The Role of Peptides in Nutrition: Insights into Metabolic, Musculoskeletal, and Behavioral Health: A Systematic Review
Abstract
1. Introduction
2. Methods
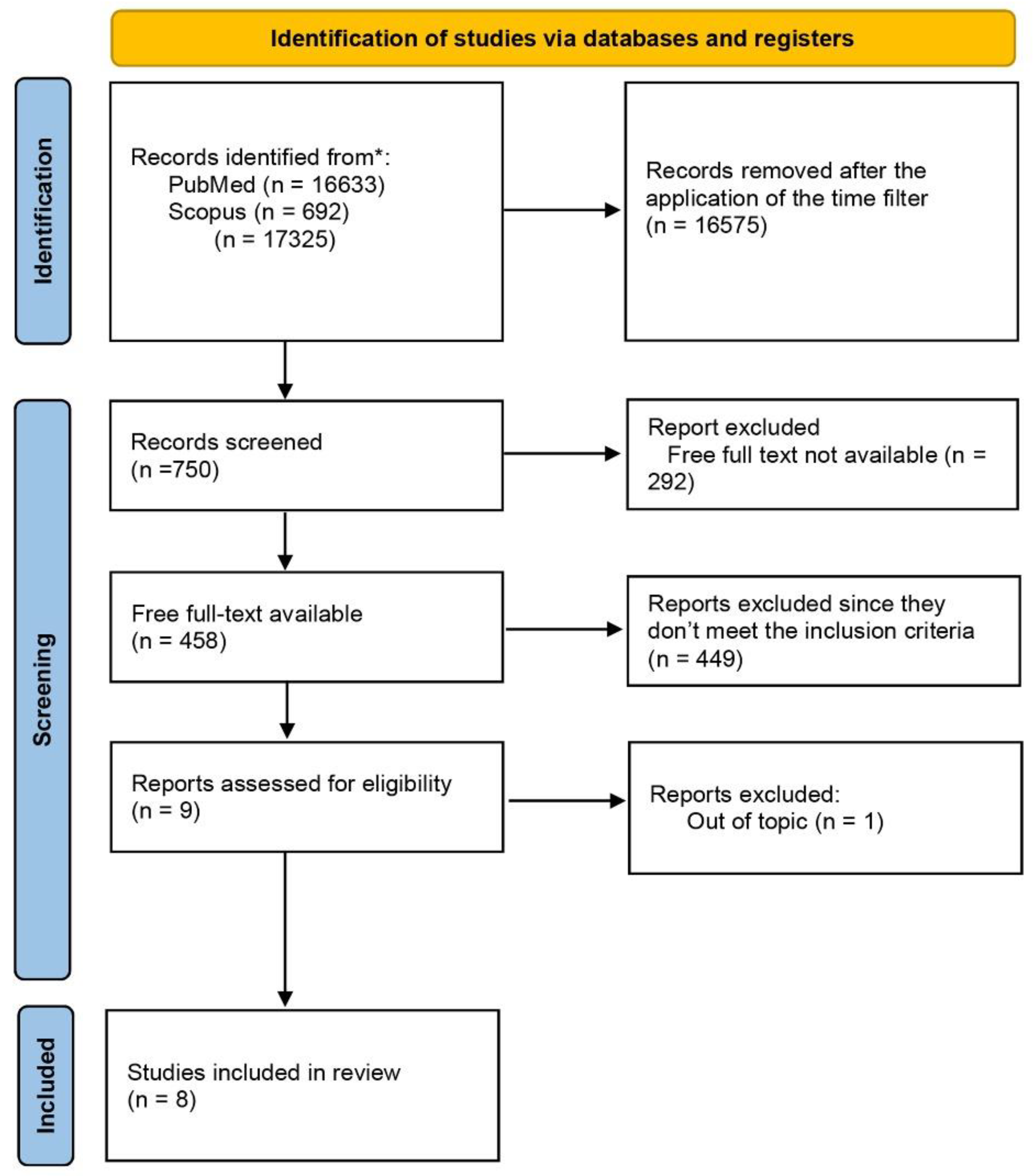
2.1. Searching for Data
2.2. Selection Criteria
2.3. Research Screening
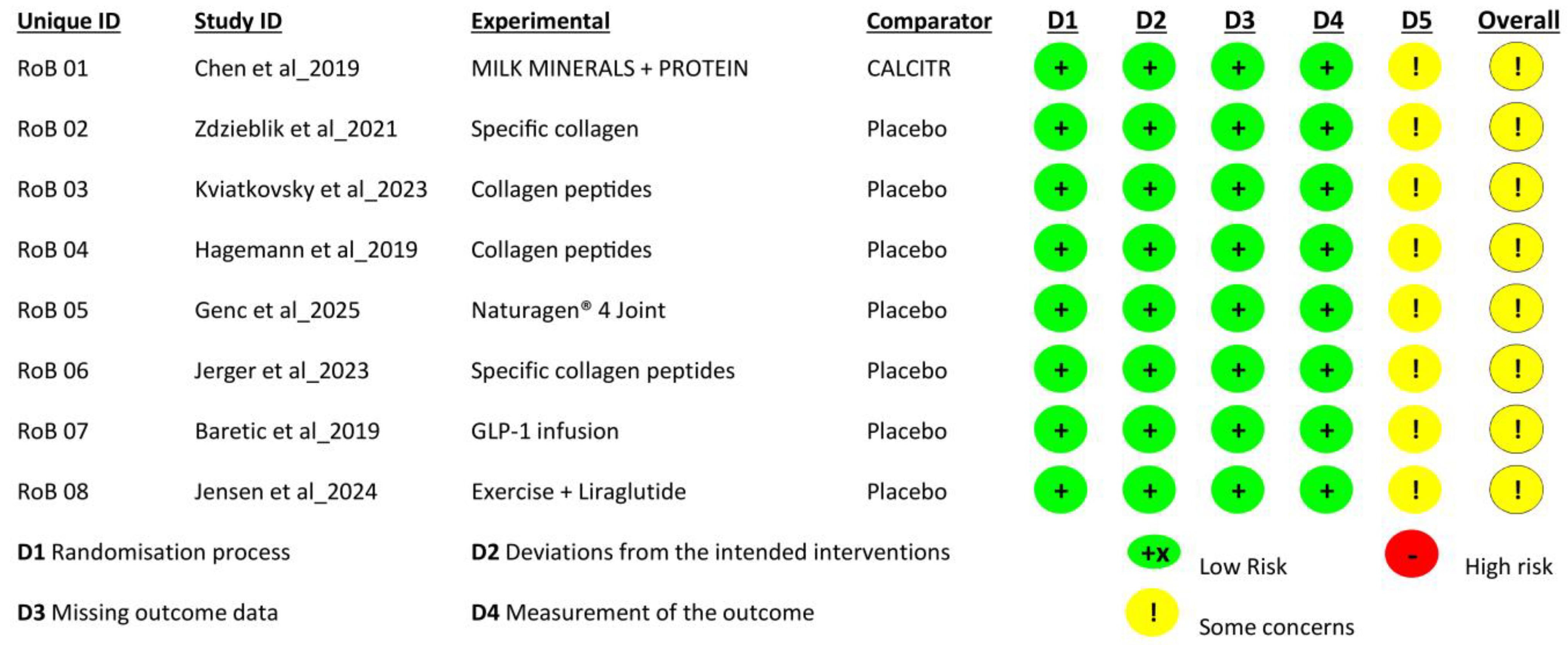
2.4. Risk of Bias Assessment
3. Results
3.1. Study Layout
3.2. Dosage
3.3. Measuring Parameters
4. Discussion
4.1. Metabolic Benefits of Peptides
4.1.1. Variations in Efficacy Among Different Treatments
4.1.2. Implications for Nutritional Approaches
4.2. Enhancement of Musculoskeletal Health
4.3. Effect on Mental and Physical Health
4.4. Influence on Taste Perception and Behavioral Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLP-1 | Glucagon-Like Peptide-1 |
| ADLs | Activities of Daily Living |
| RC | Randomized Controlled Trial |
| GIP | Glucose-dependent insulinotropic polypeptide |
| PYY | Peptide tyrosine–tyrosine |
| CP | Collagen peptide |
| Iauc | Incremental Area Under the Curve |
| GPY | Glycogen Phosphorylase Y |
| MViC | Maximal voluntary isometric contraction |
| CALCITR | Calcium Citrate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
References
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef] [PubMed]
- Alt, K.W.; Al-Ahmad, A.; Woelber, J.P. Nutrition and Health in Human Evolution-Past to Present. Nutrients 2022, 14, 3594. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.; Krishnamurthy, K. Biochemistry, Peptide; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Wang, L.; Qin, T.; Zhang, Y.; Zhang, H.; Hu, J.; Cheng, L.; Xia, X. Antimicrobial Peptides from fish: Main Forces for Reducing and Substituting Antibiotics. Turk. J. Fish. Aquat. Sci. 2023, 24, TRJFAS23922. [Google Scholar] [CrossRef]
- Shori, A.B.; Ming, K.S.; Baba, A.S. The effects of Lycium barbarum water extract and fish collagen on milk proteolysis and in vitro angiotensin I-converting enzyme inhibitory activity of yogurt. Biotechnol. Appl. Biochem. 2021, 68, 221–229. [Google Scholar] [CrossRef]
- Rodrigues, G.; Santos, L.S.; Franco, O.L. Antimicrobial peptides controlling resistant bacteria in animal production. Front. Microbiol. 2022, 13, 874153. [Google Scholar] [CrossRef]
- Shukla, P.; Chopada, K.; Sakure, A.; Hati, S. Current trends and applications of food-derived antihypertensive peptides for the management of cardiovascular disease. Protein Pept. Lett. 2022, 29, 408–428. [Google Scholar] [CrossRef]
- He, Z.; Shang, X.; Wang, X.; Xing, Y.; Zhang, T.; Yun, J. The contribution of Ca and Mg to the accumulation of amino acids in maize: From the response of physiological and biochemical processes. BMC Plant Biol. 2024, 24, 1–18. [Google Scholar] [CrossRef]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Immunonutrition: Facilitating mucosal immune response in teleost intestine with amino acids through oxidant-antioxidant balance. Front. Immunol. 2023, 14, 1241615. [Google Scholar] [CrossRef]
- Rivera-Jiménez, J.; Berraquero-García, C.; Pérez-Gálvez, R.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibit anti-inflammatory activity: Sources, structural features, and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef] [PubMed]
- Avolio, F.; Martinotti, S.; Khavinson, V.K.; Esposito, J.E.; Giambuzzi, G.; Marino, A.; Mironova, E.; Pulcini, R.; Robuffo, I.; Bologna, G.; et al. Peptides Regulating Proliferative Activity and Inflammatory Pathways in the Monocyte/Macrophage THP-1 Cell Line. Int. J. Mol. Sci. 2022, 23, 3607. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Razavi, S.H.; Yadav, H. Diabetes and seeds: New horizon to promote human nutrition and anti-diabetics compounds in grains by germination. Crit. Rev. Food Sci. Nutr. 2023, 63, 8457–8477. [Google Scholar] [CrossRef]
- Hu, M.; Du, Y.; Li, W.; Zong, X.; Du, W.; Sun, H.; Liu, H.; Zhao, K.; Li, J.; Farooq, M.Z.; et al. Interplay of Food-Derived Bioactive Peptides with Gut Microbiota: Implications for Health and Disease Management. Mol. Nutr. Food Res. 2024, 68, e2400251. [Google Scholar] [CrossRef]
