Quinazolinedione Derivatives as Potential Anticancer Agents Through Apoptosis Induction in MCF-7
Abstract
1. Introduction
2. Results
2.1. Quinazolinedione Derivatives Inhibited MCF-7 Cell Viability
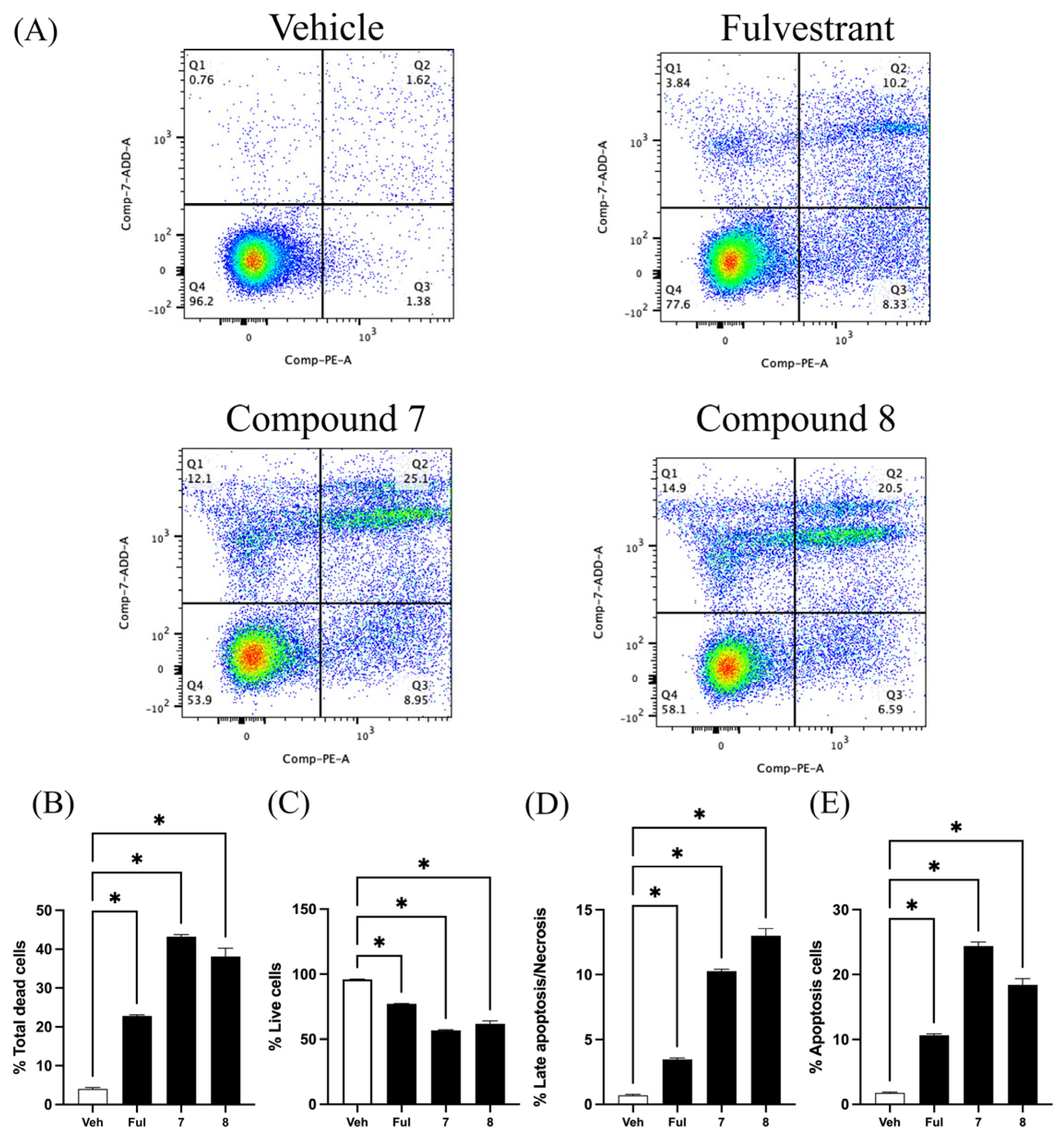
2.2. Quinazolinedione Derivatives Induce Apoptosis in MCF-7 Cells
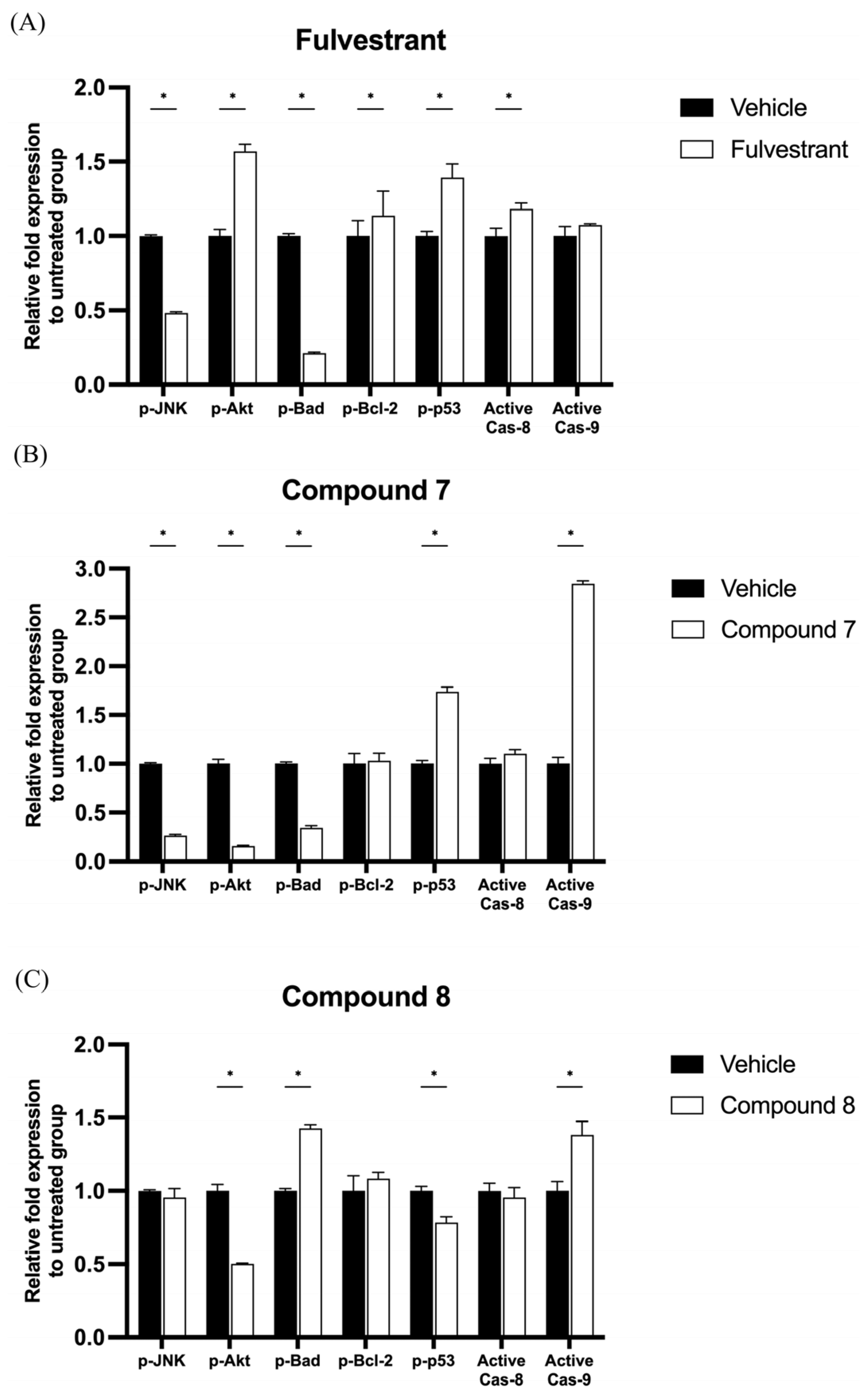
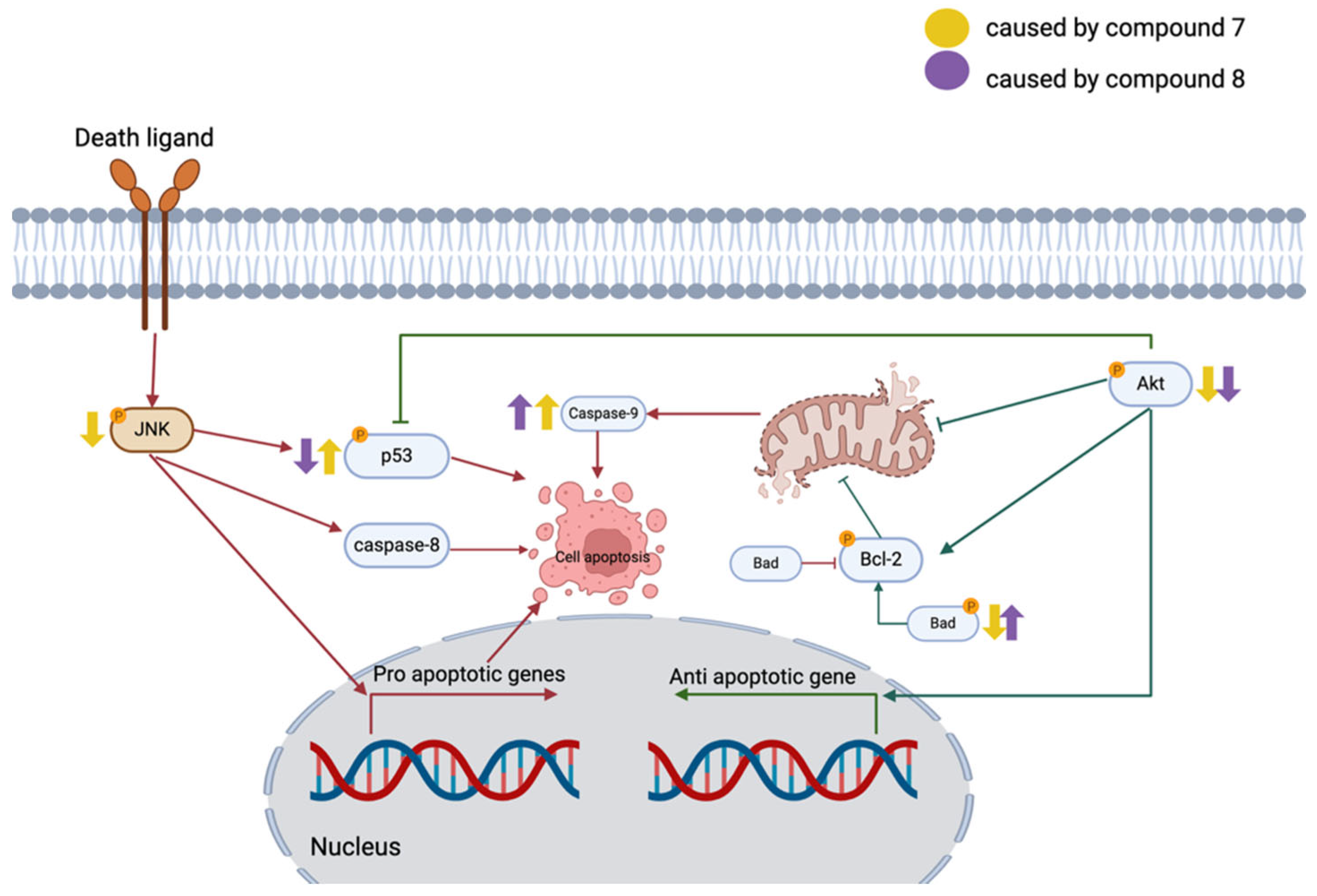
2.3. Quinazolinedione Derivatives Modulate Apoptosis-Related Protein Expression in MCF-7 Cells
3. Discussion
4. Materials and Methods
4.1. Quinazolinedione Derivatives
4.2. Cell Culture and Maintenance
4.3. Cell Viability Test (MTT Test)
4.4. Apoptosis Detection Assay
4.5. Early Apoptosis Protein Detection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA A Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: An Oestrogen Receptor Antagonist with a Novel Mechanism of Action. Br. J. Cancer 2004, 90 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef]
- Charoensutthivarakul, S.; Lohawittayanan, D.; Kanjanasirirat, P.; Jearawuttanakul, K.; Seemakhan, S.; Chabang, N.; Schlaeppi, P.; Tantivess, V.; Limboonreung, T.; Phanchana, M. Rational Design and Lead Optimisation of Potent Antimalarial Quinazolinediones and Their Cytotoxicity against MCF-7. Molecules 2023, 28, 2999. [Google Scholar] [CrossRef]
- Almela, M.J.; Lozano, S.; Lelièvre, J.; Colmenarejo, G.; Coterón, J.M.; Rodrigues, J.; Gonzalez, C.; Herreros, E. A New Set of Chemical Starting Points with Plasmodium Falciparum Transmission-Blocking Potential for Antimalarial Drug Discovery. PLoS ONE 2015, 10, e0135139. [Google Scholar] [CrossRef]
