Shedding of GPP130 by PC7 and Furin: Potential Implication in Lung Cancer Progression
Abstract
1. Introduction
2. Results
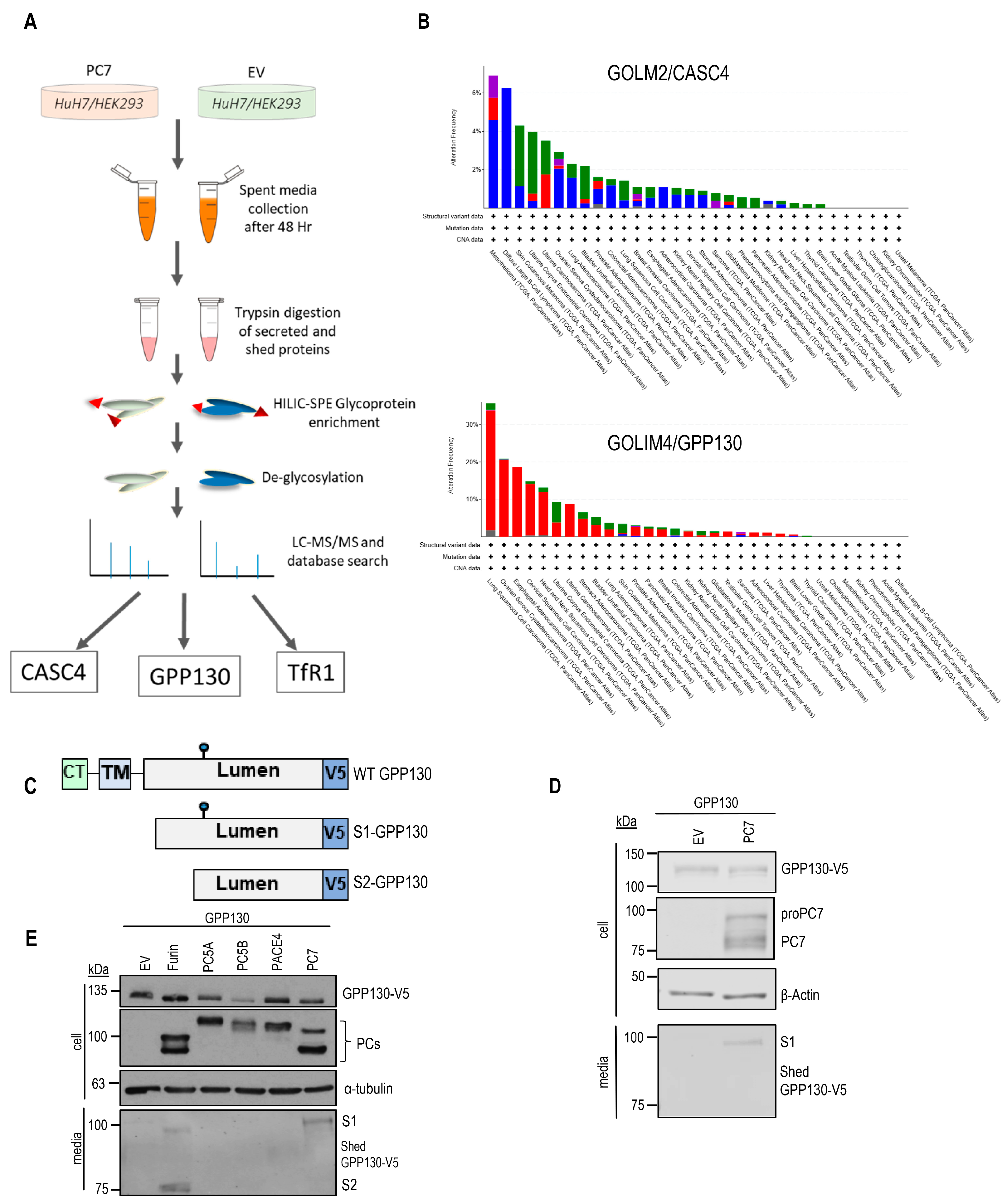