- Qiu, W.; Wang, Z.; Liu, Q.; Du, Q.; Zeng, X.; Wu, Z.; Pan, D.; Zhang, X.; Tu, M. Structure and regulatory mechanisms of food-derived peptides in inflammatory bowel disease: A review. Food Sci. Nutr. 2024, 12, 6055–6069. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, M.; Yu, C.; Yang, P.; Xu, M.; Ling, J.; Wu, Y.; Zhu, Z.; Chen, Y.; et al. Food-derived peptides as novel therapeutic strategies for NLRP3 inflammasome-related diseases: A systematic review. Crit. Rev. Food Sci. Nutr. 2025, 65, 1433–1464. [Google Scholar] [CrossRef]
- Ogori, A.F.; Girgih, A.T.; Abu, J.O.; Eke, M.O. Food derived bioactive peptides for health enhancement and management of some chronic diseases. Asian Food Sci. J. 2019, 8, 47845. [Google Scholar]
- Wang, W.; Yang, W.; Dai, Y.; Liu, J.; Chen, Z.-Y. Production of food-derived bioactive peptides with potential application in the management of diabetes and obesity: A review. J. Agric. Food Chem. 2023, 71, 5917–5943. [Google Scholar] [CrossRef]
- Zhao, L.; Li, D.; Qi, X.; Guan, K.; Chen, H.; Wang, R.; Ma, Y. Potential of food-derived bioactive peptides in alleviation and prevention of Alzheimer’s disease. Food Funct. 2022, 13, 10851–10869. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Baloda, S.; Singh, N.; Singh, S.; Bhuker, A.; Singh, P.; Yashveer, S.; et al. Identification and detection of bioactive peptides in milk and dairy products: Remarks about agro-foods. Molecules 2020, 25, 3328. [Google Scholar] [CrossRef]
- Quintieri, L.; Fanelli, F.; Monaci, L.; Fusco, V. Milk and its derivatives as sources of components and microorganisms with health-promoting properties: Probiotics and bioactive peptides. Foods 2024, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.; Ardiansyah, S. The Potential of Bioactive Peptides from Animal Protein Sources as a Mental Health Problems Prevention. Agritropica J. Agric. Sci. 2021, 4, 114–121. [Google Scholar] [CrossRef]
- Bellaver, E.H.; Kempka, A.P. Potential of milk-derived bioactive peptides as antidiabetic, antihypertensive, and xanthine oxidase inhibitors: A comprehensive bibliometric analysis and updated review. Amino Acids 2023, 55, 1829–1855. [Google Scholar] [CrossRef] [PubMed]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Sharkey, S.J.; Harnedy-Rothwell, P.A.; Allsopp, P.J.; Hollywood, L.E.; FitzGerald, R.J.; O’Harte, F.P.M. A narrative review of the anti-hyperglycemic and satiating effects of fish protein hydrolysates and their bioactive peptides. Mol. Nutr. Food Res. 2020, 64, e2000403. [Google Scholar] [CrossRef]
- Rousta, N.; Aslan, M.; Akbas, M.Y.; Ozcan, F.; Sar, T.; Taherzadeh, M.J. Effects of fungal based bioactive compounds on human health. Crit. Rev. Food Sci. Nutr. 2024, 64, 7004–7027. [Google Scholar] [CrossRef]
- Kaplan, M.; Baydemir, B.; Günar, B.B.; Arslan, A.; Duman, H.; Karav, S. Benefits of A2 milk for sports nutrition, health and performance. Front. Nutr. 2022, 9, 935344. [Google Scholar] [CrossRef]
- Saito, T. Antihypertensive peptides derived from bovine casein and whey proteins. Bioact. Compon. Milk 2008, 606, 295–317. [Google Scholar]
- Schulze, C.; Schunck, M.; Zdzieblik, D.; Oesser, S. Impact of Specific Bioactive Collagen Peptides on Joint Discomforts in the Lower Extremity during Daily Activities: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2024, 21, 687. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Elshenawy, A.I.; Abdelghany, M.; Elghaffar, H.A.A. Effects of early mobilisation program on functional capacity, daily living activities, and N-terminal prohormone brain natriuretic peptide in patients hospitalised for acute heart failure. A randomised controlled trial. Hong Kong Physiother. J. 2023, 43, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Dobenecker, B.; Böswald, L.F.; Reese, S.; Steigmeier-Raith, S.; Trillig, L.; Oesser, S.; Schunck, M.; Meyer-Lindenberg, A.; Hugenberg, J.; Tomaszewska, E. The oral intake of specific Bioactive Collagen Peptides (BCP) improves gait and quality of life in canine osteoarthritis patients—A translational large animal model for a nutritional therapy option. PLoS ONE 2024, 19, e0308378. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, P.V.; Safronova, V.N.; Duan, S.; Komlev, A.S.; Bolosov, I.A.; Kruglikov, R.N.; Kombarova, T.I.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; et al. Novel BRICHOS-Related Antimicrobial Peptides from the Marine Worm Heteromastus filiformis: Transcriptome Mining, Synthesis, Biological Activities, and Therapeutic Potential. Mar. Drugs 2023, 21, 639. [Google Scholar] [CrossRef]
- Le Huy, B.; Phuong, H.B.T.; Hai, Y.D.; Thi, K.A.T.; Thanh, B.N.T.; Hong, M.N.; Dinh, H.V. Synthesis and biological comparison of two antimicrobial peptides originated from the venom of vespa crabro and polybia paulista. Vietnam. J. Sci. Technol. 2023, 62, 222–232. [Google Scholar] [CrossRef]
- Chen, P.; Ye, T.; Li, C.; Praveen, P.; Hu, Z.; Li, W.; Shang, C. Embracing the era of antimicrobial peptides with marine organisms. Nat. Prod. Rep. 2024, 41, 331–346. [Google Scholar] [CrossRef]
- Hu, C.; Guo, T.; Zou, Y.; Gao, J.; Gao, Y.; Niu, M.; Xia, Y.; Shen, X.; Li, J. Discovery of dual S-RBD/NRP1-targeting peptides: Structure-based virtual screening, synthesis, biological evaluation, and molecular dynamics simulation studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 2212327. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as therapeutic agents: Challenges and opportunities in the green transition era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Ahmed, S.; Alam, W.; Aschner, M.; Filosa, R.; Cheang, W.S.; Jeandet, P.; Saso, L.; Khan, H. Marine Cyanobacterial Peptides in Neuroblastoma: Search for Better Therapeutic Options. Cancers 2023, 15, 2515. [Google Scholar] [CrossRef]
- Kiersnowska, K.; Jakubczyk, A. Bioactive peptides obtained from legume seeds as new compounds in metabolic syndrome prevention and diet therapy. Foods 2022, 11, 3300. [Google Scholar] [CrossRef]
- Qiao, Q.; Chen, L.; Li, X.; Lu, X.; Xu, Q.; Deng, W. Roles of Dietary bioactive peptides in redox balance and metabolic disorders. Oxidative Med. Cell. Longev. 2021, 2021, 5582245. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; Morales, D.; Miguel-Castro, M. Potential role of Bioactive proteins and peptides derived from legumes towards metabolic syndrome. Nutrients 2022, 14, 5271. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Torres, D.A.; Fernández-Velasco, D.A.; Morales-Olán, G.; Rosas-Cárdenas, F.d.F.; Luna-Suárez, S. Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment. Appl. Sci. 2020, 10, 2604. [Google Scholar] [CrossRef]
- Dwivedi, S.; Singh, V.; Sharma, K.; Sliti, A.; Baunthiyal, M.; Shin, J.-H. Significance of soy-based fermented food and their bioactive compounds against obesity, diabetes, and cardiovascular diseases. Plant Foods Hum. Nutr. 2024, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Ferjan, S.; Battelino, T.; Kovač, J.; Battelino, S.; Šuput, D.; Vovk, A.; Janež, A. Does intervention with GLP-1 receptor agonist semaglutide modulate perception of sweet taste in women with obesity: Study protocol of a randomized, single-blinded, placebo-controlled clinical trial. Trials 2021, 22, 464. [Google Scholar] [CrossRef]
- Ullah, A.; Liu, Y.; Wang, Y.; Gao, H.; Luo, Z.; Li, G. Gender differences in taste sensations based on frequency analysis of surface electromyography. Percept. Mot. Ski. 2023, 130, 938–957. [Google Scholar] [CrossRef]
- Sever, M.J.; Kovac, J.; Vovk, A.; Battelino, S.; Battelino, T.; Janez, A. 6468 Once-Weekly Semaglutide and Taste Perception in Women with Obesity. J. Endocr. Soc. 2024, 8 (Suppl. 1), bvae163-013. [Google Scholar] [CrossRef]
- Al-Ghurayr, N.K.; Al-Mowalad, A.M.; Omar, U.M.; Ashi, H.M.; Al-Shehri, S.S.; AlShaikh, A.A.; AlHarbi, S.M.; Alsufiani, H.M. Salivary Hormones Leptin, Ghrelin, Glucagon, and Glucagon-Like Peptide 1 and Their Relation to Sweet Taste Perception in Diabetic Patients. J. Diabetes Res. 2023, 2023, 7559078. [Google Scholar] [CrossRef]