- Gouhar, R.S.; Kamel, M.M. Synthesis and Reactions of Some New Quinazoline Derivatives for In Vitro Evaluation as Anticancer and Antimicrobial Agents. J. Heterocycl. Chem. 2018, 55, 2082–2089. [Google Scholar] [CrossRef]
- Patil, Y.P.; Tambade, P.J.; Jagtap, S.R.; Bhanage, B.M. Cesium Carbonate Catalyzed Efficient Synthesis of Quinazoline-2,4(1H,3H)-Diones Using Carbon Dioxide and 2-Aminobenzonitriles. Green Chem. Lett. Rev. 2008, 1, 127–132. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, M.; Yao, H.; Cao, R.; Zhao, H.; Wang, X.; Chen, X.; Xu, B. Discovery of Quinazoline-2,4(1H,3H)-Dione Derivatives as Novel PARP-1/2 Inhibitors: Design, Synthesis and Their Antitumor Activity. Org. Biomol. Chem. 2018, 16, 3189–3202. [Google Scholar] [CrossRef]
- Charoensutthivarakul, S.; Lohawittayanan, D.; Kanjanasirirat, P.; Jearawuttanakul, K.; Seemakhan, S.; Borwornpinyo, S.; Phanchana, M. A Concise Synthesis Towards Antimalarial Quinazolinedione TCMDC-125133 and Its Anti-Proliferative Activity against MCF-7. Molbank 2022, 2022, M1358. [Google Scholar] [CrossRef]
- Ricci, M.S.; Zong, W.-X. Chemotherapeutic Approaches for Targeting Cell Death Pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsapaya, J.; Grimes, J.M.; Chen, N.; Xu, X.N.; Stuart, D.I.; Jones, E.Y.; Screaton, G.R. Structure of the TRAIL-DR5 Complex Reveals Mechanisms Conferring Specificity in Apoptotic Initiation. Nat. Struct. Biol. 1999, 6, 1048–1053. [Google Scholar] [CrossRef]
- Ghavami, S.; Kerkhoff, C.; Los, M.; Hashemi, M.; Sorg, C.; Karami-Tehrani, F. Mechanism of Apoptosis Induced by S100A8/A9 in Colon Cancer Cell Lines: The Role of ROS and the Effect of Metal Ions. J. Leukoc. Biol. 2004, 76, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Akey, C.W. Apoptosome Structure, Assembly, and Procaspase Activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Shi, Y.; Nikulenkov, F.; Zawacka-Pankau, J.; Li, H.; Gabdoulline, R.; Xu, J.; Eriksson, S.; Hedström, E.; Issaeva, N.; Kel, A.; et al. ROS-Dependent Activation of JNK Converts P53 into an Efficient Inhibitor of Oncogenes Leading to Robust Apoptosis. Cell Death Differ. 2014, 21, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Perfettini, J.-L.; Castedo, M.; Nardacci, R.; Ciccosanti, F.; Boya, P.; Roumier, T.; Larochette, N.; Piacentini, M.; Kroemer, G. Essential Role of P53 Phosphorylation by P38 MAPK in Apoptosis Induction by the HIV-1 Envelope. J. Exp. Med. 2005, 201, 279–289. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Itah, Z.; Chaudhry, S.; Raju Ponny, S.; Aydemir, O.; Lee, A.; Cavanagh-Kyros, J.; Tournier, C.; Muller, W.J.; Davis, R.J. HER2-Driven Breast Cancer Suppression by the JNK Signaling Pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2218373120. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Hou, M.-F.; Chung, Y.-F.; Chen, Y.-J.; Yang, S.-F.; Chen, D.-C.; Su, J.-H.; Yuan, S.-S.F. Decreased Expression of Phosphorylated JNK in Breast Infiltrating Ductal Carcinoma Is Associated with a Better Overall Survival. Int. J. Cancer 2006, 118, 2678–2684. [Google Scholar] [CrossRef]
- Hresko, R.C.; Mueckler, M. mTOR.RICTOR Is the Ser473 Kinase for Akt/Protein Kinase B in 3T3-L1 Adipocytes. J. Biol. Chem. 2005, 280, 40406–40416. [Google Scholar] [CrossRef]
- Kondo, E.; Miyake, T.; Shibata, M.; Kimura, T.; Iwagaki, H.; Nakamura, S.-I.; Tanaka, T.; Ohara, N.; Ichimura, K.; Oka, T.; et al. Expression of Phosphorylated Ser70 of Bcl-2 Correlates with Malignancy in Human Colorectal Neoplasms. Clin. Cancer Res. 2005, 11, 7255–7263. [Google Scholar] [CrossRef] [PubMed]
