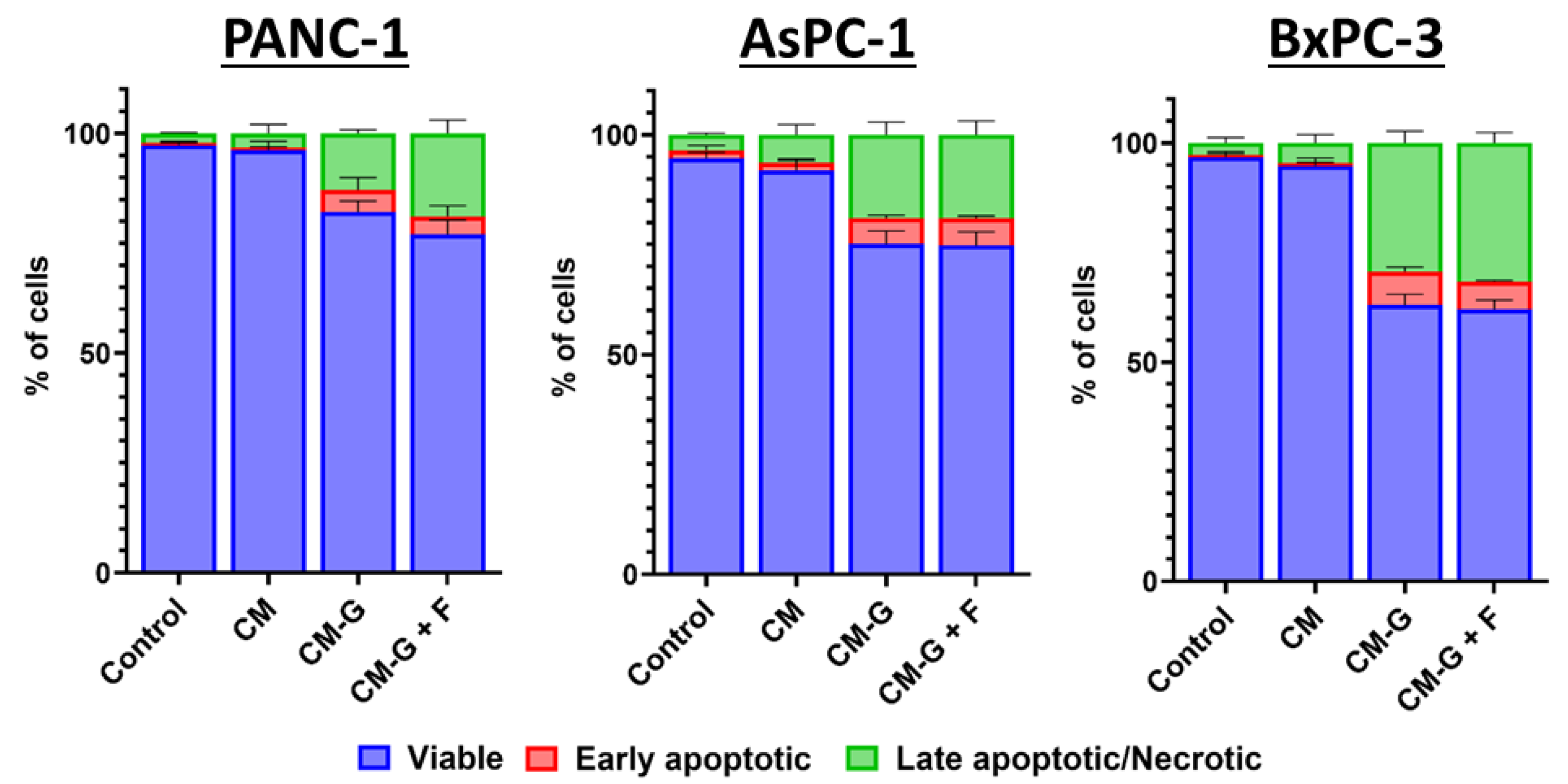
2.6. Co-Culture of GEM- and FLU-Treated BM-MSCs with PCCs
2.7. Western Blot Technique
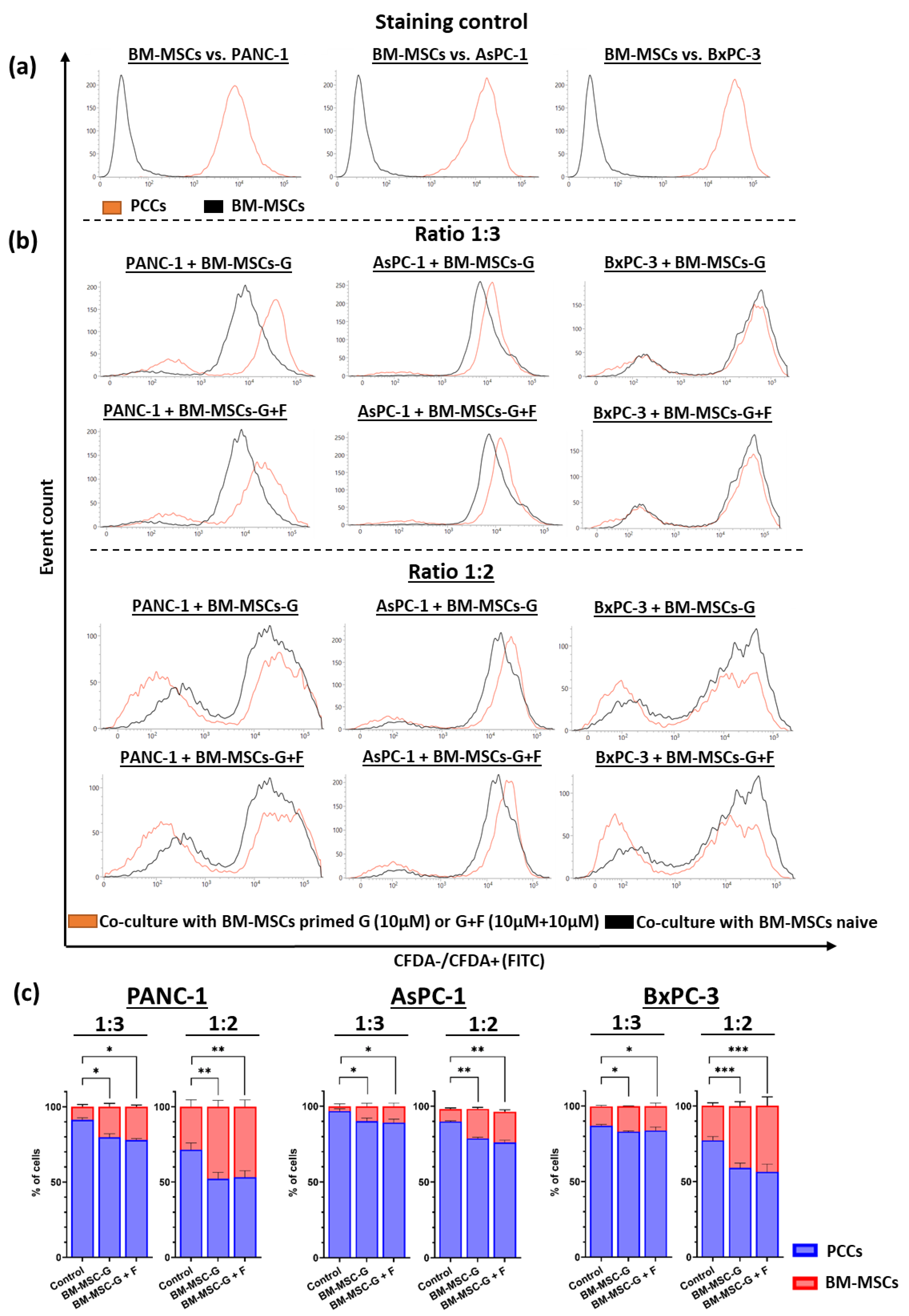

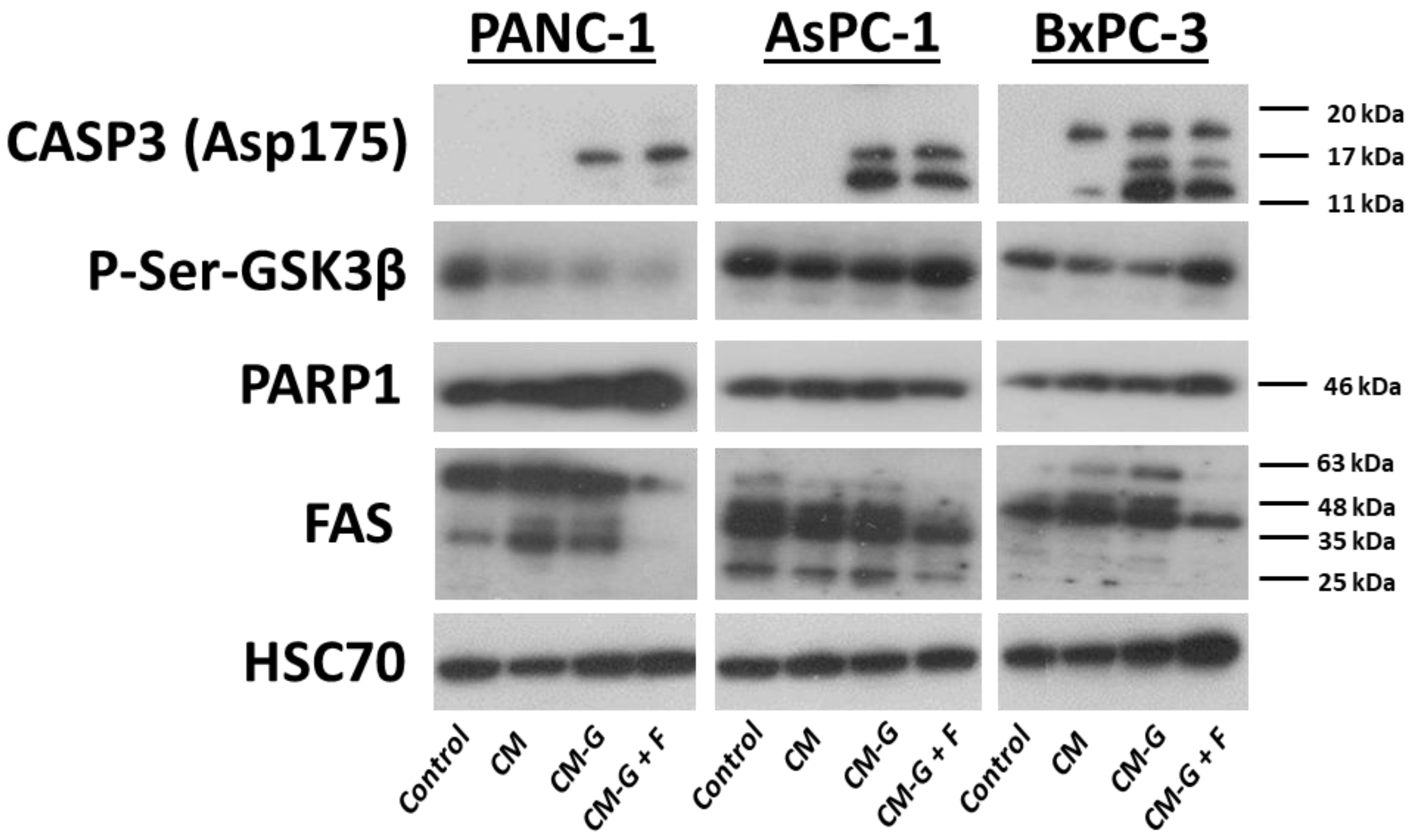
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines
4.3. BM-MSC Isolation and Phenotyping
4.4. Proliferation Assays
4.5. Annexin V and 7-AAD Assay
4.6. Cell Cycle
4.7. BM-MSC Pre-Treatment with GEM and FLU and Conditioned Media Collection
4.8. Proliferation Assay of BM-MSC-CM Priming with GEM or GEM + FLU
4.9. Annexin V and 7-AAD Assay of BM-MSC-CM Priming with GEM or GEM + FLU
4.10. CFDA Staining
4.11. BM-MSCs Co-Culture
4.12. Western Blotting
4.13. Statistical Analysis
5. Conclusions
- An interesting application of flurbiprofen as an adjuvant of gemcitabine in vitro inhibiting the growth of pancreatic cancer cells is presented;
- The combination of GEM + FLU showed significantly higher cytotoxicity towards PANC-1, AsPC-1, and BxPC-3 cells compared to gemcitabine alone;
- FLU enhanced the antiproliferative effect of GEM, inducing apoptosis and inhibiting the cell cycle;
- Western blot confirmed an increased expression of caspase-3 and a decrease in p-Ser-GSK3β and PARP1;
- The conditioned medium from BM-MSCs with drugs led to apoptosis, irrespective of the lower drug dose usage; moreover, possible interactions of BM-MSCs with cancer cells require further study;
- The combination of flurbiprofen and gemcitabine might be regarded as a potentially effective strategy in the therapy of pancreatic cancer, especially in the context of proliferation inhibition and the induction of cancer cell death.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GEM | Gemcitabine |
| FLU | Flurbiprofen |
| BM-MSCs | Bone-marrow-derived mesenchymal stem cells |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| CM | Conditioned medium |
| EVs | Extracellular vehicles |
| PDAC | Pancreatic ductal adenocarcinoma |
| NTs | Nucleoside transporters |
References
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic Cancer: A Review of Epidemiology, Trend, and Risk Factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Reiss, K.A.; O’Hara, M.H. Advancements in Systemic Therapy for Pancreatic Cancer. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e397082. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A Starring Role for Stellate Cells in the Pancreatic Cancer Microenvironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef]
