Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction
Abstract
1. Introduction
2. Results
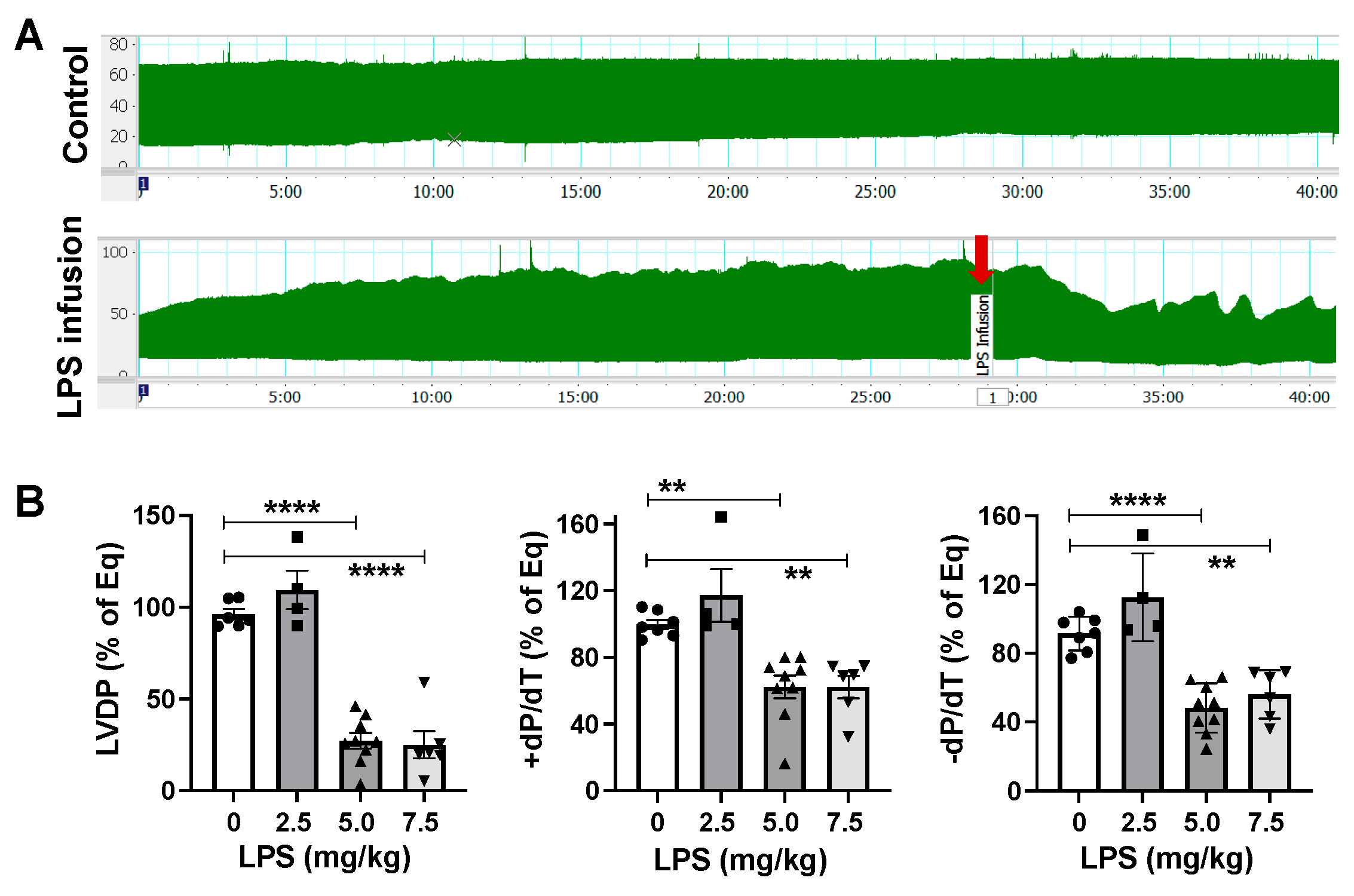
2.1. LPS Directly Induces Cardiac Functional Depression
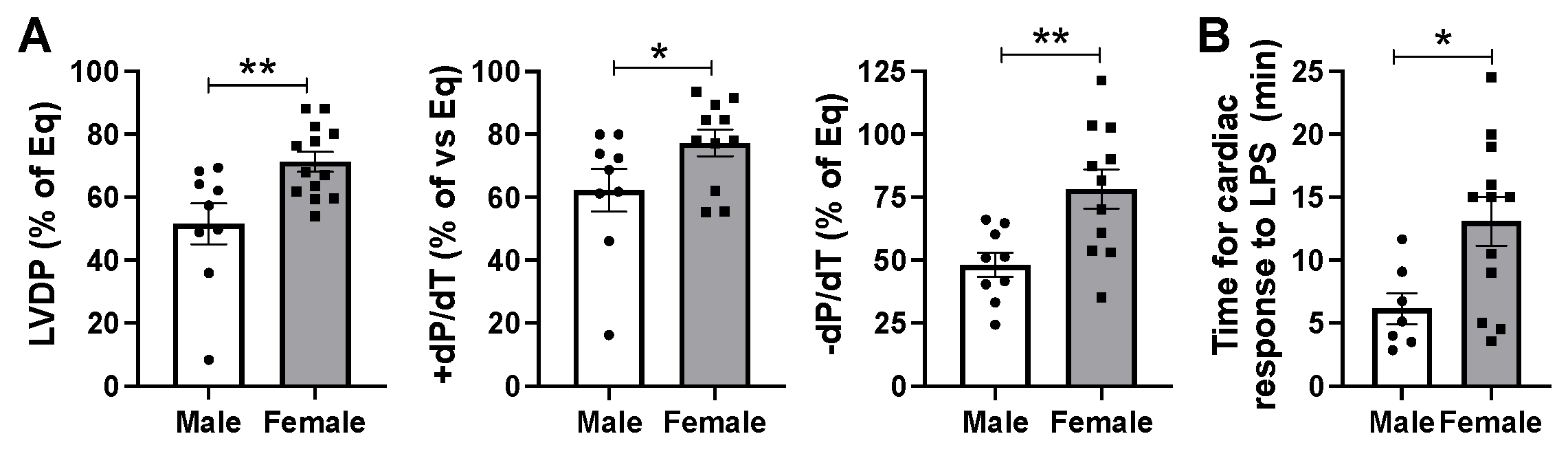
2.2. Male Hearts Experienced Greater Depression of Myocardial Function Compared to Female Hearts in Response to Infusion of LPS at 5 mg/kg Body Weight