- Low, I.C.C.; Loh, T.; Huang, Y.; Virshup, D.M.; Pervaiz, S. Ser70 Phosphorylation of Bcl-2 by Selective Tyrosine Nitration of PP2A-B56δ Stabilizes Its Antiapoptotic Activity. Blood 2014, 124, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Deng, X.; May, W.S. Phosphorylation of Bcl2 and Regulation of Apoptosis. Leukemia 2001, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 Phosphorylation by Akt Regulates the P53 Response to Oxidative Stress to Promote Cell Proliferation and Tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Dolfi, S.C.; Jäger, A.V.; Medina, D.J.; Haffty, B.G.; Yang, J.-M.; Hirshfield, K.M. Fulvestrant Treatment Alters MDM2 Protein Turnover and Sensitivity of Human Breast Carcinoma Cells to Chemotherapeutic Drugs. Cancer Lett. 2014, 350, 52–60. [Google Scholar] [CrossRef]
- Zhong, B.; Liu, M.; Bai, C.; Ruan, Y.; Wang, Y.; Qiu, L.; Hong, Y.; Wang, X.; Li, L.; Li, B. Caspase-8 Induces Lysosome-Associated Cell Death in Cancer Cells. Mol. Ther. 2020, 28, 1078–1091. [Google Scholar] [CrossRef]
- Feng, L.; Hollstein, M.; Xu, Y. Ser46 Phosphorylation Regulates P53-Dependent Apoptosis and Replicative Senescence. Cell Cycle 2006, 5, 2812–2819. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.-M.; Meinkoth, J.; Pittman, R.N. Akt Regulates Cell Survival and Apoptosis at a Postmitochondrial Level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef]
- Fu, X.; Creighton, C.J.; Biswal, N.C.; Kumar, V.; Shea, M.; Herrera, S.; Contreras, A.; Gutierrez, C.; Wang, T.; Nanda, S.; et al. Overcoming Endocrine Resistance Due to Reduced PTEN Levels in Estrogen Receptor-Positive Breast Cancer by Co-Targeting Mammalian Target of Rapamycin, Protein Kinase B, or Mitogen-Activated Protein Kinase Kinase. Breast Cancer Res. 2014, 16, 430. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S. The Quinazoline-2,4(1H,3H)-Diones Skeleton: A Key Intermediate in Drug Synthesis. Sustain. Chem. Pharm. 2022, 27, 100696. [Google Scholar] [CrossRef]
- Schwartz, P.S.; Waxman, D.J. Cyclophosphamide Induces Caspase 9-Dependent Apoptosis in 9L Tumor Cells. Mol. Pharmacol. 2001, 60, 1268–1279. [Google Scholar] [CrossRef]
- Shoda, T.; Kato, M.; Fujisato, T.; Demizu, Y.; Inoue, H.; Naito, M.; Kurihara, M. Tamoxifen and Fulvestrant Hybrids Showed Potency as Selective Estrogen Receptor Down-Regulators. Med. Chem. 2017, 13, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Mimnaugh, E.G.; Rajagopalan, S.; Myers, C.E. Adriamycin Activation and Oxygen Free Radical Formation in Human Breast Tumor Cells: Protective Role of Glutathione Peroxidase in Adriamycin Resistance. Cancer Res. 1989, 49, 3844–3848. [Google Scholar]
- Cameron, D.A.; Gabra, H.; Leonard, R.C. Continuous 5-Fluorouracil in the Treatment of Breast Cancer. Br. J. Cancer 1994, 70, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, M.; Wang, B.; Zhang, L.; Zhou, F.; Fang, M. Chemoresistance and Metastasis in Breast Cancer Molecular Mechanisms and Novel Clinical Strategies. Front. Oncol. 2021, 11, 658552. [Google Scholar] [CrossRef]
- Shao, X.Y.; Zhang, Q.Y.; Niu, Z.F.; Li, M.; Wang, J.F.; Chen, Z.Z.; Luo, R.Z.; Qiao, G.D.; Wang, J.G.; Qian, L.Y.; et al. [Phase Ⅲ, multicenter, randomized comparative study of LY01005 and Zoladex® for patients with premenopausal breast cancer]. Zhonghua Zhong Liu Za Zhi 2025, 47, 340–348. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Du, L.; Li, X.; Zhen, L.; Chen, W.; Mu, L.; Zhang, Y.; Song, A. Everolimus Inhibits Breast Cancer Cell Growth through PI3K/AKT/mTOR Signaling Pathway. Mol. Med. Rep. 2018, 17, 7163–7169. [Google Scholar] [CrossRef]
