New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm
Abstract
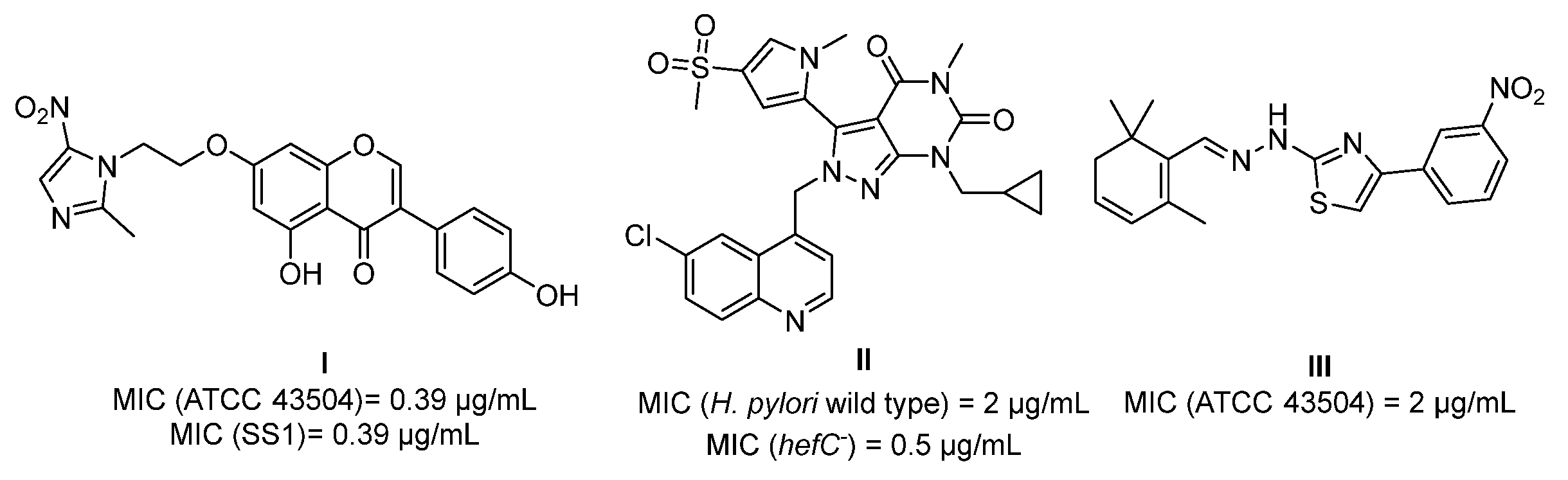
1. Introduction
2. Results and Discussion
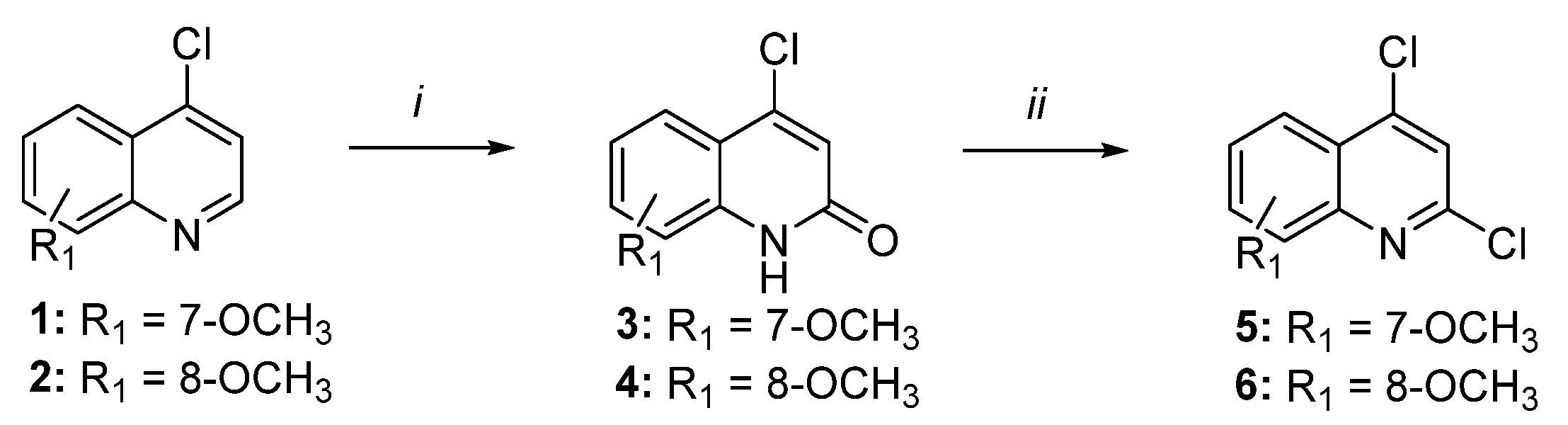
2.1. Chemistry
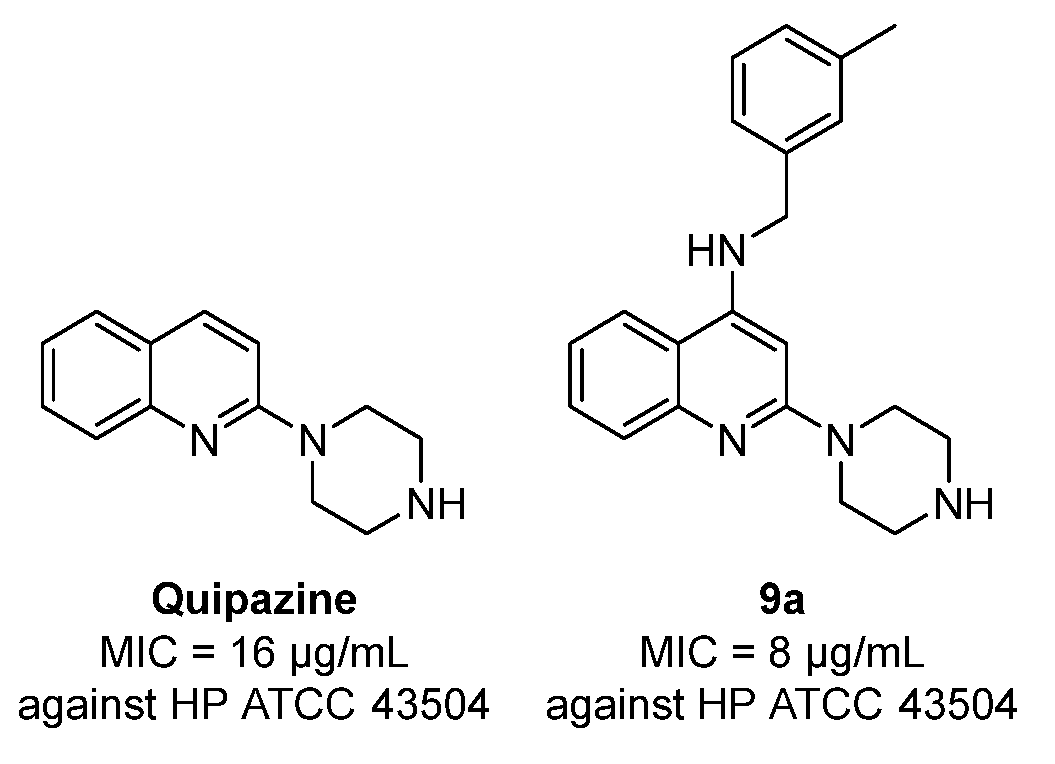
2.2. Anti-H. pylori Activity of Compounds 9a–9f and 10
2.3. Selectivity of Compound 9c over Mammalian Cells
2.4. Metabolic Stability of Compound 9c
2.5. Bacteriostatic Activity of Compound 9c
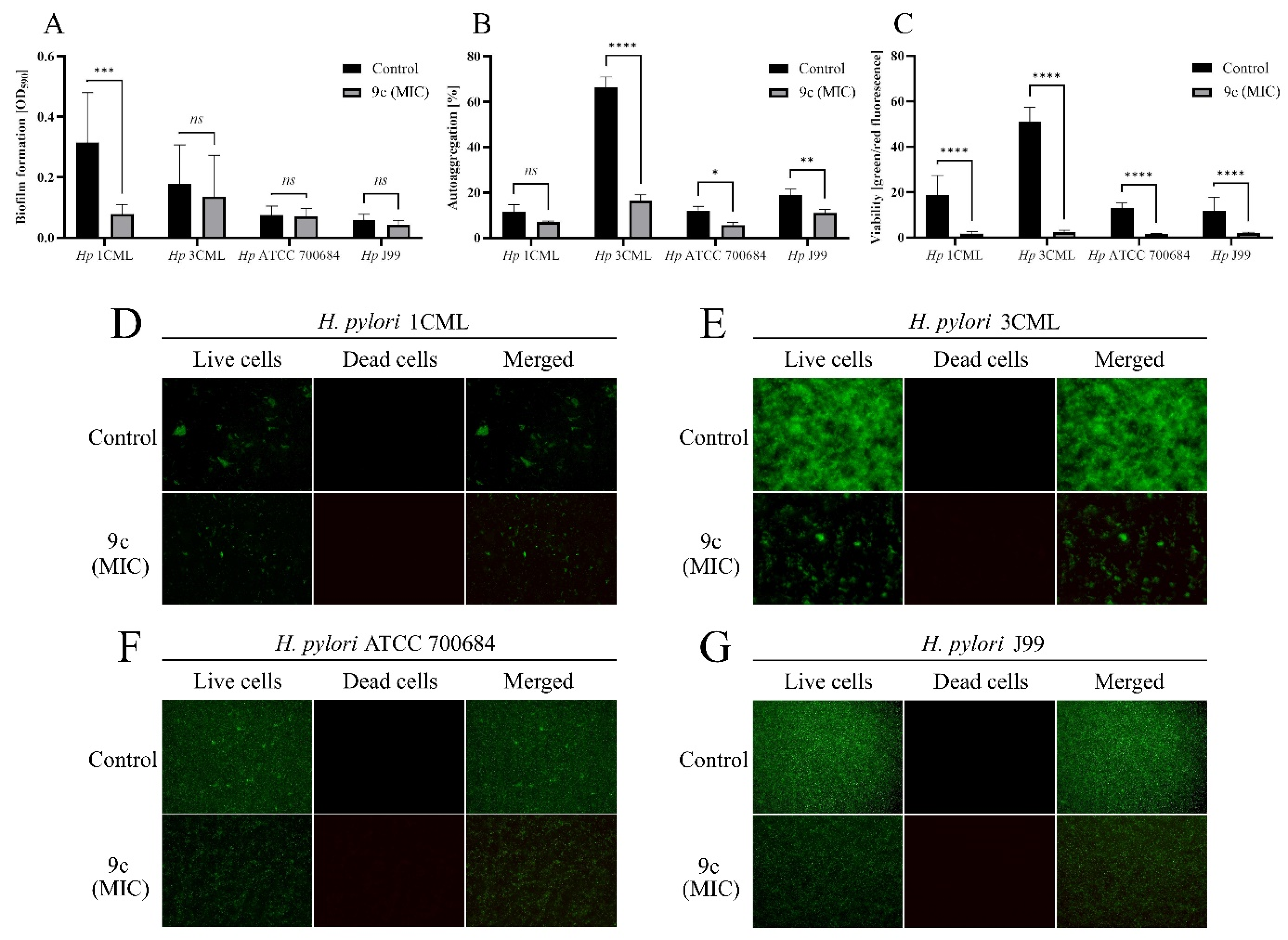
2.6. Anti-Biofilm Activity of Compound 9c
2.7. Evaluation of Antibacterial Spectrum of Compound 9c
2.8. Synergistic Effects of Compound 9c
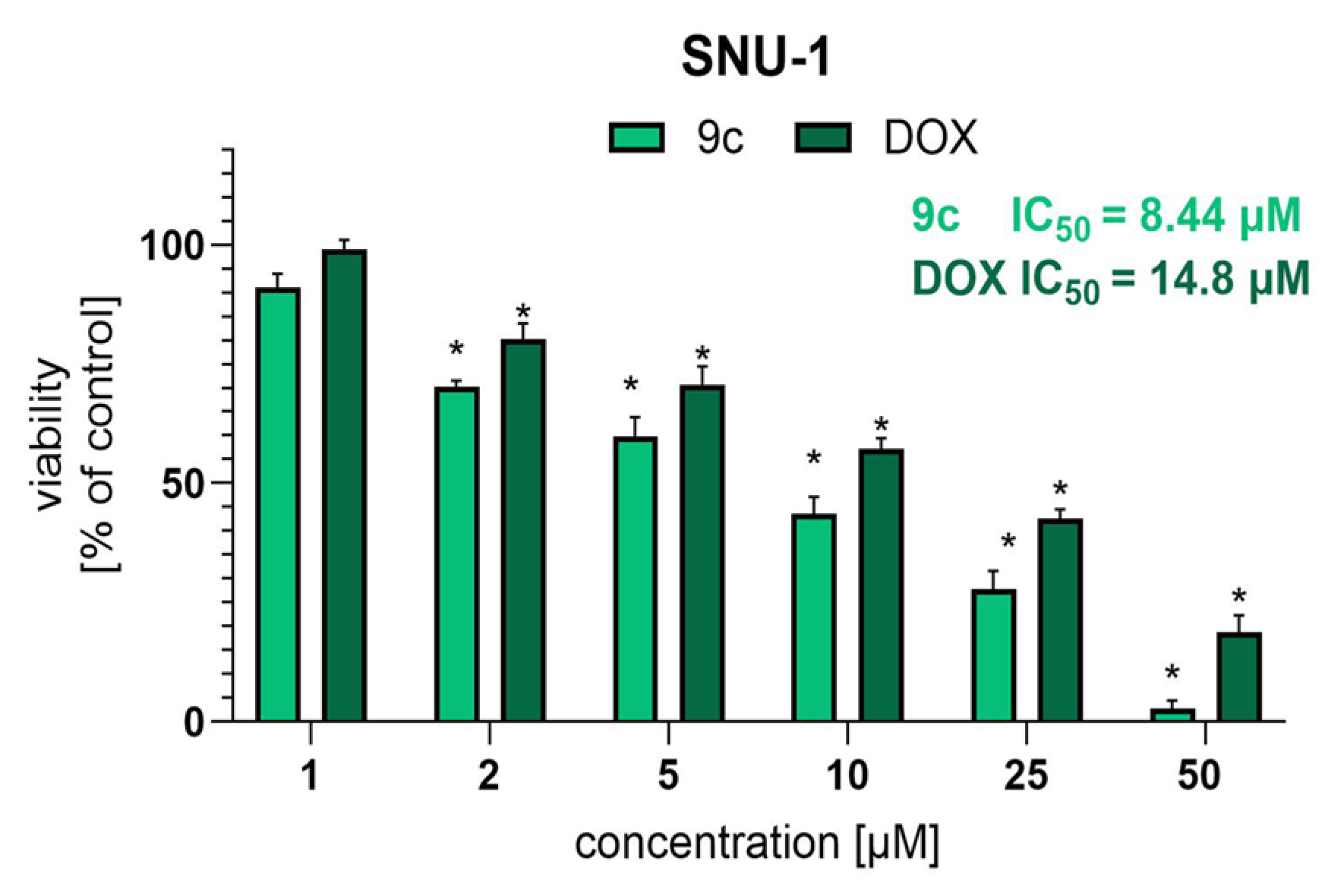
2.9. Activity of Compound 9c Against Human Gastric Cancer Cell Line SNU-1
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis General Information
3.1.2. General Procedure for Preparation of Compounds 3 and 4
3.1.3. General Procedure for Preparation of Compounds 5 and 6