- Mekapogu, A.R.; Pothula, S.P.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Multifunctional Role of Pancreatic Stellate Cells in Pancreatic Cancer. Ann. Pancreat. Cancer 2019, 2, 10. [Google Scholar] [CrossRef]
- Weniger, M.; Honselmann, K.; Liss, A. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef]
- Vicinanza, C.; Lombardi, E.; Ros, F.D.; Marangon, M.; Durante, C.; Mazzucato, M.; Agostini, F. Modified Mesenchymal Stem Cells in Cancer Therapy: A Smart Weapon Requiring Upgrades for Wider Clinical Applications. World J. Stem Cells 2022, 14, 54–75. [Google Scholar] [CrossRef]
- Nicodemou, A.; Bernátová, S.; Čeháková, M.; Danišovič, Ľ. Emerging Roles of Mesenchymal Stem/Stromal-Cell-Derived Extracellular Vesicles in Cancer Therapy. Pharmaceutics 2023, 15, 1453. [Google Scholar] [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal Stem/Stromal Cells in Cancer Therapy. J. Hematol. Oncol. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kholodenko, R.V.; Majouga, A.G.; Yarygin, K.N. Apoptotic MSCs and MSC-Derived Apoptotic Bodies as New Therapeutic Tools. Curr. Issues Mol. Biol. 2022, 44, 5153–5172. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Sordi, V.; Dugnani, E.; Ceserani, V.; Dossena, M.; Coccè, V.; Cavicchini, L.; Ciusani, E.; Bondiolotti, G.; Piovani, G.; et al. Gemcitabine-Releasing Mesenchymal Stromal Cells Inhibit in Vitro Proliferation of Human Pancreatic Carcinoma Cells. Cytotherapy 2015, 17, 1687–1695. [Google Scholar] [CrossRef]
- Klimova, D.; Jakubechova, J.; Altanerova, U.; Nicodemou, A.; Styk, J.; Szemes, T.; Repiska, V.; Altaner, C. Extracellular Vesicles Derived from Dental Mesenchymal Stem/Stromal Cells with Gemcitabine as a Cargo Have an Inhibitory Effect on the Growth of Pancreatic Carcinoma Cell Lines in Vitro. Mol. Cell. Probes 2023, 67, 101894. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-G.; Chen, T.-M.; Lu, Y.; Wang, Z.; Wang, X.-C.; Kong, Y. Human Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Loaded with Gemcitabine Inhibit Pancreatic Cancer Cell Proliferation by Enhancing Apoptosis. World J. Gastrointest. Oncol. 