Prognostic Significance of Plasma Short-Chain Fatty Acid Levels in Assessing Mortality Risk in Patients with Chronic Heart Failure and Sarcopenia
Abstract
1. Introduction
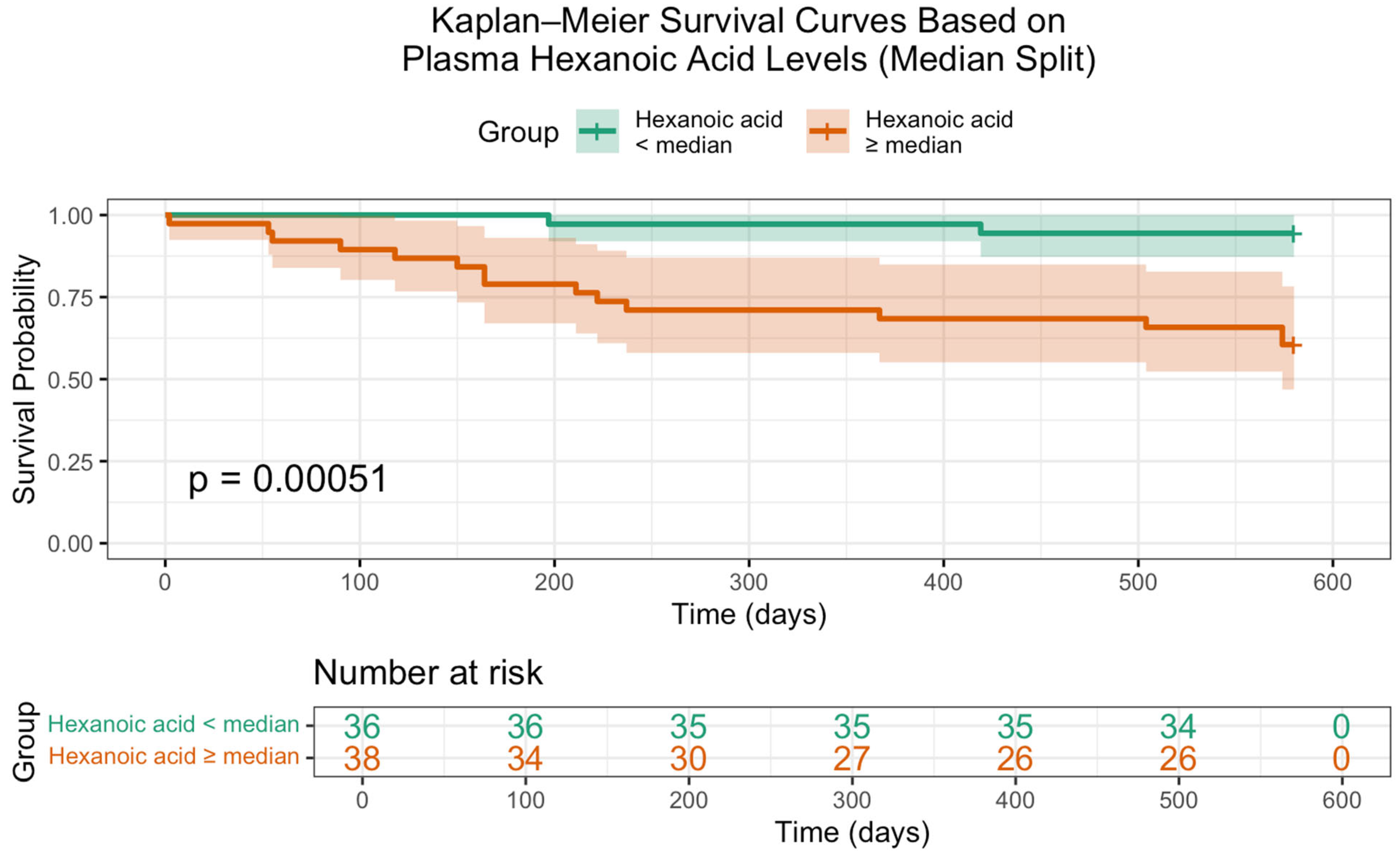
2. Results
- Arterial hypertension—81%;
- Type 2 diabetes mellitus—36%;
- Chronic kidney disease—42%;
- Coronary artery disease—67%;
- Atrial fibrillation—28%;
- Post-infarction cardiosclerosis—32%.