3.1.4. General Procedure for Preparation of Compounds 8a–8d
3.1.5. General Procedure for Preparation of Compounds 8e and 8f
3.1.6. General Procedure for Preparation of Compounds 9a–9f and 10
3.2. Antibacterial Activity of Tested Compounds Against H. pylori
3.2.1. H. pylori Strains
3.2.2. Evaluation of MIC Values of Tested Compounds
3.3. Assessment of Selectivity of Compound 9c over Mammalian Cells
3.3.1. Haemolytic Activity Assay
3.3.2. Cell Cultures
3.3.3. MTT Assay
3.3.4. Impact on Human Gastric Fibroblasts
Fibroblast Cultures
Fibroblast Viability
3.4. Metabolic Stability
3.5. Evaluation of Bacteriostatic/Bactericidal Activity of Compound 9c
3.6. Assessment of Antibiofilm Activity of Compound 9c
3.7. Evaluation of Antibacterial Spectrum of Compound 9c
3.8. Synergistic Activity of Compound 9c
3.9. Assessment of Cytotoxic Activity of 9c
3.9.1. Cell Culture
3.9.2. Evaluation of Cytotoxicity Using MTT
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHI | Brain Heart Infusion |
| CH | Clarithromycin |
| DCM | Dichloromethane |
| DMSO | Dimethylsulfoxide |
| DOX | Doxorubicin |
| FBS | Fetal bovine serum |
| FIC | Fractional inhibitory concentration |
| Hex | Hexane |
| MBC | Minimal bactericidal concentration |
| MDR | Multi-drug-resistant |
| MIC | Minimal inhibitory concentration |
| mPCBA | meta-Chloroperoxybenzoic acid |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MW | Microwave |
| MTZ | Metronidazole |
| PBS | Phosphate Buffered Saline |
| SEM | Standard error of the mean |
| TEA | Triethylamine |
| WHO | World Health Organization |
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241.
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Yuan, Y.; Forman, D.; Hunt, R.; Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 2020, 7, CD005583. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414, Erratum in Nat. Rev. Cancer 2010, 10, 593. [Google Scholar] [CrossRef]
