Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases?
Abstract
1. Voltage-Dependent Anion Channel Function and Structure
1.1. Extramitochondrial Locations of VDACs
1.2. VDACs and Mitochondrial Dynamics
1.3. The Involvement of the VDAC in Apoptosis
1.4. Involvement of pl-VDAC-1 in Neuroprotection
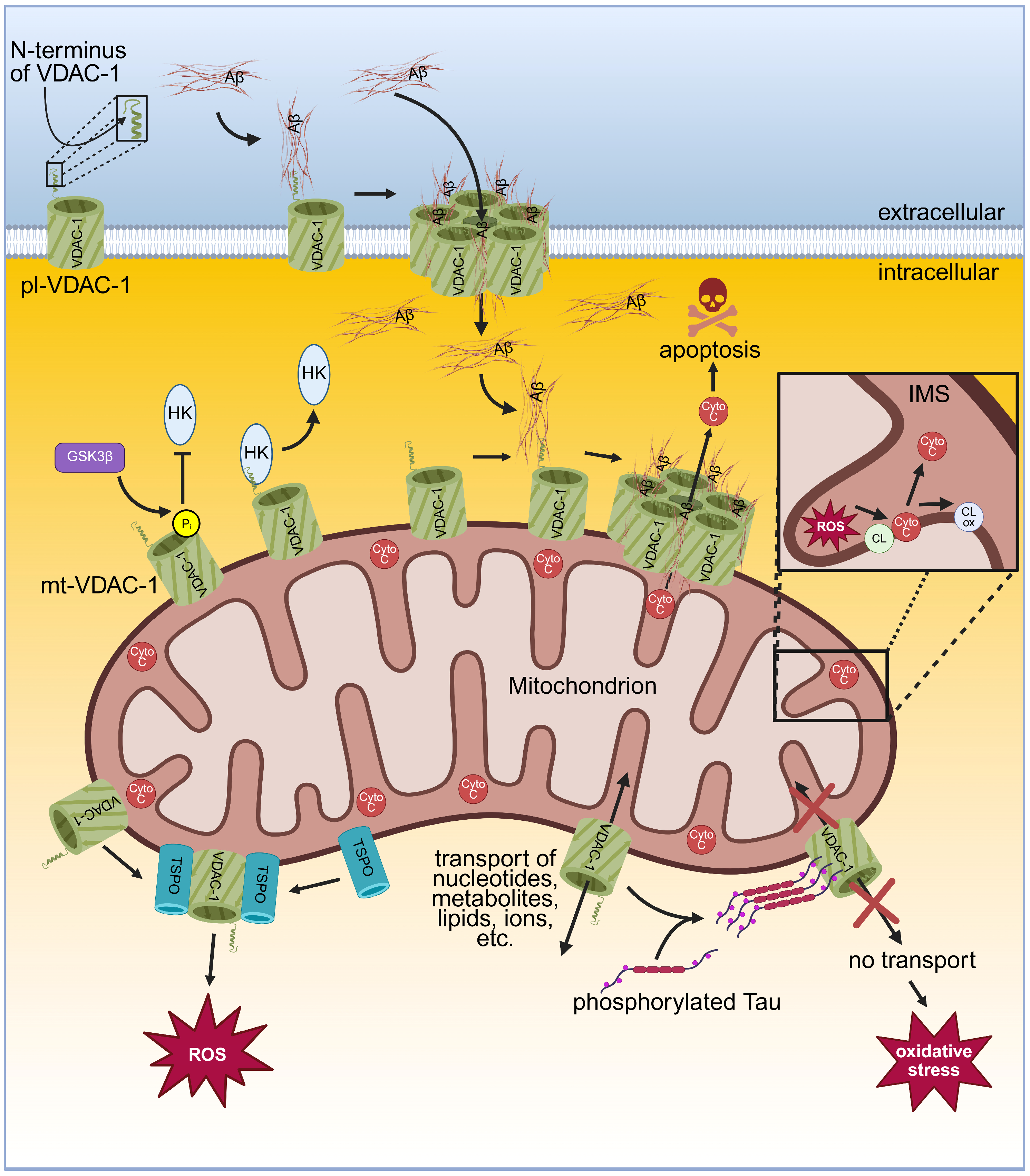
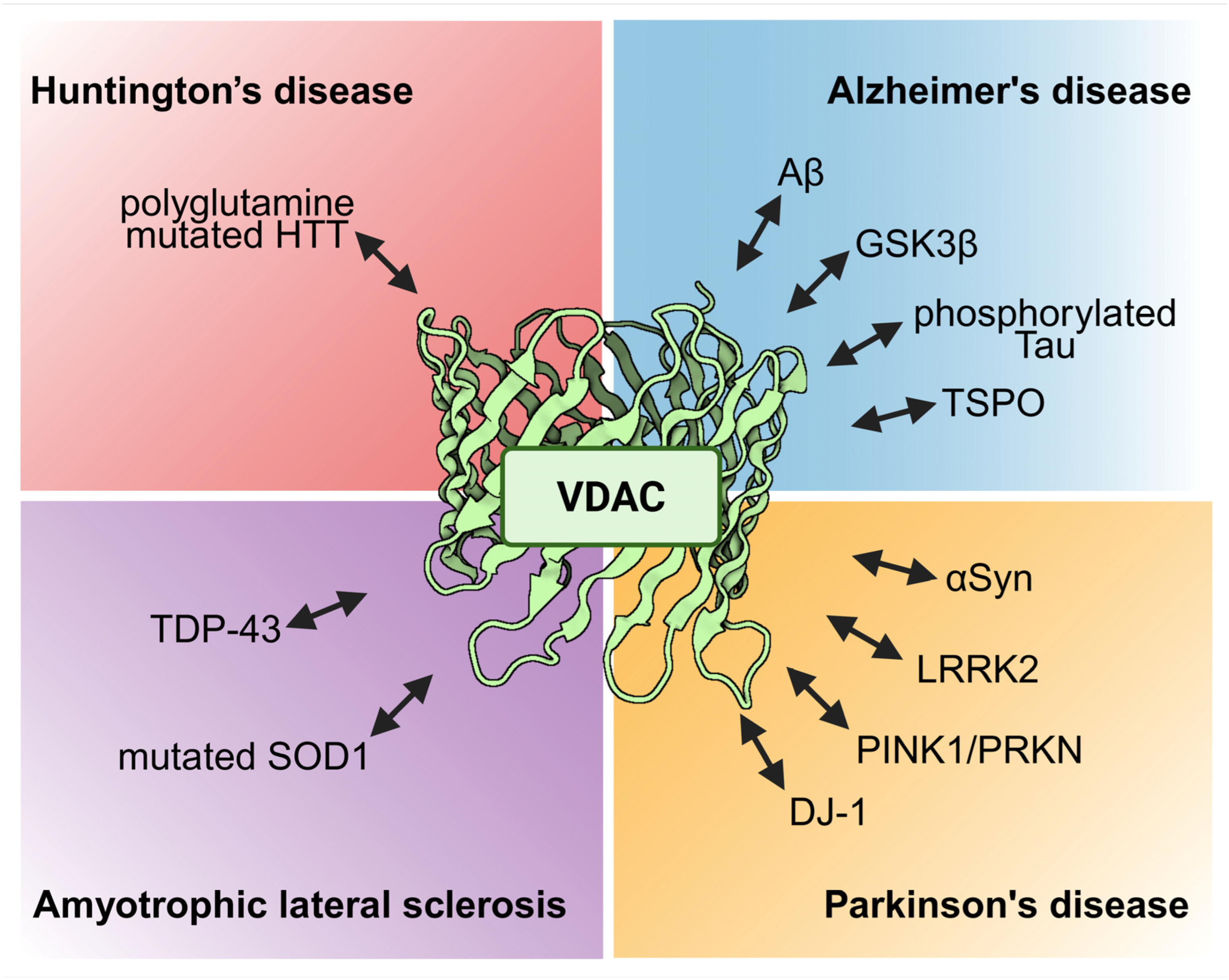
2. Alzheimer’s Disease and VDACs
3. Parkinson’s Disease and VDACs
4. Amyotrophic Lateral Sclerosis and VDACs
5. Huntington’s Disease and VDACs
6. Further Neurodegenerative Conditions and VDACs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Argueti-Ostrovsky, S.; Barel, S.; Kahn, J.; Israelson, A. Vdac1: A key player in the mitochondrial landscape of neurodegeneration. Biomolecules 2024, 15, 33. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Yang, X.; Wang, J.; Fang, Y.; Ying, X.; Zhang, M.; Wei, J.; Pan, Y. Targeting vdac: A potential therapeutic approach for mitochondrial dysfunction in alzheimer’s disease. Brain Res. 2024, 1835, 148920. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.; Reina, S.; De Pinto, V. Vdac1 as pharmacological target in cancer and neurodegeneration: Focus on its role in apoptosis. Front. Chem. 2018, 6, 108. [Google Scholar] [CrossRef]
- Magri, A.; Messina, A. Interactions of vdac with proteins involved in neurodegenerative aggregation: An opportunity for advancement on therapeutic molecules. Curr. Med. Chem. 2017, 24, 4470–4487. [Google Scholar] [CrossRef] [PubMed]
- Schein, S.J.; Colombini, M.; Finkelstein, A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol. 1976, 30, 99–120. [Google Scholar] [CrossRef]
- Colombini, M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 1979, 279, 643–645. [Google Scholar] [CrossRef]
- Craigen, W.J.; Graham, B.H. Genetic strategies for dissecting mammalian and drosophila voltage-dependent anion channel functions. J. Bioenerg. Biomembr. 2008, 40, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.J.; Ross, L.; Decker, W.K.; Craigen, W.J. A novel isoform of the mitochondrial outer membrane protein vdac3 via alternative splicing of a 3-base exon. J. Biol. Chem. 1998, 273, 30482–30486. [Google Scholar] [CrossRef]
- Colombini, M. Vdac structure, selectivity, and dynamics. Biochim. Biophys. Acta 2012, 1818, 1457–1465. [Google Scholar] [CrossRef]
- Raghavan, A.; Sheiko, T.; Graham, B.H.; Craigen, W.J. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim. Biophys. Acta 2012, 1818, 1477–1485. [Google Scholar] [CrossRef]
- Freitag, H.; Janes, M.; Neupert, W. Biosynthesis of mitochondrial porin and insertion into the outer mitochondrial membrane of neurospora crassa. Eur. J. Biochem. 1982, 126, 197–202. [Google Scholar] [CrossRef]
- Hartl, F.U.; Pfanner, N.; Nicholson, D.W.; Neupert, W. Mitochondrial protein import. Biochim. Biophys. Acta 1989, 988, 1–45. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Bykov, Y. Protein translocation in mitochondria: Sorting out the toms, tims, pams, sams and mia. FEBS Lett. 2023, 597, 1553–1554. [Google Scholar] [CrossRef]
- Benz, R. Solute transport through mitochondrial porins in vitro and in vivo. Biomolecules 2024, 14, 303. [Google Scholar] [CrossRef]
- Messina, A.; Reina, S.; Guarino, F.; De Pinto, V. Vdac isoforms in mammals. Biochim. Biophys. Acta 2012, 1818, 1466–1476. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. Vdac1, mitochondrial dysfunction, and alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Kim, I.H.; Kim, S.K.; Kim, E.H.; Kim, S.W.; Sohn, S.H.; Lee, S.C.; Choi, S.; Pyo, S.; Rhee, D.K. Korean red ginseng up-regulates c21-steroid hormone metabolism via cyp11a1 gene in senescent rat testes. J. Ginseng Res. 2011, 35, 272–282. [Google Scholar] [CrossRef]
- Heslop, K.A.; Milesi, V.; Maldonado, E.N. Vdac modulation of cancer metabolism: Advances and therapeutic challenges. Front. Physiol. 2021, 12, 742839. [Google Scholar] [CrossRef]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution structure of the integral human membrane protein vdac-1 in detergent micelles. Science 2008, 321, 1206–1210. [Google Scholar] [CrossRef]
- Bayrhuber, M.; Meins, T.; Habeck, M.; Becker, S.; Giller, K.; Villinger, S.; Vonrhein, C.; Griesinger, C.; Zweckstetter, M.; Zeth, K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 15370–15375. [Google Scholar] [CrossRef] [PubMed]
- Ujwal, R.; Cascio, D.; Colletier, J.P.; Faham, S.; Zhang, J.; Toro, L.; Ping, P.; Abramson, J. The crystal structure of mouse vdac1 at 2.3 a resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. USA 2008, 105, 17742–17747. [Google Scholar] [CrossRef] [PubMed]
- Schredelseker, J.; Paz, A.; Lopez, C.J.; Altenbach, C.; Leung, C.S.; Drexler, M.K.; Chen, J.N.; Hubbell, W.L.; Abramson, J. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J. Biol. Chem. 2014, 289, 12566–12577. [Google Scholar] [CrossRef] [PubMed]
- Benz, R. Permeation of hydrophilic solutes through mitochondrial outer membranes: Review on mitochondrial porins. Biochim. Biophys. Acta 1994, 1197, 167–196. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Maldonado, E.N.; Krelin, Y. Vdac1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress 2017, 1, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Najbauer, E.E.; Becker, S.; Giller, K.; Zweckstetter, M.; Lange, A.; Steinem, C.; de Groot, B.L.; Griesinger, C.; Andreas, L.B. Structure, gating and interactions of the voltage-dependent anion channel. Eur. Biophys. J. 2021, 50, 159–172. [Google Scholar] [CrossRef]
- Jahn, H.; Bartos, L.; Dearden, G.I.; Dittman, J.S.; Holthuis, J.C.M.; Vacha, R.; Menon, A.K. Phospholipids are imported into mitochondria by vdac, a dimeric beta barrel scramblase. Nat. Commun. 2023, 14, 8115. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M.; Hoogerheide, D.P. Regulation of mitochondrial respiration by vdac is enhanced by membrane-bound inhibitors with disordered polyanionic c-terminal domains. Int. J. Mol. Sci. 2021, 22, 7358. [Google Scholar] [CrossRef]
- Tan, W.; Colombini, M. Vdac closure increases calcium ion flux. Biochim. Biophys. Acta 2007, 1768, 2510–2515. [Google Scholar] [CrossRef]
- Checchetto, V.; Reina, S.; Magri, A.; Szabo, I.; De Pinto, V. Recombinant human voltage dependent anion selective channel isoform 3 (hvdac3) forms pores with a very small conductance. Cell Physiol. Biochem. 2014, 34, 842–853. [Google Scholar] [CrossRef]
- Sander, P.; Gudermann, T.; Schredelseker, J. A calcium guard in the outer membrane: Is vdac a regulated gatekeeper of mitochondrial calcium uptake? Int. J. Mol. Sci. 2021, 22, 946. [Google Scholar] [CrossRef]
- Shuvo, S.R.; Ferens, F.G.; Court, D.A. The n-terminus of vdac: Structure, mutational analysis, and a potential role in regulating barrel shape. Biochim. Biophys. Acta 2016, 1858, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Israelson, A. The voltage-dependent anion channel in endoplasmic/sarcoplasmic reticulum: Characterization, modulation and possible function. J. Membr. Biol. 2005, 204, 57–66. [Google Scholar] [CrossRef]
- Thinnes, F.P.; Gotz, H.; Kayser, H.; Benz, R.; Schmidt, W.E.; Kratzin, H.D.; Hilschmann, N. Identification of human porins. I. Purification of a porin from human b-lymphocytes (porin 31hl) and the topochemical proof of its expression on the plasmalemma of the progenitor cell. Biol. Chem. Hoppe Seyler 1989, 370, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Bathori, G.; Parolini, I.; Tombola, F.; Szabo, I.; Messina, A.; Oliva, M.; De Pinto, V.; Lisanti, M.; Sargiacomo, M.; Zoratti, M. Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae-related domains. J. Biol. Chem. 1999, 274, 29607–29612. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, V.; Messina, A.; Lane, D.J.; Lawen, A. Voltage-dependent anion-selective channel (vdac) in the plasma membrane. FEBS Lett. 2010, 584, 1793–1799. [Google Scholar] [CrossRef]
- Buettner, R.; Papoutsoglou, G.; Scemes, E.; Spray, D.C.; Dermietzel, R. Evidence for secretory pathway localization of a voltage-dependent anion channel isoform. Proc. Natl. Acad. Sci. USA 2000, 97, 3201–3206. [Google Scholar] [CrossRef]
- Li, L.; Yao, Y.C.; Gu, X.Q.; Che, D.; Ma, C.Q.; Dai, Z.Y.; Li, C.; Zhou, T.; Cai, W.B.; Yang, Z.H.; et al. Plasminogen kringle 5 induces endothelial cell apoptosis by triggering a voltage-dependent anion channel 1 (vdac1) positive feedback loop. J. Biol. Chem. 2014, 289, 32628–32638. [Google Scholar] [CrossRef]