3. Discussion
4. Materials and Methods
- -
- Propanoic acid (C3): 180.05 → 91.05 ng/mL;
- -
- Butanoic acid (C4) and isobutyric acid (iC4): 194.10 → 91.05 ng/mL;
- -
- Pentanoic acid (C5), 2-methylbutanoic acid (αC5), and 3-methylbutanoic acid (βC5): 208.15 → 91.05 ng/mL;
- -
- 4-Methylpentanoic acid (iC6) and hexanoic acid (C6): 222.10 → 91.05 ng/mL.
Statistical Analysis
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thing, M.; Werge, M.P.; Kimer, N.; Hetland, L.E.; Rashu, E.B.; Nabilou, P.; Junker, A.E.; Galsgaard, E.D.; Bendtsen, F.; Laupsa-Borge, J.; et al. Targeted metabolomics reveals plasma short-chain fatty acids are associated with metabolic dysfunction-associated steatotic liver disease. BMC Gastroenterol. 2024, 24, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Takaya, H.; Araki, Y.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Medium- and long-chain fatty acids differentially modulate interleukin-8 secretion in human fetal intestinal epithelial cells. J. Nutr. 2000, 130, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Sekine, S.; Kojima, K.; Aoyama, T. The application of medium-chain fatty acids: Edible oil with a suppressing effect on body fat accumulation. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 320–323. [Google Scholar] [PubMed]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829, Erratum in Immunity 2016, 44, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short. Chain. Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E (2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sam, Q.H.; Ling, H.; Yew, W.S.; Tan, Z.; Ravikumar, S.; Chang, M.W.; Chai, L.Y.A. The Divergent Immunomodulatory Effects of Short Chain Fatty Acids and Medium Chain Fatty Acids. Int. J. Mol. Sci. 2021, 22, 6453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferwerda, B.; McCall, M.B.; Alonso, S.; Giamarellos-Bourboulis, E.J.; Mouktaroudi, M.; Izagirre, N.; Syafruddin, D.; Kibiki, G.; Cristea, T.; Hijmans, A.; et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. USA 2007, 104, 16645–16650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas FJr Sumegi, B.; Veres, B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, D.S.; Madrenas, J. Evolving Bacterial Envelopes and Plasticity of TLR2-Dependent Responses: Basic Research and Translational Opportunities. Front. Immunol. 2013, 4, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, M.; Takaishi, S.; Nagasaki, M.; Onozawa, Y.; Iino, I.; Maeda, H.; Komai, T.; Oda, T. Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor. J. Biol. Chem. 2013, 288, 10684–10691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Downes, N.L.; Laham-Karam, N.; Kaikkonen, M.U.; Ylä-Herttuala, S. Differential but Complementary HIF1α and HIF2α Transcriptional Regulation. Mol. Ther. 2018, 26, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rankin, E.B.; Rha, J.; Selak, M.A.; Unger, T.L.; Keith, B.; Liu, Q.; Haase, V.H. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol. 2009, 29, 4527–4538. [Google Scholar] [CrossRef] [PubMed Central]
- Di Marca, S.; Rando, A.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Terranova, V.; Corriere, T.; Pisano, M.; Di Quattro, R.; Ronsisvalle, M.L.; et al. B-type natriuretic peptide may predict prognosis in older adults admitted with a diagnosis other than heart failure. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 60 years | Presence of electronic medical devices |