- Freire de Melo, F.; Marques, H.S.; Rocha Pinheiro, S.L.; Lemos, F.F.B.; Luz, M.S.; Teixeira, K.N.; Souza, C.L.; Oliveira, M.V. Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis. World J. Clin. Oncol. 2022, 13, 866–879. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Hunt, R.H.; Leja, M.; O’Morain, C.; Rugge, M.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, J.W. Best Helicobacter pylori Eradication Strategy in the Era of Antibiotic Resistance. Antibiotics 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, E.; Ghanbarimasir, Z.; Emami, S. A review on the structures and biological activities of anti-Helicobacter pylori agents. Eur. J. Med. Chem. 2021, 223, 113669. [Google Scholar] [CrossRef]
- Rosen, J.E.; Chen, D.; Prahalad, A.K.; Spratt, T.E.; Schluter, G.; Williams, G.M. A fluoroquinolone antibiotic with a methoxy group at the 8 position yields reduced generation of 8-oxo-7,8-dihydro-2’-deoxyguanosine after ultraviolet-A irradiation. Toxicol. Appl. Pharmacol. 1997, 145, 381–387. [Google Scholar] [CrossRef]
- Enoua, G.C.; Lahm, G.; Uray, G.; Stadlbauer, W. Syntheses and Fluorescent Properties of 6-Methoxy-2-oxoquinoline-3,4-dicarbonitriles and 6,7-Dimethoxy-2-oxoquinoline-3,4-dicarbonitriles. J. Heterocycl. Chem. 2014, 51, E263–E275. [Google Scholar] [CrossRef]
- International Standard ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. ISO: Geneva, Switzerland, 2019.
- The European Committee on Antimicrobial Susceptibility Testing. Reading Guide for Broth Microdilution. Version 5.0, 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_5.0_2024.pdf (accessed on 1 June 2025).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0, 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 1 June 2025).