- Bahamonde, M.I.; Fernandez-Fernandez, J.M.; Guix, F.X.; Vazquez, E.; Valverde, M.A. Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi cl- currents in c1300 neuroblastoma cells. J. Biol. Chem. 2003, 278, 33284–33289. [Google Scholar] [CrossRef]
- Bathori, G.; Parolini, I.; Szabo, I.; Tombola, F.; Messina, A.; Oliva, M.; Sargiacomo, M.; De Pinto, V.; Zoratti, M. Extramitochondrial porin: Facts and hypotheses. J. Bioenerg. Biomembr. 2000, 32, 79–89. [Google Scholar] [CrossRef]
- Baker, M.A.; Lane, D.J.; Ly, J.D.; De Pinto, V.; Lawen, A. Vdac1 is a transplasma membrane nadh-ferricyanide reductase. J. Biol. Chem. 2004, 279, 4811–4819. [Google Scholar] [CrossRef]
- Baker, M.A.; Ly, J.D.; Lawen, A. Characterization of vdac1 as a plasma membrane nadh-oxidoreductase. Biofactors 2004, 21, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.F.; O’Neal, W.K.; Huang, P.; Nicholas, R.A.; Ostrowski, L.E.; Craigen, W.J.; Lazarowski, E.R.; Boucher, R.C. Voltage-dependent anion channel-1 (vdac-1) contributes to atp release and cell volume regulation in murine cells. J. Gen. Physiol. 2004, 124, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Mohammed Al-Amily, I.; Mohammed, S.; Luan, C.; Asplund, O.; Ahmed, M.; Ye, Y.; Ben-Hail, D.; Soni, A.; Vishnu, N.; et al. Preserving insulin secretion in diabetes by inhibiting vdac1 overexpression and surface translocation in beta cells. Cell Metab. 2019, 29, 64–77.e6. [Google Scholar] [CrossRef]
- Hinsch, K.D.; De Pinto, V.; Aires, V.A.; Schneider, X.; Messina, A.; Hinsch, E. Voltage-dependent anion-selective channels vdac2 and vdac3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J. Biol. Chem. 2004, 279, 15281–15288. [Google Scholar] [CrossRef] [PubMed]
- Menzel, V.A.; Cassara, M.C.; Benz, R.; de Pinto, V.; Messina, A.; Cunsolo, V.; Saletti, R.; Hinsch, K.D.; Hinsch, E. Molecular and functional characterization of vdac2 purified from mammal spermatozoa. Biosci. Rep. 2009, 29, 351–362. [Google Scholar] [CrossRef]
- Paradowska, A.; Bohring, C.; Krause, E.; Krause, W. Identification of evolutionary conserved mouse sperm surface antigens by human antisperm antibodies (asa) from infertile patients. Am. J. Reprod. Immunol. 2006, 55, 321–330. [Google Scholar] [CrossRef]
- Liu, B.; Wang, P.; Wang, Z.; Zhang, W. The use of anti-vdac2 antibody for the combined assessment of human sperm acrosome integrity and ionophore a23187-induced acrosome reaction. PLoS ONE 2011, 6, e16985. [Google Scholar] [CrossRef][Green Version]
- Ning, L.; Pan, B.; Zhao, Y.P.; Liao, Q.; Zhang, T.P.; Chen, G.; Wang, W.B.; Yang, Y.C. Immuno-proteomic screening of human pancreatic cancer associated membrane antigens for early diagnosis. Zhonghua Wai Ke Za Zhi 2007, 45, 34–38. [Google Scholar]
- Valis, K.; Neubauerova, J.; Man, P.; Pompach, P.; Vohradsky, J.; Kovar, J. Vdac2 and aldolase a identified as membrane proteins of k562 cells with increased expression under iron deprivation. Mol. Cell. Biochem. 2008, 311, 225–231. [Google Scholar] [CrossRef]
- Stark, G. Functional consequences of oxidative membrane damage. J. Membr. Biol. 2005, 205, 1–16. [Google Scholar] [CrossRef]
- Budzinska, M.; Galganska, H.; Wojtkowska, M.; Stobienia, O.; Kmita, H. Effects of vdac isoforms on cuzn-superoxide dismutase activity in the intermembrane space of saccharomyces cerevisiae mitochondria. Biochem. Biophys. Res. Commun. 2007, 357, 1065–1070. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Toan, S.; Mui, D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res. Rev. 2021, 66, 101250. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Delerue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, M.; Arnaune-Pelloquin, L.; Davezac, N.; Mils, V.; Miquel, M.C.; et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016, 90, 3–19. [Google Scholar] [CrossRef]
- Nardin, A.; Schrepfer, E.; Ziviani, E. Counteracting pink/parkin deficiency in the activation of mitophagy: A potential therapeutic intervention for parkinson’s disease. Curr. Neuropharmacol. 2016, 14, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhou, H.; Sun, Y.; Fu, H.; Ran, Y.; Yang, B.; Yang, F.; Bjorklund, M.; Xu, S. Miro-1 interacts with vdac-1 to regulate mitochondrial membrane potential in caenorhabditis elegans. EMBO Rep. 2023, 24, e56297. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Vashisht, A.A.; Tchieu, J.; Wohlschlegel, J.A.; Dreier, L. Voltage-dependent anion channels (vdacs) recruit parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 2012, 287, 40652–40660. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.R.; Fullerton, M.; Chaudhuri, M. Tim50 in trypanosoma brucei possesses a dual specificity phosphatase activity and is critical for mitochondrial protein import. J. Biol. Chem. 2013, 288, 3184–3197. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, S. Phosphorylation of voltage-dependent anion channel by c-jun n-terminal kinase-3 leads to closure of the channel. Biochem. Biophys. Res. Commun. 2015, 459, 100–106. [Google Scholar] [CrossRef]
- Vijayan, M.; Alvir, R.V.; Alvir, R.V.; Bunquin, L.E.; Pradeepkiran, J.A.; Reddy, P.H. A partial reduction of vdac1 enhances mitophagy, autophagy, synaptic activities in a transgenic tau mouse model. Aging Cell 2022, 21, e13663. [Google Scholar] [CrossRef]
- Saini, N.; Lakshminarayanan, S.; Kundu, P.; Sarin, A. Notch1 modulation of cellular calcium regulates mitochondrial metabolism and anti-apoptotic activity in t-regulatory cells. Front. Immunol. 2022, 13, 832159. [Google Scholar] [CrossRef]
- Petrozzi, L.; Ricci, G.; Giglioli, N.J.; Siciliano, G.; Mancuso, M. Mitochondria and neurodegeneration. Biosci. Rep. 2007, 27, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Tschopp, J. Apoptosis induced by death receptors. Pharm. Acta Helv. 2000, 74, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Ott, M.; Robertson, J.D.; Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 2002, 99, 1259–1263. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.V.; Alekseev, A.V. Molecular mechanisms of apoptosis. Structure of cytochrome c-cardiolipin complex. Biochemistry 2013, 78, 1086–1097. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Morcillo, P. The role of cardiolipin in mitochondrial function and neurodegenerative diseases. Cells 2024, 13, 609. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W.G. Mitochondria and cell death. Nat. Cell. Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Kilbride, S.M.; Prehn, J.H. Central roles of apoptotic proteins in mitochondrial function. Oncogene 2013, 32, 2703–2711. [Google Scholar] [CrossRef]
- Repnik, U.; Turk, B. Lysosomal-mitochondrial cross-talk during cell death. Mitochondrion 2010, 10, 662–669. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Chandel, N.S.; Li, X.X.; Schumacker, P.T.; Colombini, M.; Thompson, C.B. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA 2000, 97, 4666–4671. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Stefkova, K.; Cervinkova, Z.; Kucera, O. The mitochondrial permeability transition pore-current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells 2023, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The bcl2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Antignani, A.; Youle, R.J. How do bax and bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 2006, 18, 685–689. [Google Scholar] [CrossRef]
- Lovell, J.F.; Billen, L.P.; Bindner, S.; Shamas-Din, A.; Fradin, C.; Leber, B.; Andrews, D.W. Membrane binding by tbid initiates an ordered series of events culminating in membrane permeabilization by bax. Cell 2008, 135, 1074–1084. [Google Scholar] [CrossRef]
- Banerjee, J.; Ghosh, S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tbid. Biochem. Biophys. Res. Commun. 2004, 323, 310–314. [Google Scholar] [CrossRef]