2.1. GPP130 Is Cleaved and Shed by PC7 and Furin
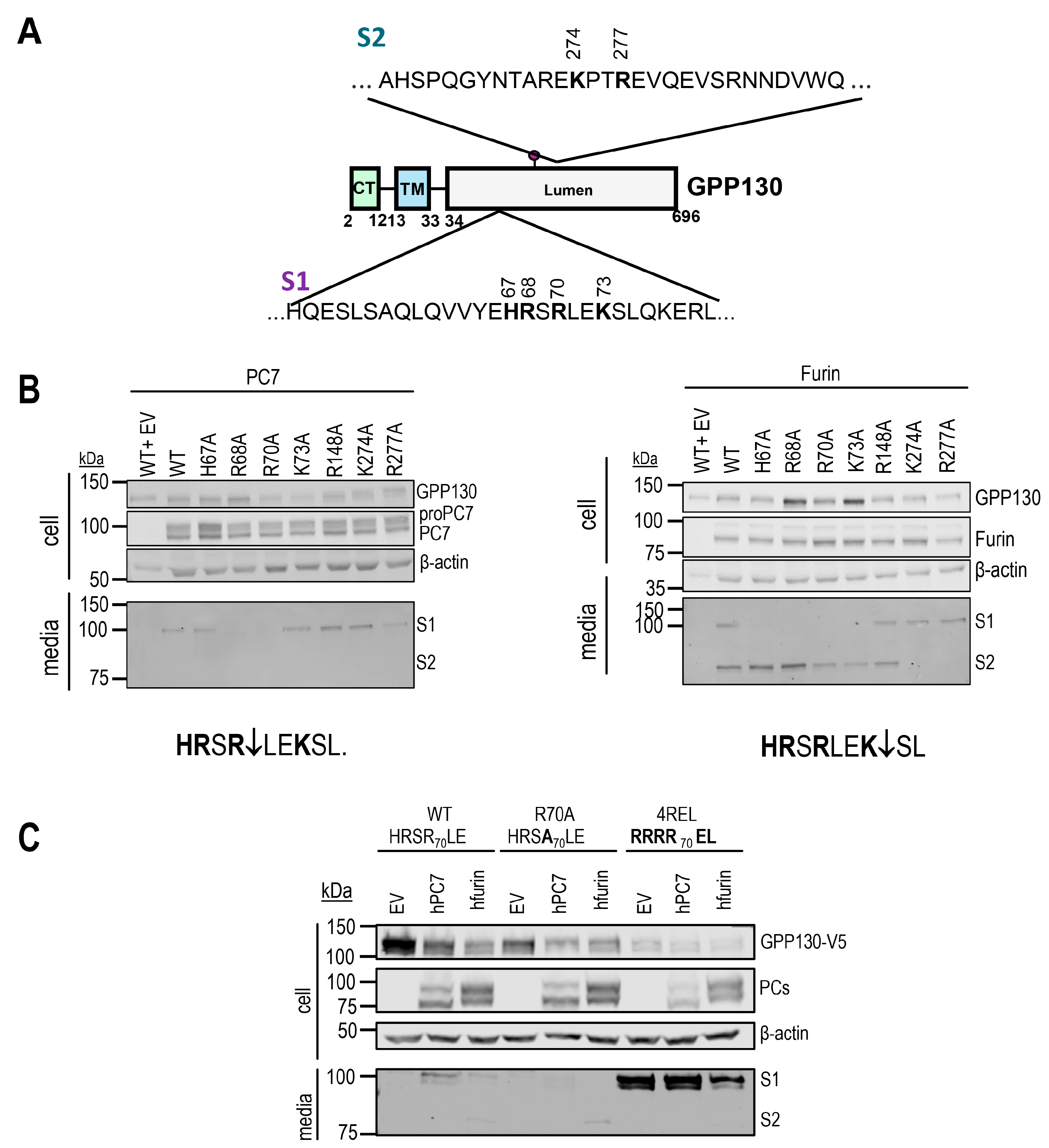
2.2. Identification of GPP130 Sites Shed by PC7 and Furin
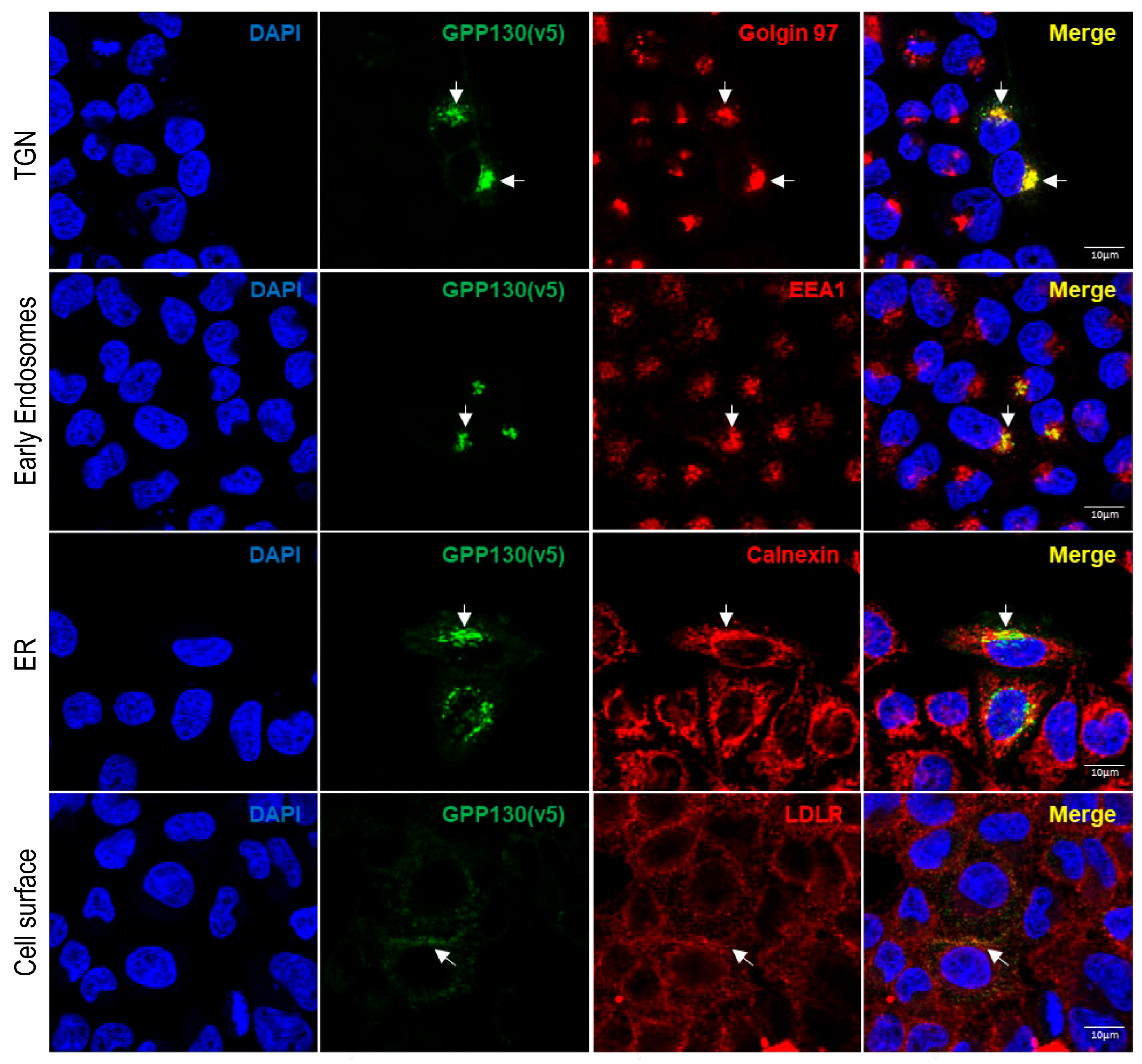