- Żelaszczyk, D.; Chmiel, A.; Gunia-Krzyżak, A.; Marona, H.; Krzyżek, P.; Dworak, K.; Skiba-Kurek, I.; Karczewska, E.; Popiół, J.; Pękala, E.; et al. Antibacterial and antibiofilm agents in the group of xanthone derivatives with piperazine moiety active against drug-resistant Helicobacter pylori strains. Bioorg. Chem. 2024, 153, 107755. [Google Scholar] [CrossRef]
- Klesiewicz, K.; Karczewska, E.; Nowak, P.; Skiba-Kurek, I.; Sito, E.; Pańczyk, K.; Koczurkiewicz, P.; Żelaszczyk, D.; Pękala, E.; Waszkielewicz, A.M.; et al. Anti-Helicobacter pylori activities of selected N-substituted cinnamamide derivatives evaluated on reference and clinical bacterial strains. J. Antibiot. 2018, 71, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Canale, V.; Trybała, W.; Chaumont-Dubel, S.; Koczurkiewicz-Adamczyk, P.; Satała, G.; Bento, O.; Blicharz-Futera, K.; Bantreil, X.; Pękala, E.; Bojarski, A.J.; et al. 1-(Arylsulfonyl-isoindol-2-yl)piperazines as 5-HT6R Antagonists: Mechanochemical Synthesis, In Vitro Pharmacological Properties and Glioprotective Activity. Biomolecules 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Y.; Richter, B.; Malhotra, R.; Vossough-Teehan, S. Treating Helicobacter pylori and Recurrent Clostridioides difficile Coinfection: A Delicate Balance in Management and a Need for Guidelines. ACG Case Rep. J. 2024, 11, e01369. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Paluch, E.; Gościniak, G. Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics 2020, 9, 658. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Jenkins, S.G.; Schuetz, A.N. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012, 87, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI M100; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2022. [Google Scholar]
- Canale, V.; Czekajewska, J.; Klesiewicz, K.; Papież, M.; Kuziak, A.; Witek, K.; Piska, K.; Niemiec, D.; Kasza, P.; Pękala, E.; et al. Design and synthesis of novel arylurea derivatives of aryloxy(1-phenylpropyl) alicyclic diamines with antimicrobial activity against multidrug-resistant Gram-positive bacteria. Eur. J. Med. Chem. 2023, 251, 115224. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Qin, S.; Yan, D.; Shen, B.; Zhang, T.; Wang, M.; Li, S.; Ampomah-Wireko, M.; Bai, M.; Zhang, E.; et al. Development of Aromatic-Linked Diamino Acid Antimicrobial Peptide Mimics with Low Hemolytic Toxicity and Excellent Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2023, 66, 7756–7771. [Google Scholar] [CrossRef]
- Singh, J.K.; Solanki, A. Comparative In-Vitro Intrinsic Clearance of Imipramine in Multiple Species Liver Microsomes: Human, Rat, Mouse and Dog. J. Drug Metab. Toxicol. 2012, 3, 126. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Ottemann, K.M. Helicobacter pylori Biofilm Involves a Multigene Stress-Biased Response, Including a Structural Role for Flagella. mBio 2018, 9, e01973-18. [Google Scholar] [CrossRef]

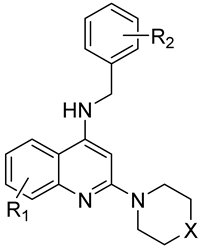 | ||||||||
| Cmpd | R1 | R2 | X | MIC [µg/mL] a | Mean MIC [µg/mL] | |||
| ATCC 43504 b | ATCC 700684 c | J99 d | 3CML e | |||||