- Shimizu, S.; Tsujimoto, Y. Proapoptotic bh3-only bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc. Natl. Acad. Sci. USA 2000, 97, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Keinan, N.; Tyomkin, D.; Shoshan-Barmatz, V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol. Cell. Biol. 2010, 30, 5698–5709. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. Vdac oligomers form mitochondrial pores to release mtdna fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Zalk, R.; Israelson, A.; Garty, E.S.; Azoulay-Zohar, H.; Shoshan-Barmatz, V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 2005, 386, 73–83. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Arbel, N.; Calo, D.; Arzoine, L.; Israelson, A.; Keinan, N.; Ben-Romano, R.; Friedman, O.; Shoshan-Barmatz, V. The vdac1 n-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009, 122, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Mizrachi, D. Vdac1: From structure to cancer therapy. Front. Oncol. 2012, 2, 164. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Mizrachi, D.; Keinan, N. Oligomerization of the mitochondrial protein vdac1: From structure to function and cancer therapy. Prog. Mol. Biol. Transl. Sci. 2013, 117, 303–334. [Google Scholar] [CrossRef]
- Keinan, N.; Pahima, H.; Ben-Hail, D.; Shoshan-Barmatz, V. The role of calcium in vdac1 oligomerization and mitochondria-mediated apoptosis. Biochim. Biophys. Acta 2013, 1833, 1745–1754. [Google Scholar] [CrossRef]
- Weisthal, S.; Keinan, N.; Ben-Hail, D.; Arif, T.; Shoshan-Barmatz, V. Ca(2+)-mediated regulation of vdac1 expression levels is associated with cell death induction. Biochim. Biophys. Acta 2014, 1843, 2270–2281. [Google Scholar] [CrossRef]
- Ben-Hail, D.; Begas-Shvartz, R.; Shalev, M.; Shteinfer-Kuzmine, A.; Gruzman, A.; Reina, S.; De Pinto, V.; Shoshan-Barmatz, V. Novel compounds targeting the mitochondrial protein vdac1 inhibit apoptosis and protect against mitochondrial dysfunction. J. Biol. Chem. 2016, 291, 24986–25003. [Google Scholar] [CrossRef]
- Geula, S.; Naveed, H.; Liang, J.; Shoshan-Barmatz, V. Structure-based analysis of vdac1 protein: Defining oligomer contact sites. J. Biol. Chem. 2012, 287, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Smilansky, A.; Dangoor, L.; Nakdimon, I.; Ben-Hail, D.; Mizrachi, D.; Shoshan-Barmatz, V. The voltage-dependent anion channel 1 mediates amyloid beta toxicity and represents a potential target for alzheimer disease therapy. J. Biol. Chem. 2015, 290, 30670–30683. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Han, J.; Ben-Hail, D.; He, L.; Li, B.; Chen, Z.; Wang, Y.; Yang, Y.; Liu, L.; Zhu, Y.; et al. A new fungal diterpene induces vdac1-dependent apoptosis in bax/bak-deficient cells. J. Biol. Chem. 2015, 290, 23563–23578. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; De, S.; Meir, A. The mitochondrial voltage-dependent anion channel 1, Ca(2+) transport, apoptosis, and their regulation. Front. Oncol. 2017, 7, 60. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A. Vdac1 functions in ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium 2018, 69, 81–100. [Google Scholar] [CrossRef]
- Arbel, N.; Shoshan-Barmatz, V. Voltage-dependent anion channel 1-based peptides interact with bcl-2 to prevent antiapoptotic activity. J. Biol. Chem. 2010, 285, 6053–6062. [Google Scholar] [CrossRef]
- Arbel, N.; Ben-Hail, D.; Shoshan-Barmatz, V. Mediation of the antiapoptotic activity of bcl-xl protein upon interaction with vdac1 protein. J. Biol. Chem. 2012, 287, 23152–23161. [Google Scholar] [CrossRef]
- Shimizu, S.; Konishi, A.; Kodama, T.; Tsujimoto, Y. Bh4 domain of antiapoptotic bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 3100–3105. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Zaid, H.; Israelson, A.; Nahon, E.; Shoshan-Barmatz, V. Hexokinase-i protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: Mapping the site of binding. J. Biol. Chem. 2008, 283, 13482–13490. [Google Scholar] [CrossRef]
- Zaid, H.; Abu-Hamad, S.; Israelson, A.; Nathan, I.; Shoshan-Barmatz, V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005, 12, 751–760. [Google Scholar] [CrossRef]
- Ghosh, T.; Pandey, N.; Maitra, A.; Brahmachari, S.K.; Pillai, B. A role for voltage-dependent anion channel vdac1 in polyglutamine-mediated neuronal cell death. PLoS ONE 2007, 2, e1170. [Google Scholar] [CrossRef] [PubMed]
- Godbole, A.; Varghese, J.; Sarin, A.; Mathew, M.K. Vdac is a conserved element of death pathways in plant and animal systems. Biochim. Biophys. Acta 2003, 1642, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.J.; Dong, C.W.; Du, C.S.; Zhang, Q.Y. Characterization and expression analysis of paralichthys olivaceus voltage-dependent anion channel (vdac) gene in response to virus infection. Fish Shellfish Immunol. 2007, 23, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Azoulay-Zohar, H.; Israelson, A.; Abu-Hamad, S.; Shoshan-Barmatz, V. In self-defence: Hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004, 377, 347–355. [Google Scholar] [CrossRef]
- Tajeddine, N.; Galluzzi, L.; Kepp, O.; Hangen, E.; Morselli, E.; Senovilla, L.; Araujo, N.; Pinna, G.; Larochette, N.; Zamzami, N.; et al. Hierarchical involvement of bak, vdac1 and bax in cisplatin-induced cell death. Oncogene 2008, 27, 4221–4232. [Google Scholar] [CrossRef]
- Elinder, F.; Akanda, N.; Tofighi, R.; Shimizu, S.; Tsujimoto, Y.; Orrenius, S.; Ceccatelli, S. Opening of plasma membrane voltage-dependent anion channels (vdac) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell Death Differ. 2005, 12, 1134–1140. [Google Scholar] [CrossRef]
- Akanda, N.; Tofighi, R.; Brask, J.; Tamm, C.; Elinder, F.; Ceccatelli, S. Voltage-dependent anion channels (vdac) in the plasma membrane play a critical role in apoptosis in differentiated hippocampal neurons but not in neural stem cells. Cell Cycle 2008, 7, 3225–3234. [Google Scholar] [CrossRef]
- Marin, R.; Ramirez, C.M.; Gonzalez, M.; Gonzalez-Munoz, E.; Zorzano, A.; Camps, M.; Alonso, R.; Diaz, M. Voltage-dependent anion channel (vdac) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons. Mol. Membr. Biol. 2007, 24, 148–160. [Google Scholar] [CrossRef]
- Koma, H.; Yamamoto, Y.; Okamura, N.; Yagami, T. A plausible involvement of plasmalemmal voltage-dependent anion channel 1 in the neurotoxicity of 15-deoxy-delta(12,14)-prostaglandin j2. Brain Behav. 2020, 10, e01866. [Google Scholar] [CrossRef]
- Liu, Z.; Bengtsson, S.; Krogh, M.; Marquez, M.; Nilsson, S.; James, P.; Aliaya, A.; Holmberg, A.R. Somatostatin effects on the proteome of the lncap cell-line. Int. J. Oncol. 2007, 30, 1173–1179. [Google Scholar] [CrossRef][Green Version]
- Thinnes, F.P. Neuroendocrine differentiation of lncap cells suggests: Vdac in the cell membrane is involved in the extrinsic apoptotic pathway. Mol. Genet. Metab. 2009, 97, 241–243. [Google Scholar] [CrossRef]
- Heumann, R.; Goemans, C.; Bartsch, D.; Lingenhohl, K.; Waldmeier, P.C.; Hengerer, B.; Allegrini, P.R.; Schellander, K.; Wagner, E.F.; Arendt, T.; et al. Transgenic activation of ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. J. Cell Biol. 2000, 151, 1537–1548. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Serchov, T.; Mann, S.A.; Dietzel, I.D.; Heumann, R. Enhancement of dopaminergic properties and protection mediated by neuronal activation of ras in mouse ventral mesencephalic neurones. Eur. J. Neurosci. 2007, 25, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Felderhoff-Mueser, U.; Bittigau, P.; Sifringer, M.; Jarosz, B.; Korobowicz, E.; Mahler, L.; Piening, T.; Moysich, A.; Grune, T.; Thor, F.; et al. Oxygen causes cell death in the developing brain. Neurobiol. Dis. 2004, 17, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Serdar, M.; Herz, J.; Kempe, K.; Winterhager, E.; Jastrow, H.; Heumann, R.; Felderhoff-Muser, U.; Bendix, I. Protection of oligodendrocytes through neuronal overexpression of the small gtpase ras in hyperoxia-induced neonatal brain injury. Front. Neurol. 2018, 9, 175. [Google Scholar] [CrossRef]
- Hansen, H.H.; Briem, T.; Dzietko, M.; Sifringer, M.; Voss, A.; Rzeski, W.; Zdzisinska, B.; Thor, F.; Heumann, R.; Stepulak, A.; et al. Mechanisms leading to disseminated apoptosis following nmda receptor blockade in the developing rat brain. Neurobiol. Dis. 2004, 16, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Makwana, M.; Serchov, T.; Hristova, M.; Bohatschek, M.; Gschwendtner, A.; Kalla, R.; Liu, Z.Q.; Heumann, R.; Raivich, G. Regulation and function of neuronal gtp-ras in facial motor nerve regeneration. J. Neurochem. 2009, 108, 1453–1463. [Google Scholar] [CrossRef]