2.3. GPP130 Is Cleaved in Post-ER Acidic Compartments
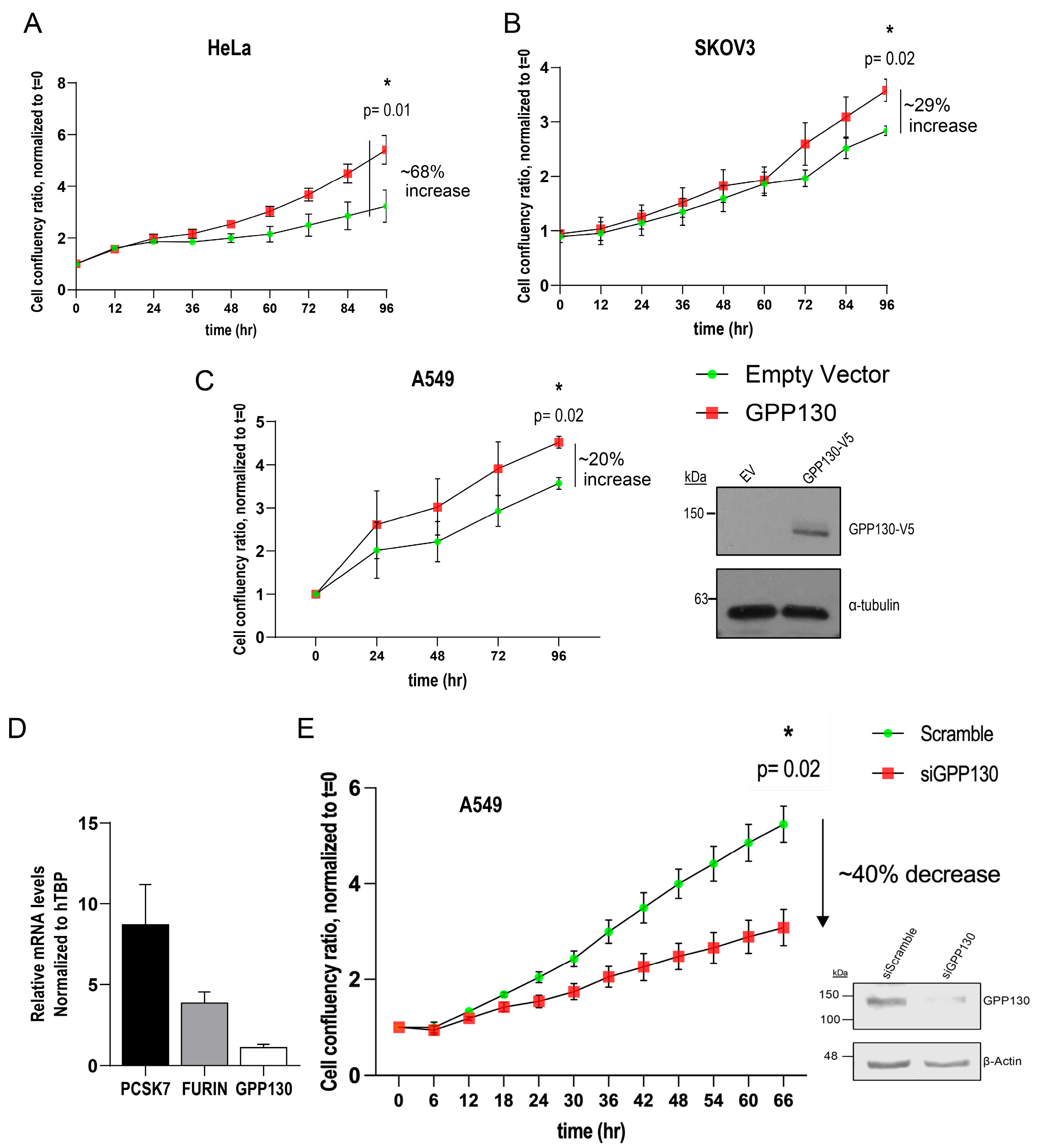
2.4. GPP130 Influences Cell Proliferation in Lung Cancer-, Ovarian Cancer- and Cervical