- Sammons, S.; Kornblum, N.S.; Blackwell, K.L. Fulvestrant-Based Combination Therapy for Second-Line Treatment of Hormone Receptor-Positive Advanced Breast Cancer. Target. Oncol. 2019, 14, 1–12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limboonreung, T.; Suansilpong, T.; Jumjitvi, P.; Lohawittayanan, D.; Krobthong, S.; Charoensutthivarakul, S. Quinazolinedione Derivatives as Potential Anticancer Agents Through Apoptosis Induction in MCF-7. Int. J. Mol. Sci. 2025, 26, 6038. https://doi.org/10.3390/ijms26136038
Limboonreung T, Suansilpong T, Jumjitvi P, Lohawittayanan D, Krobthong S, Charoensutthivarakul S. Quinazolinedione Derivatives as Potential Anticancer Agents Through Apoptosis Induction in MCF-7. International Journal of Molecular Sciences. 2025; 26(13):6038. https://doi.org/10.3390/ijms26136038
Chicago/Turabian StyleLimboonreung, Tanapol, Teetat Suansilpong, Panitan Jumjitvi, Duangporn Lohawittayanan, Sucheewin Krobthong, and Sitthivut Charoensutthivarakul. 2025. "Quinazolinedione Derivatives as Potential Anticancer Agents Through Apoptosis Induction in MCF-7" International Journal of Molecular Sciences 26, no. 13: 6038. https://doi.org/10.3390/ijms26136038
APA StyleLimboonreung, T., Suansilpong, T., Jumjitvi, P., Lohawittayanan, D., Krobthong, S., & Charoensutthivarakul, S. (2025). Quinazolinedione Derivatives as Potential Anticancer Agents Through Apoptosis Induction in MCF-7. International Journal of Molecular Sciences, 26(13), 6038. https://doi.org/10.3390/ijms26136038