- Neumann, S.; Kuteykin-Teplyakov, K.; Heumann, R. Neuronal protection by ha-ras-gtpase signaling through selective downregulation of plasmalemmal voltage-dependent anion channel-1. Int. J. Mol. Sci. 2024, 25, 3030. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Hauptmann, S.; Scherping, I.; Drose, S.; Brandt, U.; Schulz, K.L.; Jendrach, M.; Leuner, K.; Eckert, A.; Muller, W.E. Mitochondrial dysfunction: An early event in alzheimer pathology accumulates with age in ad transgenic mice. Neurobiol. Aging 2009, 30, 1574–1586. [Google Scholar] [CrossRef]
- Leuner, K.; Hauptmann, S.; Abdel-Kader, R.; Scherping, I.; Keil, U.; Strosznajder, J.B.; Eckert, A.; Muller, W.E. Mitochondrial dysfunction: The first domino in brain aging and alzheimer’s disease? Antioxid. Redox. Signal. 2007, 9, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Park, B.S.; Jung, Y.; Reddy, P.H. Differential expression of oxidative phosphorylation genes in patients with alzheimer’s disease: Implications for early mitochondrial dysfunction and oxidative damage. Neuromol. Med. 2004, 5, 147–162. [Google Scholar] [CrossRef]
- Reddy, P.H.; Tripathi, R.; Troung, Q.; Tirumala, K.; Reddy, T.P.; Anekonda, V.; Shirendeb, U.P.; Calkins, M.J.; Reddy, A.P.; Mao, P.; et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim. Biophys. Acta 2012, 1822, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Shi, Q. A mitocentric view of alzheimer’s disease suggests multi-faceted treatments. J. Alzheimer’s Dis. 2010, 20 (Suppl. S2), S591–S607. [Google Scholar] [CrossRef]
- Rao, V.K.; Carlson, E.A.; Yan, S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim. Biophys. Acta 2014, 1842, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 2010, 1802, 2–10. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Kazmierczak, A.; Strosznajder, J.B.; Gotz, J.; Eckert, A. Insights into mitochondrial dysfunction: Aging, amyloid-beta, and tau-a deleterious trio. Antioxid. Redox Signal. 2012, 16, 1456–1466. [Google Scholar] [CrossRef]
- De Pinto, V.; al Jamal, J.A.; Benz, R.; Genchi, G.; Palmieri, F. Characterization of sh groups in porin of bovine heart mitochondria. Porin cysteines are localized in the channel walls. Eur. J. Biochem. 1991, 202, 903–911. [Google Scholar] [CrossRef]
- De Pinto, V.; Reina, S.; Gupta, A.; Messina, A.; Mahalakshmi, R. Role of cysteines in mammalian vdac isoforms’ function. Biochim. Biophys. Acta 2016, 1857, 1219–1227. [Google Scholar] [CrossRef]
- Lemeshko, V.V. Vdac as a voltage-dependent mitochondrial gatekeeper under physiological conditions. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184175. [Google Scholar] [CrossRef]
- Manczak, M.; Reddy, P.H. Abnormal interaction of vdac1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in alzheimer’s disease. Hum. Mol. Genet. 2012, 21, 5131–5146. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Tejedor, M.; Vilarino, M.; Cabodevilla, F.; Del Rio, J.; Frechilla, D.; Perez-Mediavilla, A. Enhanced expression of the voltage-dependent anion channel 1 (vdac1) in alzheimer’s disease transgenic mice: An insight into the pathogenic effects of amyloid-beta. J. Alzheimer’s Dis. 2011, 23, 195–206. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Gonzalez, M.; Diaz, M.; Alonso, R.; Ferrer, I.; Santpere, G.; Puig, B.; Meyer, G.; Marin, R. Vdac and eralpha interaction in caveolae from human cortex is altered in alzheimer’s disease. Mol. Cell. Neurosci. 2009, 42, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Colurso, G.J.; Nilson, J.E.; Vervoort, L.G. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with alzheimer’s disease. Life Sci. 2003, 73, 1795–1803. [Google Scholar] [CrossRef]
- Silva, D.F.; Selfridge, J.E.; Lu, J.; Cardoso, S.M.; Swerdlow, R.H. Mitochondrial abnormalities in alzheimer’s disease: Possible targets for therapeutic intervention. Adv. Pharmacol. 2012, 64, 83–126. [Google Scholar] [CrossRef] [PubMed]
- Geula, S.; Ben-Hail, D.; Shoshan-Barmatz, V. Structure-based analysis of vdac1: N-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem. J. 2012, 444, 475–485. [Google Scholar] [CrossRef]
- Munter, L.M.; Voigt, P.; Harmeier, A.; Kaden, D.; Gottschalk, K.E.; Weise, C.; Pipkorn, R.; Schaefer, M.; Langosch, D.; Multhaup, G. Gxxxg motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of abeta42. EMBO J. 2007, 26, 1702–1712. [Google Scholar] [CrossRef]
- Thinnes, F.P. Apoptogenic interactions of plasmalemmal type-1 vdac and abeta peptides via gxxxg motifs induce alzheimer’s disease—A basic model of apoptosis? Wien. Med. Wochenschr. 2011, 161, 274–276. [Google Scholar] [CrossRef]
- Thinnes, F.P. New findings concerning vertebrate porin ii—On the relevance of glycine motifs of type-1 vdac. Mol. Genet. Metab. 2013, 108, 212–224. [Google Scholar] [CrossRef]
- Jaye, S.; Sandau, U.S.; Saugstad, J.A. Clathrin mediated endocytosis in alzheimer’s disease: Cell type specific involvement in amyloid beta pathology. Front. Aging Neurosci. 2024, 16, 1378576. [Google Scholar] [CrossRef]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Sheiko, T.; Craigen, W.J.; Reddy, P.H. Reduced vdac1 protects against alzheimer’s disease, mitochondria, and synaptic deficiencies. J. Alzheimer’s Dis. 2013, 37, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shteinfer-Kuzmine, A.; Kamenetsky, N.; Pittala, S.; Paul, A.; Nahon Crystal, E.; Ouro, A.; Chalifa-Caspi, V.; Pandey, S.K.; Monsonego, A.; et al. Targeting the overexpressed mitochondrial protein vdac1 in a mouse model of alzheimer’s disease protects against mitochondrial dysfunction and mitigates brain pathology. Transl. Neurodegener. 2022, 11, 58. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Ilzorkina, A.I.; Matveeva, L.A.; Chulkov, A.V.; Semenova, A.A.; Dubinin, M.V.; Belosludtseva, N.V. Effect of vbit-4 on the functional activity of isolated mitochondria and cell viability. Biochim. Biophys. Acta Biomembr. 2024, 1866, 184329. [Google Scholar] [CrossRef]
- Iwamoto, N.; Kobayashi, K.; Kosaka, K. The formation of prostaglandins in the postmortem cerebral cortex of alzheimer-type dementia patients. J. Neurol. 1989, 236, 80–84. [Google Scholar] [CrossRef]
- Marcone, S.; Fitzgerald, D.J. Proteomic identification of the candidate target proteins of 15-deoxy-delta12,14-prostaglandin j2. Proteomics 2013, 13, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Rockenfeller, P. Phospholipid scramblase activity of vdac dimers: New implications for cell death, autophagy and ageing. Biomolecules 2024, 14, 1218. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3 (gsk3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Hoek, J.B.; Shulga, N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase ii to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005, 65, 10545–10554. [Google Scholar] [CrossRef]
- Liu, M.; Sui, D.; Dexheimer, T.; Hovde, S.; Deng, X.; Wang, K.W.; Lin, H.L.; Chien, H.T.; Kweon, H.K.; Kuo, N.S.; et al. Hyperphosphorylation renders tau prone to aggregate and to cause cell death. Mol. Neurobiol. 2020, 57, 4704–4719. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in alzheimer’s disease. Brain Res. 2011, 1415, 136–148. [Google Scholar] [CrossRef]
- Khan, A.; Kuriachan, G.; Mahalakshmi, R. Cellular interactome of mitochondrial voltage-dependent anion channels: Oligomerization and channel (mis)regulation. ACS Chem. Neurosci. 2021, 12, 3497–3515. [Google Scholar] [CrossRef]
- Ferrer, I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in alzheimer’s disease. J. Bioenerg. Biomembr. 2009, 41, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ebneth, A.; Godemann, R.; Stamer, K.; Illenberger, S.; Trinczek, B.; Mandelkow, E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for alzheimer’s disease. J. Cell Biol. 1998, 143, 777–794. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kda) (tspo) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Gatliff, J.; East, D.; Crosby, J.; Abeti, R.; Harvey, R.; Craigen, W.; Parker, P.; Campanella, M. Tspo interacts with vdac1 and triggers a ros-mediated inhibition of mitochondrial quality control. Autophagy 2014, 10, 2279–2296. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Dennett, O.; Lau, L.C.; Chatelet, D.S.; Bottlaender, M.; Nicoll, J.A.R.; Boche, D. The mitochondrial protein tspo in alzheimer’s disease: Relation to the severity of ad pathology and the neuroinflammatory environment. J. Neuroinflamm. 2023, 20, 186. [Google Scholar] [CrossRef]