Cancer-Derived Cell Lines
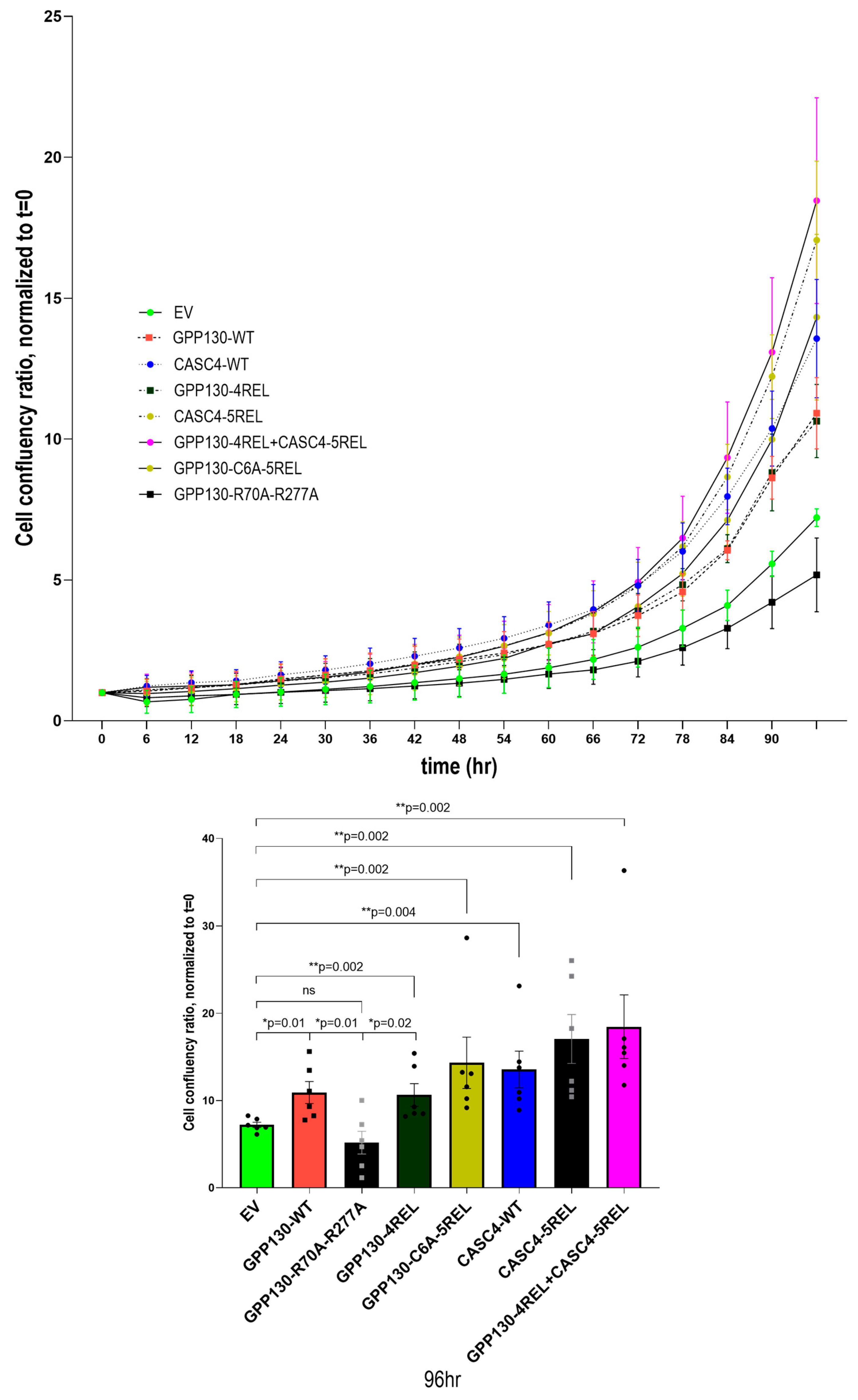
2.5. Identification of Critical Domains and Palmitoylation of GPP130 Implicated in Enhanced Proliferation
2.6. Comparative Growth of A549 Cells Overexpressing GPP130 or CASC4 Constructs
3. Discussion
- https://www.proteinatlas.org/ENSG00000173905-GOLIM4/cancer, accessed on 21 May 2025).