2024, 16, 4006–4013. [Google Scholar] [CrossRef]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery. Pharmaceuticals 2024, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Wuchter, P.; Bieback, K.; Schrezenmeier, H.; Bornhäuser, M.; Müller, L.P.; Bönig, H.; Wagner, W.; Meisel, R.; Pavel, P.; Tonn, T.; et al. Standardization of Good Manufacturing Practice-Compliant Production of Bone Marrow-Derived Human Mesenchymal Stromal Cells for Immunotherapeutic Applications. Cytotherapy 2015, 17, 128–139. [Google Scholar] [CrossRef]
- Menard, C.; Pacelli, L.; Bassi, G.; Dulong, J.; Bifari, F.; Bezier, I.; Zanoncello, J.; Ricciardi, M.; Latour, M.; Bourin, P.; et al. Clinical-Grade Mesenchymal Stromal Cells Produced under Various Good Manufacturing Practice Processes Differ in Their Immunomodulatory Properties: Standardization of Immune Quality Controls. Stem Cells Dev. 2013, 22, 1789–1801. [Google Scholar] [CrossRef]
- Nold, P.; Hackstein, H.; Riedlinger, T.; Kasper, C.; Neumann, A.; Mernberger, M.; Fölsch, C.; Schmitt, J.; Fuchs-Winkelmann, S.; Barckhausen, C.; et al. Immunosuppressive Capabilities of Mesenchymal Stromal Cells Are Maintained under Hypoxic Growth Conditions and after Gamma Irradiation. Cytotherapy 2015, 17, 152–162. [Google Scholar] [CrossRef]
- van Griensven, M.; Balmayor, E.R. Extracellular Vesicles Are Key Players in Mesenchymal Stem Cells’ Dual Potential to Regenerate and Modulate the Immune System. Adv. Drug Deliv. Rev. 2024, 207, 115203. [Google Scholar] [CrossRef]
- Liang, W.; Han, B.; Hai, Y.; Sun, D.; Yin, P. Mechanism of Action of Mesenchymal Stem Cell-Derived Exosomes in the Intervertebral Disc Degeneration Treatment and Bone Repair and Regeneration. Front. Cell Dev. Biol. 2022, 9, 833840. [Google Scholar] [CrossRef]
- Panda, B.; Sharma, Y.; Gupta, S.; Mohanty, S. Mesenchymal Stem Cell-Derived Exosomes as an Emerging Paradigm for Regenerative Therapy and Nano-Medicine: A Comprehensive Review. Life 2021, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Maity, P.; Kapat, K. The Opportunities and Challenges of Mesenchymal Stem Cells-Derived Exosomes in Theranostics and Regenerative Medicine. Cells 2024, 13, 1956. [Google Scholar] [CrossRef] [PubMed]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of Gemcitabine in Cancer Therapy. Future Oncol. 2005, 1, 7–17. [Google Scholar] [CrossRef]