- Maeda, J.; Zhang, M.R.; Okauchi, T.; Ji, B.; Ono, M.; Hattori, S.; Kumata, K.; Iwata, N.; Saido, T.C.; Trojanowski, J.Q.; et al. In vivo positron emission tomographic imaging of glial responses to amyloid-beta and tau pathologies in mouse models of alzheimer’s disease and related disorders. J. Neurosci. 2011, 31, 4720–4730. [Google Scholar] [CrossRef]
- James, M.L.; Belichenko, N.P.; Nguyen, T.V.; Andrews, L.E.; Ding, Z.; Liu, H.; Bodapati, D.; Arksey, N.; Shen, B.; Cheng, Z.; et al. Pet imaging of translocator protein (18 kda) in a mouse model of alzheimer’s disease using n-(2,5-dimethoxybenzyl)-2-18f-fluoro-n-(2-phenoxyphenyl)acetamide. J. Nucl. Med. 2015, 56, 311–316. [Google Scholar] [CrossRef]
- Ji, B.; Maeda, J.; Sawada, M.; Ono, M.; Okauchi, T.; Inaji, M.; Zhang, M.R.; Suzuki, K.; Ando, K.; Staufenbiel, M.; et al. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of alzheimer’s and other cns pathologies. J. Neurosci. 2008, 28, 12255–12267. [Google Scholar] [CrossRef]
- Mirzaei, N.; Tang, S.P.; Ashworth, S.; Coello, C.; Plisson, C.; Passchier, J.; Selvaraj, V.; Tyacke, R.J.; Nutt, D.J.; Sastre, M. In vivo imaging of microglial activation by positron emission tomography with [(11)c]pbr28 in the 5xfad model of alzheimer’s disease. Glia 2016, 64, 993–1006. [Google Scholar] [CrossRef]
- Serriere, S.; Tauber, C.; Vercouillie, J.; Mothes, C.; Pruckner, C.; Guilloteau, D.; Kassiou, M.; Domene, A.; Garreau, L.; Page, G.; et al. Amyloid load and translocator protein 18 kda in appsweps1-de9 mice: A longitudinal study. Neurobiol. Aging 2015, 36, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Tournier, B.B.; Tsartsalis, S.; Rigaud, D.; Fossey, C.; Cailly, T.; Fabis, F.; Pham, T.; Gregoire, M.C.; Kovari, E.; Moulin-Sallanon, M.; et al. Tspo and amyloid deposits in sub-regions of the hippocampus in the 3xtgad mouse model of alzheimer’s disease. Neurobiol. Dis. 2019, 121, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of mirna regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.L.; Mao, H.; Silver, D.L. Rna on the brain: Emerging layers of post-transcriptional regulation in cerebral cortex development. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e290. [Google Scholar] [CrossRef]
- Wang, F.; Qiang, Y.; Zhu, L.; Jiang, Y.; Wang, Y.; Shao, X.; Yin, L.; Chen, J.; Chen, Z. Microrna-7 downregulates the oncogene vdac1 to influence hepatocellular carcinoma proliferation and metastasis. Tumour Biol. 2016, 37, 10235–10246. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Choi, D.C.; Kabaria, S.; Tran, A.; Junn, E. Microrna-7 regulates the function of mitochondrial permeability transition pore by targeting vdac1 expression. J. Biol. Chem. 2016, 291, 6483–6493. [Google Scholar] [CrossRef]
- Fatima, M.; Prajapati, B.; Saleem, K.; Kumari, R.; Mohindar Singh Singal, C.; Seth, P. Novel insights into role of mir-320a-vdac1 axis in astrocyte-mediated neuronal damage in neuroaids. Glia 2017, 65, 250–263. [Google Scholar] [CrossRef]
- Bargaje, R.; Gupta, S.; Sarkeshik, A.; Park, R.; Xu, T.; Sarkar, M.; Halimani, M.; Roy, S.S.; Yates, J.; Pillai, B. Identification of novel targets for mir-29a using mirna proteomics. PLoS ONE 2012, 7, e43243. [Google Scholar] [CrossRef][Green Version]
- Roshan, R.; Shridhar, S.; Sarangdhar, M.A.; Banik, A.; Chawla, M.; Garg, M.; Singh, V.P.; Pillai, B. Brain-specific knockdown of mir-29 results in neuronal cell death and ataxia in mice. RNA 2014, 20, 1287–1297. [Google Scholar] [CrossRef]
- Hebert, S.S.; Horre, K.; Nicolai, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microrna cluster mir-29a/b-1 in sporadic alzheimer’s disease correlates with increased bace1/beta-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Stary, C.M.; Sun, X.; Ouyang, Y.; Li, L.; Giffard, R.G. Mir-29a differentially regulates cell survival in astrocytes from cornu ammonis 1 and dentate gyrus by targeting vdac1. Mitochondrion 2016, 30, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Yaffe, K.; Cauley, J.A.; Rolka, D.B.; Blackwell, T.L.; Narayan, K.M.; Cummings, S.R. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of osteoporotic fractures research group. Arch. Intern. Med. 2000, 160, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.N.N.; Lima-Filho, R.A.S.; De Felice, F.G. Connecting alzheimer’s disease to diabetes: Underlying mechanisms and potential therapeutic targets. Neuropharmacology 2018, 136, 160–171. [Google Scholar] [CrossRef]
- Watson, G.S.; Craft, S. The role of insulin resistance in the pathogenesis of alzheimer’s disease: Implications for treatment. CNS Drugs 2003, 17, 27–45. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimer’s Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Folstein, M.F. Insulin, insulin-degrading enzyme and amyloid-beta peptide in alzheimer’s disease: Review and hypothesis. Neurobiol. Aging 2006, 27, 190–198. [Google Scholar] [CrossRef]
- Kickstein, E.; Krauss, S.; Thornhill, P.; Rutschow, D.; Zeller, R.; Sharkey, J.; Williamson, R.; Fuchs, M.; Kohler, A.; Glossmann, H.; et al. Biguanide metformin acts on tau phosphorylation via mtor/protein phosphatase 2a (pp2a) signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21830–21835. [Google Scholar] [CrossRef]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by alzheimer’s disease- associated abeta oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in alzheimer’s disease patients is associated with igf-1 resistance, irs-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef]
- Kciuk, M.; Kruczkowska, W.; Galeziewska, J.; Wanke, K.; Kaluzinska-Kolat, Z.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s disease as type 3 diabetes: Understanding the link and implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Gupta, S. Molecular and biochemical trajectories from diabetes to alzheimer’s disease: A critical appraisal. World J. Diabetes 2015, 6, 1223–1242. [Google Scholar] [CrossRef]
- Baglietto-Vargas, D.; Shi, J.; Yaeger, D.M.; Ager, R.; LaFerla, F.M. Diabetes and alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016, 64, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N. Bidirectional interactions between diabetes and alzheimer’s disease. Neurochem. Int. 2017, 108, 296–302. [Google Scholar] [CrossRef]
- Perez-Gracia, E.; Torrejon-Escribano, B.; Ferrer, I. Dystrophic neurites of senile plaques in alzheimer’s disease are deficient in cytochrome c oxidase. Acta Neuropathol. 2008, 116, 261–268. [Google Scholar] [CrossRef]
- Ahmed, M.; Muhammed, S.J.; Kessler, B.; Salehi, A. Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic beta-cells exposed to high glucose. Islets 2010, 2, 283–292. [Google Scholar] [CrossRef]
- Sasaki, K.; Donthamsetty, R.; Heldak, M.; Cho, Y.E.; Scott, B.T.; Makino, A. Vdac: Old protein with new roles in diabetes. Am. J. Physiol. Cell Physiol. 2012, 303, C1055–C1060. [Google Scholar] [CrossRef]
- Picone, P.; Vilasi, S.; Librizzi, F.; Contardi, M.; Nuzzo, D.; Caruana, L.; Baldassano, S.; Amato, A.; Mule, F.; San Biagio, P.L.; et al. Biological and biophysics aspects of metformin-induced effects: Cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging 2016, 8, 1718–1734. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.; Ferreira, J.J.; Rocha, J.F.; Rascol, O.; Poewe, W.; Gama, H.; Soares-da-Silva, P. Safety profile of opicapone in the management of parkinson’s disease. J. Park. Dis. 2019, 9, 733–740. [Google Scholar] [CrossRef]
- Stefani, A.; Hogl, B. Sleep in parkinson’s disease. Neuropsychopharmacology 2020, 45, 121–128. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. Mds research criteria for prodromal parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. Ii. Patterns of loss of dopamine-containing neurons in parkinson’s disease. Brain 1999, 122 Pt 8, 1437–1448. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Cortico-basal ganglia-cortical circuitry in parkinson’s disease reconsidered. Exp. Neurol. 2008, 212, 226–229. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139 (Suppl. S1), 325–337. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Moratalla, R.; Herrero, M.T.; Chakrabarty, K.; Drucker-Colin, R.; Garcia-Montes, J.R.; Simola, N.; Morelli, M. Dyskinesia in parkinson’s disease: Mechanisms and current non-pharmacological interventions. J. Neurochem. 2014, 130, 472–489. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.R.; Arduino, D.M.; Silva, D.F.; Oliveira, C.R.; Cardoso, S.M. Mitochondrial dysfunction: The road to alpha-synuclein oligomerization in pd. Park. Dis. 2011, 2011, 693761. [Google Scholar] [CrossRef]