4. Materials and Methods
4.1. Plasmids
4.2. Cell Culture, Transfections, and Cell Treatments
4.3. Cell Treatments
4.4. Immunofluorescence
4.5. Western Blot Analysis and Antibodies Used
4.6. Cell Proliferation Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidah, N.G.; Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012, 11, 367–383. [Google Scholar] [CrossRef] [PubMed]
- François, A.; Descarpentrie, J.; Badiola, I.; Siegfried, G.; Evrard, S.; Pernot, S.; Khatib, A.M. Reprogramming immune cells activity by furin-like enzymes as emerging strategy for enhanced immunotherapy in cancer. Br. J. Cancer 2023, 128, 1189–1195. [Google Scholar] [CrossRef]
- He, Z.; Khatib, A.M.; Creemers, J.W.M. The proprotein convertase furin in cancer: More than an oncogene. Oncogene 2022, 41, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Abu-Thuraia, A.; Elkholi, I.E.; Chen, R.; Seebun, D.; Mayne, J.; Côté, J.F.; Figeys, D.; Seidah, N.G. Shedding of cancer susceptibility candidate 4 by the convertases PC7/furin unravels a novel secretory protein implicated in cancer progression. Cell Death Dis. 2020, 11, 665. [Google Scholar] [CrossRef]
- Couture, F.; Sabbagh, R.; Kwiatkowska, A.; Desjardins, R.; Guay, S.P.; Bouchard, L.; Day, R. PACE4 Undergoes an Oncogenic Alternative Splicing Switch in Cancer. Cancer Res. 2017, 77, 6863–6879. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, C.; Mastrofrancesco, A.; Metze, D.; Kemper, B.; Stegemann, A.; Picardo, M.; Klein-Szanto, A.J.P.; Böhm, M. Paired Basic Amino Acid-cleaving Enzyme 4 (PCSK6): An Emerging New Target Molecule in Human Melanoma. Acta Derm. Venereol. 2020, 100, adv00157. [Google Scholar] [CrossRef]
- Navals, P.; Kwiatkowska, A.; Mekdad, N.; Couture, F.; Desjardins, R.; Day, R.; Dory, Y.L. Enhancing the Drug-Like Profile of a Potent Peptide PACE4 Inhibitor by the Formation of a Host-Guest Inclusion Complex with β-Cyclodextrin. Mol. Pharm. 2023, 20, 4559–4573. [Google Scholar] [CrossRef]
- Sun, X.; Essalmani, R.; Day, R.; Khatib, A.M.; Seidah, N.G.; Prat, A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia 2012, 14, 1122–1131. [Google Scholar] [CrossRef]
- Shu, Z.; Fan, M.; Tu, B.; Tang, Z.; Wang, H.; Li, H.; Li, H.; Yuan, M.; Bai, J.; Huo, S.; et al. The Lin28b/Wnt5a axis drives pancreas cancer through crosstalk between cancer associated fibroblasts and tumor epithelium. Nat. Commun. 2023, 14, 6885. [Google Scholar] [CrossRef]
- Libby, P.; Tokgözoğlu, L. Chasing LDL cholesterol to the bottom–PCSK9 in perspective. Nat. Cardiovasc. Res. 2022, 1, 554–561. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Laufs, U. PCSK9-directed therapies: An update. Curr. Opin. Lipidol. 2024, 35, 117–125. [Google Scholar] [CrossRef]
- Seidah, N.G.; Hamelin, J.; Mamarbachi, M.; Dong, W.; Tardos, H.; Mbikay, M.; Chretien, M.; Day, R. cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc. Natl. Acad. Sci. USA 1996, 93, 3388–3393. [Google Scholar] [CrossRef]
- Sachan, V.; Le Dévéhat, M.; Roubtsova, A.; Essalmani, R.; Laurendeau, J.F.; Garçon, D.; Susan-Resiga, D.; Duval, S.; Mikaeeli, S.; Hamelin, J.; et al. PCSK7: A novel regulator of apolipoprotein B and a potential target against non-alcoholic fatty liver disease. Metabolism 2024, 150, 155736. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Targher, G.; Romeo, S.; Pajvani, U.B.; Zheng, M.H.; Aghemo, A.; Valenti, L.V.C. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024, 44, 1526–1536. [Google Scholar] [CrossRef]