| Confirmed diagnosis of chronic heart failure (CHF) | Any severe, decompensated, or unstable somatic diseases or conditions that, in the opinion of the researcher, threaten the life of the patient or worsen the prognosis of the disease (including anemia, autoimmune, endocrine, oncological diseases, hepatitis, connective tissue diseases, etc.). |
| Confirmed diagnosis of sarcopenia | Severe congenital or acquired musculoskeletal disorders |
| Signed informed consent for participation in the clinical study | Presence of concomitant neurosurgical or neurological pathology |
| Abuse of alcohol, drugs, narcotics | |
| Mental illness, incapacity | |
| Glomerular filtration rate < 45 mL/min |
| Parameter | Mean ± SD / Median (IQR) | Units |
|---|---|---|
| White blood cells (WBC) | 6.79 ± 1.89 | ×109/L |
| –Lymphocytes (absolute count) | 1.88 ± 0.74 | ×109/L |
| –Neutrophils (absolute count) | 4.15 ± 1.21 | ×109/L |
| –Monocytes (absolute count) | 0.60 ± 1.03 | ×109/L |
| Platelets (PLT) | 225 ± 72 | ×109/L |
| High-sensitivity C-reactive protein (hs-CRP) | 5.8 ± 2.4 | mg/L |
| Neutrophil-to-lymphocyte ratio (NLR) | 1.85 (1.50–2.64) | — |
| Systemic immune-inflammation index (SII) | 419 (288–634) | — |
| Platelet-to-lymphocyte ratio (PLR) | 121 (96–163) | — |
| Monocyte-to-lymphocyte ratio (MLR) | 0.27 (0.21–0.33) | — |
| Lymphocyte-to-monocyte ratio (LMR) | 3.72 (3.00–4.68) | — |
| SCFA | Minimum | 5th Percentile | 25th Percentile | Median | 75th Percentile | 95th Percentile | Maximum |
|---|---|---|---|---|---|---|---|
| Butanoic acid | 6370 | 7860.5 | 9752.5 | 12,300 | 14,500 | 22,735 | 45,000 |
| Propanoic acid | 1980 | 2113.0 | 2815.0 | 3745 | 6110.0 | 8419.5 | 17,900 |
| Isobutyric acid | 549 | 606.2 | 937.8 | 1860 | 10,800 | 18,085 | 76,205 |
| 2-Methylbutanoic acid | 197 | 231.9 | 419.5 | 1655 | 7947.5 | 14,090 | 27,000 |
| 3-Methylbutanoic acid | 93.5 | 138.6 | 234.2 | 638.5 | 12,725 | 23,595 | 29,000 |
| Hexanoic acid | 206 | 243.1 | 455.0 | 622.5 | 1285.0 | 2659.0 | 4060 |
| Pentanoic acid | 105 | 203.9 | 278.8 | 376.5 | 491.2 | 832.0 | 1253 |
| 4-Methylpentanoic acid | 52.7 | 58.8 | 97.5 | 114.6 | 145.4 | 267.6 | 390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, A.V.; Dragunov, D.O.; Klimova, A.V.; Golubev, Y.V.; Shmigol, T.A.; Negrebetsky, V.V.; Arutyunov, G.P. Prognostic Significance of Plasma Short-Chain Fatty Acid Levels in Assessing Mortality Risk in Patients with Chronic Heart Failure and Sarcopenia. Int. J. Mol. Sci. 2025, 26, 5984. https://doi.org/10.3390/ijms26135984
Sokolova AV, Dragunov DO, Klimova AV, Golubev YV, Shmigol TA, Negrebetsky VV, Arutyunov GP. Prognostic Significance of Plasma Short-Chain Fatty Acid Levels in Assessing Mortality Risk in Patients with Chronic Heart Failure and Sarcopenia. International Journal of Molecular Sciences. 2025; 26(13):5984. https://doi.org/10.3390/ijms26135984
Chicago/Turabian StyleSokolova, Anna V., Dmitrii O. Dragunov, Anastasiya V. Klimova, Yaroslav V. Golubev, Tatiana A. Shmigol, Vadim V. Negrebetsky, and Gregory P. Arutyunov. 2025. "Prognostic Significance of Plasma Short-Chain Fatty Acid Levels in Assessing Mortality Risk in Patients with Chronic Heart Failure and Sarcopenia" International Journal of Molecular Sciences 26, no. 13: 5984. https://doi.org/10.3390/ijms26135984
APA StyleSokolova, A. V., Dragunov, D. O., Klimova, A. V., Golubev, Y. V., Shmigol, T. A., Negrebetsky, V. V., & Arutyunov, G. P. (2025). Prognostic Significance of Plasma Short-Chain Fatty Acid Levels in Assessing Mortality Risk in Patients with Chronic Heart Failure and Sarcopenia. International Journal of Molecular Sciences, 26(13), 5984. https://doi.org/10.3390/ijms26135984