- Bartels, T.; Ahlstrom, L.S.; Leftin, A.; Kamp, F.; Haass, C.; Brown, M.F.; Beyer, K. The n-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys. J. 2010, 99, 2116–2124. [Google Scholar] [CrossRef]
- Waxman, E.A.; Mazzulli, J.R.; Giasson, B.I. Characterization of hydrophobic residue requirements for alpha-synuclein fibrillization. Biochemistry 2009, 48, 9427–9436. [Google Scholar] [CrossRef]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic lewy body disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. Alpha-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Bockmann, A.; Meier, B.H.; et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef]
- Angot, E.; Steiner, J.A.; Hansen, C.; Li, J.Y.; Brundin, P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010, 9, 1128–1138. [Google Scholar] [CrossRef]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Oikawa, T.; Arai, T.; Akiyama, H.; Mann, D.M.; Hasegawa, M. Prion-like spreading of pathological alpha-synuclein in brain. Brain 2013, 136, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in parkinson’s disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef]
- Danzer, K.M.; Haasen, D.; Karow, A.R.; Moussaud, S.; Habeck, M.; Giese, A.; Kretzschmar, H.; Hengerer, B.; Kostka, M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007, 27, 9220–9232. [Google Scholar] [CrossRef]
- Luth, E.S.; Stavrovskaya, I.G.; Bartels, T.; Kristal, B.S.; Selkoe, D.J. Soluble, prefibrillar alpha-synuclein oligomers promote complex i-dependent, ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 2014, 289, 21490–21507. [Google Scholar] [CrossRef] [PubMed]
- Rosencrans, W.M.; Khuntia, H.; Ghahari Larimi, M.; Mahalakshmi, R.; Yu, T.Y.; Bezrukov, S.M.; Rostovtseva, T.K. Conformational plasticity of mitochondrial vdac2 controls the kinetics of its interaction with cytosolic proteins. Sci. Adv. 2025, 11, eadv4410. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Protchenko, O.; Hoogerheide, D.P.; Yap, T.L.; Philpott, C.C.; Lee, J.C.; Bezrukov, S.M. Alpha-synuclein shows high affinity interaction with voltage-dependent anion channel, suggesting mechanisms of mitochondrial regulation and toxicity in parkinson disease. J. Biol. Chem. 2015, 290, 18467–18477. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial import and accumulation of alpha-synuclein impair complex i in human dopaminergic neuronal cultures and parkinson disease brain. J. Biol. Chem. 2008, 283, 9089–9100. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, C.; Yin, J.; Li, X.; Cheng, F.; Li, Y.; Yang, H.; Ueda, K.; Chan, P.; Yu, S. Alpha-synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex i activity. Neurosci. Lett. 2009, 454, 187–192. [Google Scholar] [CrossRef]
- Ellis, C.E.; Murphy, E.J.; Mitchell, D.C.; Golovko, M.Y.; Scaglia, F.; Barcelo-Coblijn, G.C.; Nussbaum, R.L. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol. Cell. Biol. 2005, 25, 10190–10201. [Google Scholar] [CrossRef]
- Elkon, H.; Don, J.; Melamed, E.; Ziv, I.; Shirvan, A.; Offen, D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome c oxidase. J. Mol. Neurosci. 2002, 18, 229–238. [Google Scholar] [CrossRef]
- Nakamura, K. Alpha-synuclein and mitochondria: Partners in crime? Neurotherapeutics 2013, 10, 391–399. [Google Scholar] [CrossRef]
- Hoogerheide, D.P.; Rostovtseva, T.K.; Bezrukov, S.M. Exploring lipid-dependent conformations of membrane-bound alpha-synuclein with the vdac nanopore. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183643. [Google Scholar] [CrossRef]
- Rosencrans, W.M.; Aguilella, V.M.; Rostovtseva, T.K.; Bezrukov, S.M. Alpha-synuclein emerges as a potent regulator of vdac-facilitated calcium transport. Cell Calcium 2021, 95, 102355. [Google Scholar] [CrossRef] [PubMed]
- Queralt-Martin, M.; Bergdoll, L.; Teijido, O.; Munshi, N.; Jacobs, D.; Kuszak, A.J.; Protchenko, O.; Reina, S.; Magri, A.; De Pinto, V.; et al. A lower affinity to cytosolic proteins reveals vdac3 isoform-specific role in mitochondrial biology. J. Gen. Physiol. 2020, 152, e201912501. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, C.; Cai, Q.; Lu, Q.; Duan, C.; Zhu, Y.; Yang, H. Voltage-dependent anion channel involved in the alpha-synuclein-induced dopaminergic neuron toxicity in rats. Acta Biochim. Biophys. Sin. 2013, 45, 170–178. [Google Scholar] [CrossRef]
- Martin, L.J.; Semenkow, S.; Hanaford, A.; Wong, M. Mitochondrial permeability transition pore regulates parkinson’s disease development in mutant alpha-synuclein transgenic mice. Neurobiol. Aging 2014, 35, 1132–1152. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Goldman, J.G.; Kelly, L.; He, Y.; Waliczek, T.; Kordower, J.H. Abnormal alpha-synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental parkinson’s disease. Neurobiol. Dis. 2014, 69, 1–14. [Google Scholar] [CrossRef]
- Alberio, T.; Mammucari, C.; D’Agostino, G.; Rizzuto, R.; Fasano, M. Altered dopamine homeostasis differentially affects mitochondrial voltage-dependent anion channels turnover. Biochim. Biophys. Acta 2014, 1842, 1816–1822. [Google Scholar] [CrossRef]
- Premkumar, A.; Simantov, R. Mitochondrial voltage-dependent anion channel is involved in dopamine-induced apoptosis. J. Neurochem. 2002, 82, 345–352. [Google Scholar] [CrossRef]
- Xiong, Y.; Ding, H.; Xu, M.; Gao, J. Protective effects of asiatic acid on rotenone- or h2o2-induced injury in sh-sy5y cells. Neurochem. Res. 2009, 34, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Burte, F.; De Girolamo, L.A.; Hargreaves, A.J.; Billett, E.E. Alterations in the mitochondrial proteome of neuroblastoma cells in response to complex 1 inhibition. J. Proteome Res. 2011, 10, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Somanath, S.D.; Ramdas, P.; Haleagrahara, N.; Radhakrishnan, A.K. 6-hydroxydopamine induces neurodegeneration in terminally differentiated sh-sy5y neuroblastoma cells via enrichment of the nucleosomal degradation pathway: A global proteomics approach. J. Mol. Neurosci. 2022, 72, 1026–1046. [Google Scholar] [CrossRef] [PubMed]
- Araujo de Lima, L.; Oliveira Cunha, P.L.; Felicio Calou, I.B.; Tavares Neves, K.R.; Facundo, H.T.; Socorro de Barros Viana, G. Effects of vitamin d (vd3) supplementation on the brain mitochondrial function of male rats, in the 6-ohda-induced model of parkinson’s disease. Neurochem. Int. 2022, 154, 105280. [Google Scholar] [CrossRef]
- Junn, E.; Lee, K.W.; Jeong, B.S.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of alpha-synuclein expression and toxicity by microrna-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef]
- Leggio, L.; Vivarelli, S.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Marchetti, B.; Iraci, N. Micrornas in parkinson’s disease: From pathogenesis to novel diagnostic and therapeutic approaches. Int. J. Mol. Sci. 2017, 18, 2698. [Google Scholar] [CrossRef]
- McMillan, K.J.; Murray, T.K.; Bengoa-Vergniory, N.; Cordero-Llana, O.; Cooper, J.; Buckley, A.; Wade-Martins, R.; Uney, J.B.; O’Neill, M.J.; Wong, L.F.; et al. Loss of microrna-7 regulation leads to alpha-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol. Ther. 2017, 25, 2404–2414. [Google Scholar] [CrossRef]
- Rovini, A.; Gurnev, P.A.; Beilina, A.; Queralt-Martin, M.; Rosencrans, W.; Cookson, M.R.; Bezrukov, S.M.; Rostovtseva, T.K. Molecular mechanism of olesoxime-mediated neuroprotection through targeting alpha-synuclein interaction with mitochondrial vdac. Cell. Mol. Life Sci. 2020, 77, 3611–3626. [Google Scholar] [CrossRef]
- Bordet, T.; Buisson, B.; Michaud, M.; Drouot, C.; Galea, P.; Delaage, P.; Akentieva, N.P.; Evers, A.S.; Covey, D.F.; Ostuni, M.A.; et al. Identification and characterization of cholest-4-en-3-one, oxime (tro19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef]
- Rajendran, M.; Queralt-Martin, M.; Gurnev, P.A.; Rosencrans, W.M.; Rovini, A.; Jacobs, D.; Abrantes, K.; Hoogerheide, D.P.; Bezrukov, S.M.; Rostovtseva, T.K. Restricting alpha-synuclein transport into mitochondria by inhibition of alpha-synuclein-vdac complexation as a potential therapeutic target for parkinson’s disease treatment. Cell. Mol. Life Sci. 2022, 79, 368. [Google Scholar] [CrossRef]