- Linstedt, A.D.; Mehta, A.; Suhan, J.; Reggio, H.; Hauri, H.P. Sequence and overexpression of GPP130/GIMPc: Evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol. Biol. Cell 1997, 8, 1073–1087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natarajan, R.; Linstedt, A.D. A cycling cis-Golgi protein mediates endosome-to-Golgi traffic. Mol. Biol. Cell 2004, 15, 4798–4806. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Fimmel, C.; Linstedt, A.D. Endosomal trafficking and proprotein convertase cleavage of cis Golgi protein GP73 produces marker for hepatocellular carcinoma. Traffic 2007, 8, 1415–1423. [Google Scholar] [CrossRef]
- Puri, S.; Bachert, C.; Fimmel, C.J.; Linstedt, A.D. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 2002, 3, 641–653. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Redler, B.; Linstedt, A.D. Shiga toxin-binding site for host cell receptor GPP130 reveals unexpected divergence in toxin-trafficking mechanisms. Mol. Biol. Cell 2013, 24, 2311–2318. [Google Scholar] [CrossRef]
- Mallard, F.; Johannes, L. Shiga toxin B-subunit as a tool to study retrograde transport. Methods Mol. Med. 2003, 73, 209–220. [Google Scholar]
- Mukhopadhyay, S.; Bachert, C.; Smith, D.R.; Linstedt, A.D. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol. Biol. Cell 2010, 21, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Jarvela, T.; Linstedt, A.D. Manganese induces oligomerization to promote down-regulation of the intracellular trafficking receptor used by Shiga toxin. Mol. Biol. Cell 2014, 25, 3049–3058. [Google Scholar] [CrossRef]
- Tan, X.; Wang, S.; Xiao, G.Y.; Wu, C.; Liu, X.; Zhou, B.; Yu, J.; Duose, D.Y.; Xi, Y.; Wang, J.; et al. Chromosomal 3q amplicon encodes essential regulators of secretory vesicles that drive secretory addiction in cancer. J. Clin. Investig. 2024, 134, e176355. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer 2017, 17, 367–380. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, X.; Gao, D.; Wang, Y.; Wang, B.; Wang, W. Golgi integral membrane protein 4 manipulates cellular proliferation, apoptosis, and cell cycle in human head and neck cancer. Biosci. Rep. 2018, 38, BSR20180454. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Liu, C.; Shi, E.; Jin, Q.; Zhao, W.; Wang, J.; Ji, R. MiR-105-3p acts as an oncogene to promote the proliferation and metastasis of breast cancer cells by targeting GOLIM4. BMC Cancer 2021, 21, 275. [Google Scholar] [CrossRef]
- Kan, B.; Yan, G.; Shao, Y.; Zhang, Z.; Xue, H. CircRNA RNF10 inhibits tumorigenicity by targeting miR-942-5p/GOLIM4 axis in breast cancer. Env. Mol. Mutagen. 2022, 63, 362–372. [Google Scholar] [CrossRef]
- Taylor, A.H.; Konje, J.C.; Ayakannu, T. Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach. Cancers 2023, 15, 4665. [Google Scholar] [CrossRef]
- Ginefra, P.; Filippi, B.G.H.; Donovan, P.; Bessonnard, S.; Constam, D.B. Compartment-Specific Biosensors Reveal a Complementary Subcellular Distribution of Bioactive Furin and PC7. Cell Rep. 2018, 22, 2176–2189. [Google Scholar] [CrossRef]
- Guillemot, J.; Canuel, M.; Essalmani, R.; Prat, A.; Seidah, N.G. Implication of the proprotein convertases in iron homeostasis: Proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology 2013, 57, 2514–2524. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Yuan, L.; Tipper, C.; Amherdt, M.; Orci, L.; Klausner, R.D. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 1991, 67, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Aridor, M.; Bannykh, S.I.; Rowe, T.; Balch, W.E. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 1995, 131, 875–893. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Chao, T.L.; Li, C.L.; Chiu, M.F.; Kao, H.C.; Wang, S.H.; Pang, Y.H.; Lin, C.H.; Tsai, Y.M.; Lee, W.H.; et al. Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Chretien, M. Proprotein and prohormone convertases: A family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999, 848, 45–62. [Google Scholar] [CrossRef]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 753–766. [Google Scholar] [CrossRef]