| 9a | H | 3-CH3 | NH | 8 | 16 | 8 | 16 | 12 |
| 9b | H | 2-Cl | NH | 4 | 16 | 4 | 16 | 10 |
| 9c | H | 3-Cl | NH | 4 | 4 | 2 | 4 | 3.5 |
| 9d | H | 4-Cl | NH | 4 | 16 | 4 | 16 | 10 |
| 9e | 7-OCH3 | 3-Cl | NH | 4 | 16 | 8 | 16 | 11 |
| 9f | 8-OCH3 | 3-Cl | NH | 4 | 16 | 4 | 16 | 10 |
| 10 | H | 3-Cl | O | 4 | 16 | 4 | 16 | 10 |
| MTZ f | 16 | 4 | 1 | 16 | 9.25 | |||
| QPZ g | 16 | nth | >16 | nt h | - | |||
| H. pylori Strain | MBC [µg/mL] | MIC [µg/mL] | MBC/MIC a Ratio |
|---|---|---|---|
| ATCC 43504 b | >32 | 4 | >8 |
| ATCC 700684 c | 32 | 4 | 8 |
| J99 d | 16 | 2 | 8 |
| Compd | MIC [µg/mL] a | ||||
|---|---|---|---|---|---|
| E. coli ATCC 25922 | S. aureus ATCC 25923 | S. aureus ATCC 29213 | E. faecalis ATCC 29212 | L. paracasei BAA 52 | |
| 9c | 16 | >32 | >32 | >32 | 32 |
| Gentamycin | 0.5 | 1 | 0.5 | 8 | 16 |
| Compd | MIC [µg/mL] a | |
|---|---|---|
| ATCC 700684 b (Resistant to CH) | 3CML c | |
| CH d | 16 | 16 |
| MTZ e | 2 | 128 |
| 9c | 4 | 4 |
| CH + 9c (MIC) | 1 | 2 |
| CH + 9c (1/2 × MIC) | 4 | 4 |
| CH + 9c (1/4 × MIC) | 16 | 16 |
| MTZ + 9c (MIC) | nt | 8 |
| MTZ + 9c (1/2 × MIC) | nt | 16 |
| MTZ + 9c (1/4 × MIC) | nt | 64–128 |
| Tested Compounds | ATCC 700684 a | |
|---|---|---|
| FIC b | Interpretation [24] | |
| CH c + 9c (MIC) | 1.0625 | Indifference |
| CH + 9c (1/2 × MIC) | 0.75 | Additive |
| Tested Compounds | 3CML a | |
|---|---|---|
| FIC b | Interpretation [24] | |
| CH c + 9c (MIC) | 0.625 | Additive |
| CH + 9c (1/2 × MIC) | 0.75 | Additive |
| MTZ d + 9c (MIC) | 1.0625 | Indifference |
| MTZ + 9c (1/2 × MIC) | 0.625 | Additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grychowska, K.; Klesiewicz, K.; Pęgiel, J.; Kuziak, A.; Skiba-Kurek, I.; Canale, V.; Krzysiek-Mączka, G.; Ptak-Belowska, A.; Piska, K.; Koczurkiewicz-Adamczyk, P.; et al. New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm. Int. J. Mol. Sci. 2025, 26, 5997. https://doi.org/10.3390/ijms26135997
Grychowska K, Klesiewicz K, Pęgiel J, Kuziak A, Skiba-Kurek I, Canale V, Krzysiek-Mączka G, Ptak-Belowska A, Piska K, Koczurkiewicz-Adamczyk P, et al. New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm. International Journal of Molecular Sciences. 2025; 26(13):5997. https://doi.org/10.3390/ijms26135997
Chicago/Turabian StyleGrychowska, Katarzyna, Karolina Klesiewicz, Joanna Pęgiel, Agata Kuziak, Iwona Skiba-Kurek, Vittorio Canale, Gracjana Krzysiek-Mączka, Agata Ptak-Belowska, Kamil Piska, Paulina Koczurkiewicz-Adamczyk, and et al. 2025. "New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm" International Journal of Molecular Sciences 26, no. 13: 5997. https://doi.org/10.3390/ijms26135997
APA StyleGrychowska, K., Klesiewicz, K., Pęgiel, J., Kuziak, A., Skiba-Kurek, I., Canale, V., Krzysiek-Mączka, G., Ptak-Belowska, A., Piska, K., Koczurkiewicz-Adamczyk, P., Krzyżek, P., Brzozowski, T., Zajdel, P., & Karczewska, E. (2025). New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm. International Journal of Molecular Sciences, 26(13), 5997. https://doi.org/10.3390/ijms26135997