- Feng, S.; Gui, J.; Qin, B.; Ye, J.; Zhao, Q.; Guo, A.; Sang, M.; Sun, X. Resveratrol inhibits vdac1-mediated mitochondrial dysfunction to mitigate pathological progression in parkinson’s disease model. Mol. Neurobiol. 2024, 62, 6636–6654. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, C.; Crosio, C.; Vitale, C.; Sanna, G.; Carri, M.T.; Barone, P. Apoptotic mechanisms in mutant lrrk2-mediated cell death. Hum. Mol. Genet. 2007, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, M.; Niu, J.; Yue, Z.; Xu, Z. Expression of leucine-rich repeat kinase 2 (lrrk2) inhibits the processing of umtck to induce cell death in a cell culture model system. Biosci. Rep. 2011, 31, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Shimura, H.; Hattori, N.; Kubo, S.; Mizuno, Y.; Asakawa, S.; Minoshima, S.; Shimizu, N.; Iwai, K.; Chiba, T.; Tanaka, K.; et al. Familial parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000, 25, 302–305. [Google Scholar] [CrossRef]
- Rogaeva, E.; Johnson, J.; Lang, A.E.; Gulick, C.; Gwinn-Hardy, K.; Kawarai, T.; Sato, C.; Morgan, A.; Werner, J.; Nussbaum, R.; et al. Analysis of the pink1 gene in a large cohort of cases with parkinson disease. Arch. Neurol. 2004, 61, 1898–1904. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary early-onset parkinson’s disease caused by mutations in pink1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of pink1, parkin, and mitochondrial fidelity in parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Sun, K.; Jing, X.; Guo, J.; Yao, X.; Guo, F. Mitophagy in degenerative joint diseases. Autophagy 2021, 17, 2082–2092. [Google Scholar] [CrossRef]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. Pink1/parkin-mediated mitophagy is dependent on vdac1 and p62/sqstm1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Callegari, S.; Kirk, N.S.; Gan, Z.Y.; Dite, T.; Cobbold, S.A.; Leis, A.; Dagley, L.F.; Glukhova, A.; Komander, D. Structure of human pink1 at a mitochondrial tom-vdac array. Science 2025, 388, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.J.; Lee, D.; Yoo, H.; Jun, K.; Shin, H.; Chung, J. Decision between mitophagy and apoptosis by parkin via vdac1 ubiquitination. Proc. Natl. Acad. Sci. USA 2020, 117, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Fan, C.; Gu, L.; Gao, H.; Liu, Q.; Zhang, T.; Qi, Z.; Zhao, C.; Zhao, H.; Cai, Q.; et al. Silencing of pink1 induces mitophagy via mitochondrial permeability transition in dopaminergic mn9d cells. Brain Res. 2011, 1394, 1–13. [Google Scholar] [CrossRef]
- Yan, J.; Sun, W.; Shen, M.; Zhang, Y.; Jiang, M.; Liu, A.; Ma, H.; Lai, X.; Wu, J. Idebenone improves motor dysfunction, learning and memory by regulating mitophagy in mptp-treated mice. Cell Death Discov. 2022, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; Jalali Sefid Dashti, Z.; Christoffels, A.; Loos, B.; Bardien, S. Evidence for a common biological pathway linking three parkinson’s disease-causing genes: Parkin, pink1 and dj-1. Eur. J. Neurosci. 2015, 41, 1113–1125. [Google Scholar] [CrossRef]
- Ottolini, D.; Cali, T.; Negro, A.; Brini, M. The parkinson disease-related protein dj-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum. Mol. Genet. 2013, 22, 2152–2168. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Fujioka, H.; Liu, J.; Chen, S.; Zhu, X. Dj-1 regulates the integrity and function of er-mitochondria association through interaction with ip3r3-grp75-vdac1. Proc. Natl. Acad. Sci. USA 2019, 116, 25322–25328. [Google Scholar] [CrossRef]
- Basso, V.; Marchesan, E.; Ziviani, E. A trio has turned into a quartet: Dj-1 interacts with the ip3r-grp75-vdac complex to control er-mitochondria interaction. Cell Calcium 2020, 87, 102186. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Rothstein, J.D. From charcot to lou gehrig: Deciphering selective motor neuron death in als. Nat. Rev. Neurosci. 2001, 2, 806–819. [Google Scholar] [CrossRef]
- Mulder, D.W.; Kurland, L.T.; Offord, K.P.; Beard, C.M. Familial adult motor neuron disease: Amyotrophic lateral sclerosis. Neurology 1986, 36, 511–517. [Google Scholar] [CrossRef]
- Gibbons, C.; Pagnini, F.; Friede, T.; Young, C.A. Treatment of fatigue in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2018, 1, CD011005. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, L.; Deng, M. Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front. Neurosci. 2023, 17, 1170996. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and cellular mechanisms affected in als. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in cu/zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, N.; Tranmer, G.K. Hydrogen peroxide and amyotrophic lateral sclerosis: From biochemistry to pathophysiology. Antioxidants 2021, 11, 52. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Kahn, J.; Leyton-Jaimes, M.F.; Rosenblatt, J.; Israelson, A. Misfolded sod1 accumulation and mitochondrial association contribute to the selective vulnerability of motor neurons in familial als: Correlation to human disease. ACS Chem. Neurosci. 2017, 8, 2225–2234. [Google Scholar] [CrossRef]
- Israelson, A.; Arbel, N.; Da Cruz, S.; Ilieva, H.; Yamanaka, K.; Shoshan-Barmatz, V.; Cleveland, D.W. Misfolded mutant sod1 directly inhibits vdac1 conductance in a mouse model of inherited als. Neuron 2010, 67, 575–587. [Google Scholar] [CrossRef]
- Li, Q.; Vande Velde, C.; Israelson, A.; Xie, J.; Bailey, A.O.; Dong, M.Q.; Chun, S.J.; Roy, T.; Winer, L.; Yates, J.R.; et al. Als-linked mutant superoxide dismutase 1 (sod1) alters mitochondrial protein composition and decreases protein import. Proc. Natl. Acad. Sci. USA 2010, 107, 21146–21151. [Google Scholar] [CrossRef]
- Shteinfer-Kuzmine, A.; Argueti-Ostrovsky, S.; Leyton-Jaimes, M.F.; Anand, U.; Abu-Hamad, S.; Zalk, R.; Shoshan-Barmatz, V.; Israelson, A. Targeting the mitochondrial protein vdac1 as a potential therapeutic strategy in als. Int. J. Mol. Sci. 2022, 23, 9946. [Google Scholar] [CrossRef]
- Tan, W.; Naniche, N.; Bogush, A.; Pedrini, S.; Trotti, D.; Pasinelli, P. Small peptides against the mutant sod1/bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant sod1-mediated als. J. Neurosci. 2013, 33, 11588–11598. [Google Scholar] [CrossRef]
- Pedrini, S.; Sau, D.; Guareschi, S.; Bogush, M.; Brown, R.H., Jr.; Naniche, N.; Kia, A.; Trotti, D.; Pasinelli, P. Als-linked mutant sod1 damages mitochondria by promoting conformational changes in bcl-2. Hum. Mol. Genet. 2010, 19, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Shteinfer-Kuzmine, A.; Argueti, S.; Gupta, R.; Shvil, N.; Abu-Hamad, S.; Gropper, Y.; Hoeber, J.; Magri, A.; Messina, A.; Kozlova, E.N.; et al. A vdac1-derived n-terminal peptide inhibits mutant sod1-vdac1 interactions and toxicity in the sod1 model of als. Front. Cell. Neurosci. 2019, 13, 346. [Google Scholar] [CrossRef]
- Magri, A.; Belfiore, R.; Reina, S.; Tomasello, M.F.; Di Rosa, M.C.; Guarino, F.; Leggio, L.; De Pinto, V.; Messina, A. Hexokinase i n-terminal based peptide prevents the vdac1-sod1 g93a interaction and re-establishes als cell viability. Sci. Rep. 2016, 6, 34802. [Google Scholar] [CrossRef]
- Magri, A.; Risiglione, P.; Caccamo, A.; Formicola, B.; Tomasello, M.F.; Arrigoni, C.; Zimbone, S.; Guarino, F.; Re, F.; Messina, A. Small hexokinase 1 peptide against toxic sod1 g93a mitochondrial accumulation in als rescues the atp-related respiration. Biomedicines 2021, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Fukada, K.; Zhang, F.; Vien, A.; Cashman, N.R.; Zhu, H. Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis. Mol. Cell. Proteom. 2004, 3, 1211–1223. [Google Scholar] [CrossRef]
- Pittala, M.G.G.; Reina, S.; Cubisino, S.A.M.; Cucina, A.; Formicola, B.; Cunsolo, V.; Foti, S.; Saletti, R.; Messina, A. Post-translational modification analysis of vdac1 in als-sod1 model cells reveals specific asparagine and glutamine deamidation. Antioxidants 2020, 9, 1218. [Google Scholar] [CrossRef]
- Pittala, M.G.G.; Reina, S.; Nibali, S.C.; Cucina, A.; Cubisino, S.A.M.; Cunsolo, V.; Amodeo, G.F.; Foti, S.; De Pinto, V.; Saletti, R.; et al. Specific post-translational modifications of vdac3 in als-sod1 model cells identified by high-resolution mass spectrometry. Int. J. Mol. Sci. 2022, 23, 15853. [Google Scholar] [CrossRef]