- Guan, X.; Fierke, C.A. Understanding Protein Palmitoylation: Biological Significance and Enzymology. Sci. China Chem. 2011, 54, 1888–1897. [Google Scholar] [CrossRef]
- Lin, D.T.; Conibear, E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife 2015, 4, e11306. [Google Scholar] [CrossRef]
- Venkat, S.; Linstedt, A.D. Manganese-induced trafficking and turnover of GPP130 is mediated by sortilin. Mol. Biol. Cell 2017, 28, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Mitok, K.A.; Keller, M.P.; Attie, A.D. Sorting through the extensive and confusing roles of sortilin in metabolic disease. J. Lipid Res. 2022, 63, 100243. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signal 2021, 19, 60. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xu, H.; Zhang, Y.W. The γ-secretase complex: From structure to function. Front. Cell Neurosci. 2014, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Liu, Y.; Li, X.; Rao, M.; Li, D.; Ruan, X.; Li, S.; Jiang, Z.; Zhang, Q. Palmitoylation landscapes across human cancers reveal a role of palmitoylation in tumorigenesis. J. Transl. Med. 2023, 21, 826. [Google Scholar] [CrossRef] [PubMed]
- Hann, H.W.; Wang, M.; Hafner, J.; Long, R.E.; Kim, S.H.; Ahn, M.; Park, S.; Comunale, M.A.; Block, T.M.; Mehta, A. Analysis of GP73 in patients with HCC as a function of anti-cancer treatment. Cancer Biomark. 2010, 7, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Romano, P.R.; Nikolaeva, O.; Steel, L.; Mehta, A.; Fimmel, C.J.; Comunale, M.A.; D’Amelio, A.; Lok, A.S.; Block, T.M. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J. Hepatol. 2005, 43, 1007–1012. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Gong, Y.; Testa, C.L.; Klein-Szanto, A.J. Regulation of HIF-1 alpha by the proprotein convertases furin and PC7 in human squamous carcinoma cells. Mol. Carcinog. 2015, 54, 698–706. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhala, P.; Duval, S.; Evagelidis, A.; Le Dévéhat, M.; Sachan, V.; Seidah, N.G. Shedding of GPP130 by PC7 and Furin: Potential Implication in Lung Cancer Progression. Int. J. Mol. Sci. 2025, 26, 6164. https://doi.org/10.3390/ijms26136164
Prabhala P, Duval S, Evagelidis A, Le Dévéhat M, Sachan V, Seidah NG. Shedding of GPP130 by PC7 and Furin: Potential Implication in Lung Cancer Progression. International Journal of Molecular Sciences. 2025; 26(13):6164. https://doi.org/10.3390/ijms26136164
Chicago/Turabian StylePrabhala, Priyanka, Stephanie Duval, Alexandra Evagelidis, Maïlys Le Dévéhat, Vatsal Sachan, and Nabil G. Seidah. 2025. "Shedding of GPP130 by PC7 and Furin: Potential Implication in Lung Cancer Progression" International Journal of Molecular Sciences 26, no. 13: 6164. https://doi.org/10.3390/ijms26136164
APA StylePrabhala, P., Duval, S., Evagelidis, A., Le Dévéhat, M., Sachan, V., & Seidah, N. G. (2025). Shedding of GPP130 by PC7 and Furin: Potential Implication in Lung Cancer Progression. International Journal of Molecular Sciences, 26(13), 6164. https://doi.org/10.3390/ijms26136164