- Singh, A. A Review on Gemcitabine Modification. Mater. Today Proc. 2020, 37, 3301–3304. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; Yuan, C.; Hamada, T.; Cao, Y.; Babic, A.; Morales-Oyarvide, V.; Kraft, P.; Ng, K.; Giovannucci, E.; Ogino, S.; et al. Regular Use of Aspirin or Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs Is Not Associated With Risk of Incident Pancreatic Cancer in Two Large Cohort Studies. Gastroenterology 2018, 154, 1380–1390.e5. [Google Scholar] [CrossRef]
- Ruder, E.H.; Laiyemo, A.O.; Graubard, B.I.; Hollenbeck, A.R.; Schatzkin, A.; Cross, A.J. Non-Steroidal Anti-Inflammatory Drugs and Colorectal Cancer Risk in a Large, Prospective Cohort. Am. J. Gastroenterol. 2011, 106, 1340–1350. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sørensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-Inflammatory Drug Use and Colorectal Cancer Risk. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef]
- Pathi, S.; Jutooru, I.; Chadalapaka, G.; Nair, V.; Lee, S.-O.; Safe, S. Aspirin Inhibits Colon Cancer Cell and Tumor Growth and Downregulates Specificity Protein (Sp) Transcription Factors. PLoS ONE 2012, 7, e48208. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, W.; Sangha, S.; Albert, A.; Chang, A.J.; Liu, T.C.; Wolfe, M.M. Effects of Nonselective Cyclooxygenase Inhibition with Low-Dose Ibuprofen on Tumor Growth, Angiogenesis, Metastasis, and Survival in a Mouse Model of Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1618–1628. [Google Scholar] [CrossRef]
- Petrick, J.L.; Sahasrabuddhe, V.V.; Chan, A.T.; Alavanja, M.C.; Beane-Freeman, L.E.; Buring, J.E.; Chen, J.; Chong, D.Q.; Freedman, N.D.; Fuchs, C.S.; et al. NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer Prev. Res. 2015, 8, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Evaluation of Ibuprofen Loaded Solid Lipid Nanoparticles and Its Combination Regimens for Pancreatic Cancer Chemoprevention. Int. J. Oncol. 2015, 46, 1827–1834. [Google Scholar] [CrossRef]
- Trabert, B.; Ness, R.B.; Lo-Ciganic, W.-H.; Murphy, M.A.; Goode, E.L.; Poole, E.M.; Brinton, L.A.; Webb, P.M.; Nagle, C.M.; Jordan, S.J.; et al. Aspirin, Nonaspirin Nonsteroidal Anti-Inflammatory Drug, and Acetaminophen Use and Risk of Invasive Epithelial Ovarian Cancer: A Pooled Analysis in the Ovarian Cancer Association Consortium. J. Natl. Cancer Inst. 2014, 106, djt431. [Google Scholar] [CrossRef]