- Khan, R.; Doty, R.L. GLP-1 receptor agonists significantly impair taste function. Physiol. Behav. 2025, 291, 114793. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Pereira, A.J.; de Campos, L.J.; Xing, H.; Conda-Sheridan, M. Peptide-based therapeutics: Challenges and solutions. Med Chem Res 2024, 33, 1275–1280. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Smith, H.A.; Hengist, A.; Chrzanowski-Smith, O.J.; Mikkelsen, U.R.; Carroll, H.A.; Betts, J.A.; Thompson, D.; Saunders, J.; Gonzalez, J.T. Co-ingestion of whey protein hydrolysate with milk minerals rich in calcium potently stimulates glucagon-like peptide-1 secretion: An RCT in healthy adults. Eur. J. Nutr. 2020, 59, 2449–2462. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Jendricke, P.; Oesser, S.; Gollhofer, A.; König, D. The influence of specific bioactive collagen peptides on body composition and muscle strength in middle-aged, untrained men: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 4837. [Google Scholar] [CrossRef]
- Kviatkovsky, S.A.; Hickner, R.C.; Cabre, H.E.; Small, S.D.; Ormsbee, M.J. Collagen peptides supplementation improves function, pain, and physical and mental outcomes in active adults. J. Int. Soc. Sports Nutr. 2023, 20, 2243252. [Google Scholar] [CrossRef]
- Oertzen-Hagemann, V.; Kirmse, M.; Eggers, B.; Pfeiffer, K.; Marcus, K.; de Marées, M.; Platen, P. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients 2019, 11, 1072. [Google Scholar] [CrossRef]
- Genç, A.S.; Yılmaz, A.K.; Anıl, B.; Salkılıç, E.K.; Akdemir, E.; Güzel, B.; Mor, A.; Yarar, H.A.; Güzel, N.; Kehribar, L. The effect of supplementation with type I and type III collagen peptide and type II hydrolyzed collagen on pain, quality of life and physical function in patients with meniscopathy: A randomized, double-blind, placebo-controlled study. BMC Musculoskelet. Disord. 2025, 26, 17. [Google Scholar] [CrossRef]
- Jerger, S.; Centner, C.; Lauber, B.; Seynnes, O.; Friedrich, T.; Lolli, D.; Gollhofer, A.; König, D. Specific collagen peptides increase adaptions of patellar tendon morphology following 14-weeks of high-load resistance training: A randomized-controlled trial. Eur. J. Sport Sci. 2023, 23, 2329–2339. [Google Scholar] [CrossRef]
- Baretić, M.; Kušec, V.; Uroić, V.; Pavlić-Renar, I.; Altabas, V. Glucagon-like peptide-1 affects taste perception differently in women: A randomized, placebo-controlled crossover study. Acta Clin. Croat. 2019, 58, 240–247. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Blond, M.B.; Sandsdal, R.M.; Olsen, L.M.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; Holst, J.J.; Madsbad, S.; et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: A post-treatment analysis of a randomised placebo-controlled trial. eClinicalMedicine 2024, 69, 102475. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Arapidi, G.P.; Urban, A.S.; Osetrova, M.S.; Shender, V.O.; Butenko, I.O.; Bukato, O.N.; Kuznetsov, A.A.; Saveleva, T.M.; Nos, G.A.; Ivanova, O.M.; et al. Non-human peptides revealed in blood reflect the composition of intestinal microbiota. BMC Biol. 2024, 22, 178. [Google Scholar] [CrossRef] [PubMed]
- Nasri, A.; Kowaluk, M.; Widenmaier, S.B.; Unniappan, S. Nesfatin-1 and nesfatin-1-like peptide attenuate hepatocyte lipid accumulation and nucleobindin-1 disruption modulates lipid metabolic pathways. Commun. Biol. 2024, 7, 623. [Google Scholar] [CrossRef]
- Rosa, S.; Tagliani, A.; Bertaso, C.; Tadini, L.; Visentin, C.; Gourlay, L.J.; Pricl, S.; Feni, L.; Pellegrino, S.; Pesaresi, P.; et al. The cyclic peptide G4CP2 enables the modulation of galactose metabolism in yeast by interfering with GAL4 transcriptional activity. Front. Mol. Biosci. 2023, 10, 1017757. [Google Scholar] [CrossRef]
- Pandey, K.N. Molecular Signaling Mechanisms and Function of Natriuretic Peptide Receptor-A in the Pathophysiology of Cardiovascular Homeostasis. Front. Physiol. 2021, 12, 693099. [Google Scholar] [CrossRef]