- Sunyach, C.; Michaud, M.; Arnoux, T.; Bernard-Marissal, N.; Aebischer, J.; Latyszenok, V.; Gouarne, C.; Raoul, C.; Pruss, R.M.; Bordet, T.; et al. Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an als mouse model. Neuropharmacology 2012, 62, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, T.; Lacomblez, L.; Abitbol, J.L.; Ludolph, A.; Mora, J.S.; Robberecht, W.; Shaw, P.J.; Pruss, R.M.; Cuvier, V.; Meininger, V.; et al. A phase ii–iii trial of olesoxime in subjects with amyotrophic lateral sclerosis. Eur. J. Neurol. 2014, 21, 529–536. [Google Scholar] [CrossRef]
- Magri, A.; Lipari, C.L.R.; Caccamo, A.; Battiato, G.; Conti Nibali, S.; De Pinto, V.; Guarino, F.; Messina, A. Aav-mediated upregulation of vdac1 rescues the mitochondrial respiration and sirtuins expression in a sod1 mouse model of inherited als. Cell Death Discov. 2024, 10, 178. [Google Scholar] [CrossRef]
- Pappalardo, X.G.; Jansen, G.; Amaradio, M.; Costanza, J.; Umeton, R.; Guarino, F.; De Pinto, V.; Oliver, S.G.; Messina, A.; Nicosia, G. Inferring gene regulatory networks of als from blood transcriptome profiles. Heliyon 2024, 10, e40696. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. Tdp-43 triggers mitochondrial DNA release via mptp to activate cgas/sting in als. Cell 2020, 183, 636–649. [Google Scholar] [CrossRef]
- Davis, S.A.; Itaman, S.; Khalid-Janney, C.M.; Sherard, J.A.; Dowell, J.A.; Cairns, N.J.; Gitcho, M.A. Tdp-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics. Neurosci. Lett. 2018, 678, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, F.; Schmitz, A.; Maharjan, N.; Diab, R.; Odriozola, A.; Tripathi, P.; Yamoah, A.; Scheidegger, O.; Oestmann, A.; Dennys, C.N.; et al. Polyga targets the er stress-adaptive response by impairing grp75 function at the mam in c9orf72-als/ftd. Acta Neuropathol. 2022, 144, 939–966. [Google Scholar] [CrossRef]
- Illarioshkin, S.N.; Klyushnikov, S.A.; Vigont, V.A.; Seliverstov, Y.A.; Kaznacheyeva, E.V. Molecular pathogenesis in huntington’s disease. Biochemistry 2018, 83, 1030–1039. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Munoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential disease-modifying therapies for huntington’s disease: Lessons learned and future opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, D. Factors contributing to clinical picture and progression of huntington’s disease. Neural Regen. Res. 2018, 13, 1364–1365. [Google Scholar] [CrossRef]
- Jurcau, A. Molecular pathophysiological mechanisms in huntington’s disease. Biomedicines 2022, 10, 1432. [Google Scholar] [CrossRef]
- Clemens, L.E.; Weber, J.J.; Wlodkowski, T.T.; Yu-Taeger, L.; Michaud, M.; Calaminus, C.; Eckert, S.H.; Gaca, J.; Weiss, A.; Magg, J.C.; et al. Olesoxime suppresses calpain activation and mutant huntingtin fragmentation in the bachd rat. Brain 2015, 138, 3632–3653. [Google Scholar] [CrossRef]
- Lou, S.; Lepak, V.C.; Eberly, L.E.; Roth, B.; Cui, W.; Zhu, X.H.; Oz, G.; Dubinsky, J.M. Oxygen consumption deficit in huntington disease mouse brain under metabolic stress. Hum. Mol. Genet. 2016, 25, 2813–2826. [Google Scholar] [CrossRef]
- Napoli, E.; Wong, S.; Hung, C.; Ross-Inta, C.; Bomdica, P.; Giulivi, C. Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in huntington’s disease. Hum. Mol. Genet. 2013, 22, 989–1004. [Google Scholar] [CrossRef]
- Perluigi, M.; Poon, H.F.; Maragos, W.; Pierce, W.M.; Klein, J.B.; Calabrese, V.; Cini, C.; De Marco, C.; Butterfield, D.A. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: A model of huntington disease. Mol. Cell. Proteom. 2005, 4, 1849–1861. [Google Scholar] [CrossRef]
- Karachitos, A.; Grobys, D.; Kulczynska, K.; Sobusiak, A.; Kmita, H. The association of vdac with cell viability of pc12 model of huntington’s disease. Front. Oncol. 2016, 6, 238. [Google Scholar] [CrossRef]
- Beatriz, M.; Vilaca, R.; Anjo, S.I.; Manadas, B.; Januario, C.; Rego, A.C.; Lopes, C. Defective mitochondria-lysosomal axis enhances the release of extracellular vesicles containing mitochondrial DNA and proteins in huntington’s disease. J. Extracell. Biol. 2022, 1, e65. [Google Scholar] [CrossRef]
- Brondani, M.; Roginski, A.C.; Ribeiro, R.T.; de Medeiros, M.P.; Hoffmann, C.I.H.; Wajner, M.; Leipnitz, G.; Seminotti, B. Mitochondrial dysfunction, oxidative stress, er stress and mitochondria-er crosstalk alterations in a chemical rat model of huntington’s disease: Potential benefits of bezafibrate. Toxicol. Lett. 2023, 381, 48–59. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, M.A.; Sheta, E.; El-Abhar, H.S.; Abdallah, D.M.; El Kerdawy, A.M.; Eldehna, W.M.; Gowayed, M.A. Morin suppresses mtorc1/ire-1alpha/jnk and ip3r-vdac-1 pathways: Crucial mechanisms in apoptosis and mitophagy inhibition in experimental huntington’s disease, supported by in silico molecular docking simulations. Life Sci. 2024, 338, 122362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, B.; Zou, M.; Peng, B.; Rao, Y. Neuronal ceroid lipofuscinosis-concepts, classification, and avenues for therapy. CNS Neurosci. Ther. 2025, 31, e70261. [Google Scholar] [CrossRef] [PubMed]
- Kielar, C.; Wishart, T.M.; Palmer, A.; Dihanich, S.; Wong, A.M.; Macauley, S.L.; Chan, C.H.; Sands, M.S.; Pearce, D.A.; Cooper, J.D.; et al. Molecular correlates of axonal and synaptic pathology in mouse models of batten disease. Hum. Mol. Genet. 2009, 18, 4066–4080. [Google Scholar] [CrossRef]
- Homewood, J.; Bond, N.W. Thiamin deficiency and korsakoff’s syndrome: Failure to find memory impairments following nonalcoholic wernicke’s encephalopathy. Alcohol 1999, 19, 75–84. [Google Scholar] [CrossRef]
- Hazell, A.S.; Butterworth, R.F. Update of cell damage mechanisms in thiamine deficiency: Focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol. 2009, 44, 141–147. [Google Scholar] [CrossRef]
- Bueno, K.O.; de Souza Resende, L.; Ribeiro, A.F.; Dos Santos, D.M.; Goncalves, E.C.; Vigil, F.A.; de Oliveira Silva, I.F.; Ferreira, L.F.; de Castro Pimenta, A.M.; Ribeiro, A.M. Spatial cognitive deficits in an animal model of wernicke-korsakoff syndrome are related to changes in thalamic vdac protein concentrations. Neuroscience 2015, 294, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, E.; Thakur, M.K. Alterations in hippocampal mitochondrial dynamics are associated with neurodegeneration and recognition memory decline in old male mice. Biogerontology 2022, 23, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Elyaman, W.; Stern, L.J.; Jiang, N.; Dressman, D.; Bradley, P.; Klatzmann, D.; Bradshaw, E.M.; Farber, D.L.; Kent, S.C.; Chizari, S.; et al. Exploring the role of t cells in alzheimer’s and other neurodegenerative diseases: Emerging therapeutic insights from the t cells in the brain symposium. Alzheimer’s Dement. 2025, 21, e14548. [Google Scholar] [CrossRef] [PubMed]



| Feature | VDAC-1 | VDAC-2 | VDAC-3 |
|---|---|---|---|
| Exons in mammals | 9 | 10 | 9 |
| Gene encoded in nucleus | yes | yes | yes |
| Splice variants | 2 | 1 | 1 |
| cDNA homology to VDAC-1 | 100% | 90% | 68% |
| Expression level | Highest expression level | Lower expression | Lowest expression |
| Tissue distribution | Brain Heart Liver Skeletal muscles | Brain Heart Liver Skeletal muscles | Liver Lung Spleen Ovary adrenal gland Testis |
| Three-dimensional structure solved | For human and mice | For zebrafish | No structure so far |
| Conductance in artificial membranes in vitro | High | High | Low |
| Extramitochondrial locations | Sarcoplasmic reticulum Endoplasmic reticulum Plasma membrane | Plasma membrane acrosomal membrane of spermatozoa Outer dense fibres of sperm flagellum | Outer dense fibres of sperm flagellum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, S.; Heumann, R. Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases? Int. J. Mol. Sci. 2025, 26, 6138. https://doi.org/10.3390/ijms26136138
Neumann S, Heumann R. Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases? International Journal of Molecular Sciences. 2025; 26(13):6138. https://doi.org/10.3390/ijms26136138
Chicago/Turabian StyleNeumann, Sebastian, and Rolf Heumann. 2025. "Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases?" International Journal of Molecular Sciences 26, no. 13: 6138. https://doi.org/10.3390/ijms26136138
APA StyleNeumann, S., & Heumann, R. (2025). Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases? International Journal of Molecular Sciences, 26(13), 6138. https://doi.org/10.3390/ijms26136138