- Kim, S.; Shore, D.L.; Wilson, L.E.; Sanniez, E.I.; Kim, J.H.; Taylor, J.A.; Sandler, D.P. Lifetime Use of Nonsteroidal Anti-Inflammatory Drugs and Breast Cancer Risk: Results from a Prospective Study of Women with a Sister with Breast Cancer. BMC Cancer 2015, 15, 960. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Howard, L.E.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freedland, S.J. Aspirin, NSAIDs, and Risk of Prostate Cancer: Results from the REDUCE Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.-J.; Bringer, J.-P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Prostate Cancer Risk: Results from the EPICAP Study. Cancer Med. 2017, 6, 2461–2470. [Google Scholar] [CrossRef]
- Shebl, F.M.; Sakoda, L.C.; Black, A.; Koshiol, J.; Andriole, G.L.; Grubb, R.; Church, T.R.; Chia, D.; Zhou, C.; Chu, L.W.; et al. Aspirin but Not Ibuprofen Use Is Associated with Reduced Risk of Prostate Cancer: A PLCO Study. Br. J. Cancer 2012, 107, 207–214. [Google Scholar] [CrossRef]
- Shi, J.; Leng, W.; Zhao, L.; Xu, C.; Wang, J.; Chen, X.; Wang, Y.; Peng, X. Nonsteroidal Anti-Inflammatory Drugs Using and Risk of Head and Neck Cancer: A Dose-Response Meta Analysis of Prospective Cohort Studies. Oncotarget 2017, 8, 99066–99074. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S. Why Cancer and Inflammation? Yale J. Biol. Med. 2006, 79, 123–130. [Google Scholar]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Han, T.; Zhuo, M.; Wu, L.; Yuan, C.; Wu, L.; Lei, W.; Jiao, F.; Wang, L.-W. Elevated COX-2 Expression Promotes Angiogenesis Through EGFR/P38-MAPK/Sp1-Dependent Signalling in Pancreatic Cancer. Sci. Rep. 2017, 7, 470. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Tacconelli, S.; Bruno, A.; Grande, R.; Ballerini, P.; Patrignani, P. Nonsteroidal Anti-Inflammatory Drugs and Cardiovascular Safety–Translating Pharmacological Data into Clinical Readouts. Expert Opin. Drug Saf. 2017, 16, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Thiruchenthooran, V.; Sánchez-López, E.; Gliszczyńska, A. Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers 2023, 15, 475. [Google Scholar] [CrossRef]
- Yang, N.; Liang, Y.; Yang, P.; Jiang, L. Flurbiprofen Inhibits Cell Proliferation in Thyroid Cancer through Interrupting HIP1R-Induced Endocytosis of PTEN. Eur. J. Med. Res. 2022, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Zhang, Y.; Ji, F. Flurbiprofen Suppresses the Inflammation, Proliferation, Invasion and Migration of Colorectal Cancer Cells via COX2. Oncol. Lett. 2020, 20, 132. [Google Scholar] [CrossRef]