- Hulsmann, A. Peptidases: Structure, function, and modulation of peptide-mediated effects in the human lung. Clin. Exp. Allergy 2001, 29, 445–456. [Google Scholar]
- Catt, K.J.; Harwood, J.P.; Aguilera, G.; Dufau, M.L. Hormonal regulation of peptide receptors and target cell responses. Nature 1979, 280, 109–116. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef]
- Kremsmayr, T.; Aljnabi, A.; Blanco-Canosa, J.B.; Tran, H.N.T.; Emidio, N.B.; Muttenthaler, M. On the utility of chemical strategies to improve peptide gut stability. J. Med. Chem. 2022, 65, 6191–6206. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Wang, Z.-J.; Yan, F.; Zhang, Y.; Huo, L. Recent advances in discovery, bioengineering, and bioactivity-evaluation of ribosomally synthesized and post-translationally modified peptides. ACS Bio. Med. Chem. Au 2022, 3, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Song, J.; Chen, S.; Fu, C.; Li, Z.; Weng, W.; Shi, L.; Ren, Z. Improving surimi gel quality by corn oligopeptide-chitosan stabilized high-internal phase Pickering emulsions. Food Hydrocoll. 2025, 166, 111268. [Google Scholar] [CrossRef]
- Watkins, J.D.; Smith, H.A.; Hengist, A.; Brunsgaard, L.H.; Mikkelsen, U.R.; Koumanov, F.; Betts, J.A.; Gonzalez, J.T. Plasma glucagon-like peptide-1 responses to ingestion of protein with increasing doses of milk minerals rich in calcium. Br. J. Nutr. 2021, 128, 55–63. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Wu, X.; Liu, Y.; Wang, J. A review of food-derived peptides for antidiabetic applications. Food Med. Health 2024, 6, 100028. [Google Scholar]
- Zhang, Y.; Li, Q.; Huang, L.; Zhao, Y.; Chen, M. Antihypertensive peptides: Emerging insights from food proteins and AI-based discovery. Food Med. Health 2024, 6, 100030. [Google Scholar]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Neves, R.P.; Ohanenye, I.C.; Udenigwe, C.C. Peptide–mineral complexes: Understanding their chemical interactions, bioavailability, and potential application in mitigating micronutrient deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef]
- Ma, W.; Li, N.; Lin, L.; Wen, J.; Zhao, C.; Wang, F. Research progress in lipid metabolic regulation of bioactive peptides. Food Prod. Process. Nutr. 2023, 5, 10. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2012, 33, 75–81. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Küster, L.; Münzel, T.; Daiber, A.; Steven, S. Glucagon-like peptide-1 (GLP-1) receptor agonists and their cardiovascular benefits-The role of the GLP-1 receptor. Br. J. Pharmacol. 2022, 179, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.-S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Renaud, V.; Faucher, M.; Dubois, M.-J.; Pilon, G.; Varin, T.; Marette, A.; Bazinet, L. Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice. Int. J. Mol. Sci. 2023, 24, 12968. [Google Scholar] [CrossRef]
- Watkins, J.D.; Smith, H.A.; Hengist, A.; Nielsen, S.B.; Mikkelsen, U.R.; Saunders, J.; Koumanov, F.; Betts, J.A.; Gonzalez, J.T. Effects of physical form of β-lactoglobulin and calcium ingestion on GLP-1 secretion, gastric emptying, and energy intake in humans: A randomised crossover trial. Br. J. Nutr. 2024, 131, 1730–1739. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Funnell, M.P.; McDermott, E.; Maden-Wilkinson, T.M.; Abela, S.; Quteishat, B.; Edsey, M.; James, L.J.; Folland, J.P. The effect of specific bioactive collagen peptides on function and muscle remodeling during human resistance training. Acta Physiol. 2023, 237, e13903. [Google Scholar] [CrossRef]
- Bischof, K.; Stafilidis, S.; Bundschuh, L.; Oesser, S.; Baca, A.; König, D. Reduction in systemic muscle stress markers after exercise-induced muscle damage following concurrent training and supplementation with specific collagen peptides—A randomized controlled trial. Front. Nutr. 2024, 11, 1384112. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Xiang, M.; Han, Q.; Chen, Y.; Duan, S.; Han, X.; Sui, X.; Ren, C.; Wang, Q. Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial. Metabolites 2024, 14, 538. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2019, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Dossat, A.M.; Kokoska, M.M.; Whitaker-Fornek, J.R.; Sniffen, S.E.; Kulkarni, A.S.; Levitt, E.S.; Wesson, D.W. Glucagon-like peptide-1 receptors in the gustatory cortex influence food intake. J. Neurosci. 2023, 43, 4251–4261. [Google Scholar] [CrossRef] [PubMed]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do nutritional behaviors depend on biological sex and cultural gender. Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Feraco, A.; Armani, A.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Bellia, A. Gender differences in body composition, dietary patterns, and physical activity: Insights from a cross-sectional study. Front. Nutr. 2024, 11, 1414217. [Google Scholar] [CrossRef]
| Search Category | Phrases and Keywords Used |
|---|---|
| Peptides related terms | Peptides, nano peptides, short peptides, Biopeptides, Bioactive peptides, food-derived peptides |
| Nutrition-related terms | Nutrition, Supplements, Diet |
| Fields searched | Title, Abstract |
| Search operators | AND, OR |
| Database | PubMed, Scopus |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Clinical Trial | Other Publication Type |
| Random Controlled Trial | |
| Article included for the first time | Duplicate |
| Articles published between 2019 and 2024 | Articles published before 2019 |
| Human studies | Animal, in vitro, and silico studies |
| Free full-text articles | Full-text to buy |
| Articles published in English | Articles published in other languages |
| Off-topic articles |
| Author | Study Methodology | Measuring Parameters | Findings | Summary |
|---|---|---|---|---|
| Chen et al. 2019 [55] | Three randomized crossover trials with healthy adults were studied to examine the effects of calcium sources and their co-ingestion with protein hydrolysate on energy consumption, appetite, and gut hormone responses. Participants were treated with calcium citrate, milk minerals with hydrolysate protein, and milk minerals alone. Blood samples were collected every 120 min. | Baseline; GLP-1, GIP, PYY, Visual analogues scales (VAS); Plasma glucose pressure, blood pressurePrimary: Incremental area under the curve (iAUC)Secondary: Indirect calorimetry, respiratory exchange ratio (RER) | Study 1:
| GLP-1 secretion is significantly increased by protein hydrolysate with calcium-containing milk minerals, increases energy consumption, and mildly reduces diastolic blood pressure in adults compared to milk minerals and calcium citrate. |
| Zdzieblik et al. 2021 [56] | In this study, three groups of overweight men received either peptide supplements, whey proteins, or placebo supplements. They followed a 12-week training program that involved daily supplement intake. | Body composition was measured by dual-energy X-ray Absorption Spectroscopy (DXA), waist circumference and body weight were measured by Anthropometric measurements, and creatine kinase and urea levels were used as blood parameters. | The study showed that, compared to the placebo group, the collagen peptide group observed an increase in fat-free mass (3.42 ± 2.54 kg) and a decrease in fat mass (−5.28 ± 3.19 kg). Muscle strength improved in all participants, with the collagen peptide group bearing the highest increment (168 ± 189 N). This study concluded that bioactive collagen peptides are more efficient when bound with resistance training than a placebo. | Fifteen grams of collagen peptides significantly decreased fat mass compared to placebo in untrained men. Bioactive collagen peptide also enhanced muscle strength in all participants. Collagen peptides and whey proteins show similar behavior. Bone mineral content and waist circumference increased in all participants. |