- Atlıhan, İ.; Koçyiğit Sevinç, S.; Orun, O.; Yılmaz, Ö.; Güniz Küçükgüzel, Ş.; Mega Tiber, P. Effect of Flurbiprofen Derivative (SGK597) on Cell Proliferation and Apoptosis of Breast Cancer Cell Lines. İstanbul J. Pharm. 2022, 52, 265–270. [Google Scholar] [CrossRef]
- Hmood, K.S.; Kubba, A.A.R.M.; Al-bayati, R.I.; Saleh, A.M. Synthesis, and Anti-Tumor Evaluation of Some New Flurbiprofen Derivatives Against MCF-7 and WRL-68 Cell Lines. Indones. J. Pharm. 2021, 32, 17–34. [Google Scholar] [CrossRef]
- Ou, Y.; Zhu, W.; Li, Y.; Qiu, P.; Huang, Y.; Xie, J.; He, S.; Zheng, X.; Leng, T.; Xu, D.; et al. Aspirin Inhibits Proliferation of Gemcitabine-Resistant Human Pancreatic Cancer Cells and Augments Gemcitabine-Induced Cytotoxicity. Acta Pharmacol. Sin. 2010, 31, 73–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, H.; Yun, X.; Shu, Y.; Xu, K. Aspirin Increases the Efficacy of Gemcitabine in Pancreatic Cancer by Modulating the PI3K/AKT/mTOR Signaling Pathway and Reversing Epithelial-mesenchymal Transition. Oncol. Lett. 2023, 25, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Qin, L.; Yu, Z.; Wu, X.; Liu, Z.; Zheng, Y. Gemcitabine and Celecoxib Synergistically Promote Long-Lasting Antitumor Efficacy of Pd-1 Antibody by Triggering Immunogenic Cell Death. Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Gridelli, C.; Gallo, C.; Ceribelli, A.; Gebbia, V.; Gamucci, T.; Ciardiello, F.; Carozza, F.; Favaretto, A.; Daniele, B.; Galetta, D.; et al. Factorial Phase III Randomised Trial of Rofecoxib and Prolonged Constant Infusion of Gemcitabine in Advanced Non-Small-Cell Lung Cancer: The GEmcitabine-COxib in NSCLC (GECO) Study. Lancet Oncol. 2007, 8, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Hiľovská, l.; Jendželovský, r.; Fedoročko, p. Potency of Non-Steroidal Anti-Inflammatory Drugs in Chemotherapy. Mol. Clin. Oncol. 2015, 3, 3–12. [Google Scholar] [CrossRef]