| Kviatkovsky et al. 2023 [57] | In this study, a placebo-controlled, double-blind, randomized test was conducted. All participants were divided into placebo, collagen peptide (10 g/day), and collagen peptide 20 g/day groups over 3, 6, and 9 months to measure health, pain, and physical function. | Three-day food record, physical activity survey, Veterans Rand 12 Item Health Survey (VR-12), and Knee Injury and Osteoarthritis Outcome Score (KOOS) | ADLs (p = 0.031) and Pain (p = 0.037) were enhanced by 10 g/day of collagen peptide supplements, and Physical component score (PSC) in females (p = 0.013) was improved by 20 g/day CP. 10 g/day of collagen peptides over 3–9 months also enhanced mental component score (p = 0.17). No notable improvements were observed in the placebo group. | This study indicates that collagen peptide supplementation (10–20 g/day) for 6–9 months is effective in improving daily living, physical and mental health, and pain. A daily dose of 10 g was particularly effective for individuals who exercise more frequently. |
| Hagemann et al. 2019 [58] | This research involved a 12-week, randomized, double-blind, placebo-controlled study. The first group received 15 g of collagen hydrolysate, and the second group received a placebo. All participants engaged in resistance training three times a week. | A bioelectrical impedance analysis system is used to measure body mass, fat-free mass, and fat mass. A Dynamometer is used to measure leg extension maximal voluntary isometric contraction (MViC), and liquid chromatography-mass spectrometry (LC-MS) is used for muscle proteome analysis. | Muscle proteome analysis showed that the collagen group contained 221 more abundant proteins, primarily related to contractile fibers, compared to only 44 in the placebo group. Significant elevations were observed in the collagen group in the pathways related to cell cycle, immune response, protein metabolism, and muscle contraction. | Collagen peptide dosage combined with resistance training significantly enhances fat-free mass, body mass, and muscle strength compared to training without these supplements. |
| Genç et al. 2025 [59] | This study concluded a double-blind, controlled clinical trial that involved 32 individuals divided into two groups. In this controlled study, one group was treated with collagen supplements while the other received a placebo for 8 weeks. | Physical function tests, such as Timed Up and Go (TUG), a 6 min walk, the Berg balance scale (BBS), and a stair-climbing Test, were carried out. The Tampa Scale was used for Kinesiophobia (TSK). The Visual Analog Scale (VAS), WOMAC total, KOOS-PS, and Foot Function Index (FFI) were used to measure pain, quality of life, and physical function. | SAfter eight weeks, a first group treated with collagen had significant gains in Kinesiophobia, quality of life and physical functions, and pain. In addition, leg strength is also remarkably enhanced. The placebo group exhibited no substantial changes in any studied behavior. | In meniscopathy patients, collagen enhances pain and quality of life functions. In the early stage of meniscus injury, collagen supplements might be an effective nonsurgical option for addressing symptoms. |
| Jerger et al. 2023 [60] | This study was a double-blind, randomized clinical trial. All participants completed 14 weeks of resistance training. One group received a daily dose of 5 g of placebo, while the other received 5 g of specific collagen peptides. | Magnetic resonance imaging (MRI) measures the Patellar Tendon and the rectus femoris muscle Cross-Sectional Area (CSA). The stiffness evaluates the patellar tendon’s stiffness. 1-repetition maximum (1 RM) tests assess maximal muscle strength. | In the collagen group, a remarkable outcome indicates a 60% (+11.4%) increase in patellar tendon CSA and 70% (+12.3%) elevation in tendon length in contrast with placebo (+4.6% and +6.1%). In both groups, 1 RM strength (20–30%) and tendon stiffness CSA (7–8%) values showed similar enhancement. | This trial summarizes that the patellar tendon cross-sections are increased significantly in the collagen group, particularly in the proximal area of the tendon, in contrast with the placebo group. However, similar outcomes were reported in tendon stiffness and muscle strength. |
| Baretić et al. 2019 [61] | This research involved a double-blind and placebo-controlled crossover study. All subjects were treated with Glucagon-Like-Peptide-1 (1.5 pmol/kg/min) and a placebo (0.9% saline). At the end, participants had to choose their favorite foods from the list of five tastes. | A bioelectrical impedance analyzer was used for body composition analysis. A Homeostasis Model Assessment (HOMA) calculator was used for resistance estimation and insulin sensitivity, and an ELISA ALPCO kit was used for calculating glucagon-like peptide (GLP-1). | Seven out of fourteen participants reported a change in their taste after the GLP-1 infusion compared to the placebo, which showed no change. In females, a positive correlation between insulin and GLP-1 infusion was found, which shows that individuals with higher insulin resistance have a higher response to GLP-1. These findings show that taste perception and insulin sensitivity are observed differently due to different hormonal effects. | This study concluded that taste perception affects both males and females differently. A Glucagon-Like Bioelectrical Impedance analysis system is used. Peptide infusion is more likely to change taste perception in women than men. All these outcomes from this study show some basic understanding of physiological differences in metabolic responses to eating habits among men and women. |
| Jensen 2024 [62] | This investigation involved a randomized, placebo-controlled trial in which all participants were treated with a low-calorie diet. In a second step, they were treated with liraglutide for one year. | Dual-Energy X-ray Absorptiometry (DXA) is used to measure body weight, and a cycle ergometer calculates VO2 max, and accelerometers assess physical activity level. | After one year of restricted treatment, the exercise group regained 3 kg in weight while the liraglutide group regained 9 kg. In contrast, the group that received combined treatment observed a 5.1 kg gain. | During active treatment, liraglutide contributes significantly to weight loss. However, its benefits diminish once treatment is discontinued. Regular physical activity leads to weight maintenance and other targeted benefits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakir, S.K.; Jawed, B.; Esposito, J.E.; Kanwal, R.; Pulcini, R.; Martinotti, R.; Ceci, E.; Botteghi, M.; Gaudio, F.; Toniato, E.; et al. The Role of Peptides in Nutrition: Insights into Metabolic, Musculoskeletal, and Behavioral Health: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 6043. https://doi.org/10.3390/ijms26136043
Zakir SK, Jawed B, Esposito JE, Kanwal R, Pulcini R, Martinotti R, Ceci E, Botteghi M, Gaudio F, Toniato E, et al. The Role of Peptides in Nutrition: Insights into Metabolic, Musculoskeletal, and Behavioral Health: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(13):6043. https://doi.org/10.3390/ijms26136043
Chicago/Turabian StyleZakir, Syed Khuram, Bilal Jawed, Jessica Elisabetta Esposito, Rimsha Kanwal, Riccardo Pulcini, Riccardo Martinotti, Edmondo Ceci, Matteo Botteghi, Francesco Gaudio, Elena Toniato, and et al. 2025. "The Role of Peptides in Nutrition: Insights into Metabolic, Musculoskeletal, and Behavioral Health: A Systematic Review" International Journal of Molecular Sciences 26, no. 13: 6043. https://doi.org/10.3390/ijms26136043
APA StyleZakir, S. K., Jawed, B., Esposito, J. E., Kanwal, R., Pulcini, R., Martinotti, R., Ceci, E., Botteghi, M., Gaudio, F., Toniato, E., & Martinotti, S. (2025). The Role of Peptides in Nutrition: Insights into Metabolic, Musculoskeletal, and Behavioral Health: A Systematic Review. International Journal of Molecular Sciences, 26(13), 6043. https://doi.org/10.3390/ijms26136043