- Cerviño, R.H.; Gómez, N.; Sahores, A.; Gouts, A.; González, B.; Shayo, C.; Davio, C.; Yaneff, A. Flurbiprofen Inhibits cAMP Transport by MRP4/ABCC4 Increasing the Potency of Gemcitabine Treatment in PDAC Cell Models. Int. J. Biol. Macromol. 2024, 280, 136386. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Fan, P.; Bauer, N.; Gladkich, J.; Ryschich, E.; Bazhin, A.V.; Giese, N.A.; Strobel, O.; Hackert, T.; et al. Aspirin Counteracts Cancer Stem Cell Features, Desmoplasia and Gemcitabine Resistance in Pancreatic Cancer. Oncotarget 2015, 6, 9999–10015. [Google Scholar] [CrossRef]
- Amrite, A.C.; Kompella, U.B. Celecoxib Inhibits Proliferation of Retinal Pigment Epithelial and Choroid-Retinal Endothelial Cells by a Cyclooxygenase-2-Independent Mechanism. J. Pharmacol. Exp. Ther. 2008, 324, 749–758. [Google Scholar] [CrossRef]
- Derissen, E.J.B.; Huitema, A.D.R.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H. Intracellular Pharmacokinetics of Gemcitabine, Its Deaminated Metabolite 2′,2′-difluorodeoxyuridine and Their Nucleotides. Br. J. Clin. Pharmacol. 2018, 84, 1279–1289. [Google Scholar] [CrossRef]
- Hodge, L.S.; Taub, M.E.; Tracy, T.S. Effect of Its Deaminated Metabolite, 2’,2’-Difluorodeoxyuridine, on the Transport and Toxicity of Gemcitabine in HeLa Cells. Biochem. Pharmacol. 2011, 81, 950–956. [Google Scholar] [CrossRef]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal Stem Cells as a Double-Edged Sword in Tumor Growth: Focusing on MSC-Derived Cytokines. Cell. Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-Neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef]
- Slama, Y.; Ah-Pine, F.; Khettab, M.; Arcambal, A.; Begue, M.; Dutheil, F.; Gasque, P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 13511. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Ishiwata, T.; Kleeff, J.; Beger, H.G.; Korc, M. Fas and Fas-Ligand Expression in Human Pancreatic Cancer. Ann. Surg. 2000, 231, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Elmadbouh, O.H.M.; Pandol, S.J.; Edderkaoui, M. Glycogen Synthase Kinase 3β: A True Foe in Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 14133. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.A.d.M.; Floresta, L.G.; Gopalsamy, R.G.; Brasileiro, B.F.; Trento, C.L.; Borges, L.P. AlphaFold: An Emerging Tool for Drug Development in Oral Cancer. J. Stomatol. Oral Maxillofac. Surg. 2025, 102414. [Google Scholar] [CrossRef]
- Guo, S.-B.; Meng, Y.; Lin, L.; Zhou, Z.-Z.; Li, H.-L.; Tian, X.-P.; Huang, W.-J. Artificial Intelligence Alphafold Model for Molecular Biology and Drug Discovery: A Machine-Learning-Driven Informatics Investigation. Mol. Cancer 2024, 23, 223. [Google Scholar] [CrossRef]
- Bonomi, A.; Steimberg, N.; Benetti, A.; Berenzi, A.; Alessandri, G.; Pascucci, L.; Boniotti, J.; Coccè, V.; Sordi, V.; Pessina, A.; et al. Paclitaxel-Releasing Mesenchymal Stromal Cells Inhibit the Growth of Multiple Myeloma Cells in a Dynamic 3D Culture System. Hematol. Oncol. 2017, 35, 693–702. [Google Scholar] [CrossRef]
- Zajkowicz, A.; Gdowicz-Kłosok, A.; Krześniak, M.; Ścieglińska, D.; Rusin, M. Actinomycin D and Nutlin-3a Synergistically Promote Phosphorylation of P53 on Serine 46 in Cancer Cell Lines of Different Origin. Cell. Signal. 2015, 27, 1677–1687. [Google Scholar] [CrossRef]
| Compound | IC50 [μM] ± SD | |||
|---|---|---|---|---|
| PANC-1 | AsPC-1 | BxPC-3 | BM-MSCs | |
| GEM | 228.2 ± 16.3 | 194.6 ± 11.2 | 98.6 ± 8.8 | 240 ± 19.8 |
| GEM + FLU | 109.3 ± 9.6 | 104.3 ± 7.3 | 1.9 ± 0.5 | 179.1 ± 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawulok, A.; Borzdziłowska, P.; Głowala-Kosińska, M.; Fidyk, W.; Smagur, A.; Łasut-Szyszka, B.; Gdowicz-Kłosok, A.; Mitrus, I.; Wilkiewicz, M.; Chwieduk, A.; et al. Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells. Int. J. Mol. Sci. 2025, 26, 6212. https://doi.org/10.3390/ijms26136212
Kawulok A, Borzdziłowska P, Głowala-Kosińska M, Fidyk W, Smagur A, Łasut-Szyszka B, Gdowicz-Kłosok A, Mitrus I, Wilkiewicz M, Chwieduk A, et al. Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2025; 26(13):6212. https://doi.org/10.3390/ijms26136212
Chicago/Turabian StyleKawulok, Agata, Paulina Borzdziłowska, Magdalena Głowala-Kosińska, Wojciech Fidyk, Andrzej Smagur, Barbara Łasut-Szyszka, Agnieszka Gdowicz-Kłosok, Iwona Mitrus, Marcin Wilkiewicz, Agata Chwieduk, and et al. 2025. "Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells" International Journal of Molecular Sciences 26, no. 13: 6212. https://doi.org/10.3390/ijms26136212
APA StyleKawulok, A., Borzdziłowska, P., Głowala-Kosińska, M., Fidyk, W., Smagur, A., Łasut-Szyszka, B., Gdowicz-Kłosok, A., Mitrus, I., Wilkiewicz, M., Chwieduk, A., Burdalska, D., Korfanty, J., Giebel, S., Rojkiewicz, M., Bak, A., & Kozik, V. (2025). Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells. International Journal of Molecular Sciences, 26(13), 6212. https://doi.org/10.3390/ijms26136212