2.3. Effects of Estrous Cycle on LPS Caused Myocardial Dysfunction in Females and LPS’ Role in Modulating Expression of Estrogen Receptors in Male and Female Hearts
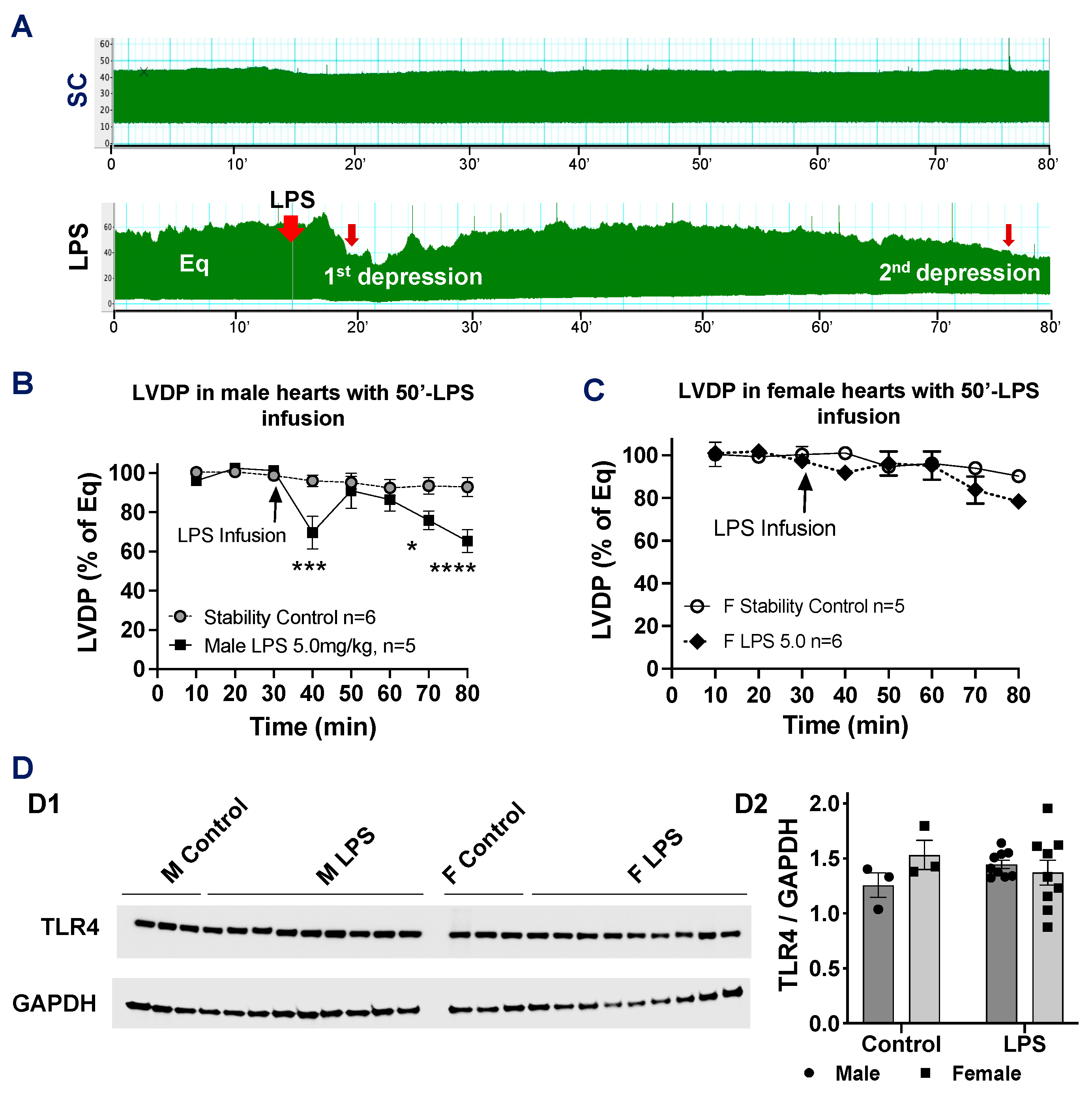
2.4. Effects of Longer LPS Infusion on Isolated Male and Female Heart Function and on Myocardial Expression of Toll-like Receptor 4
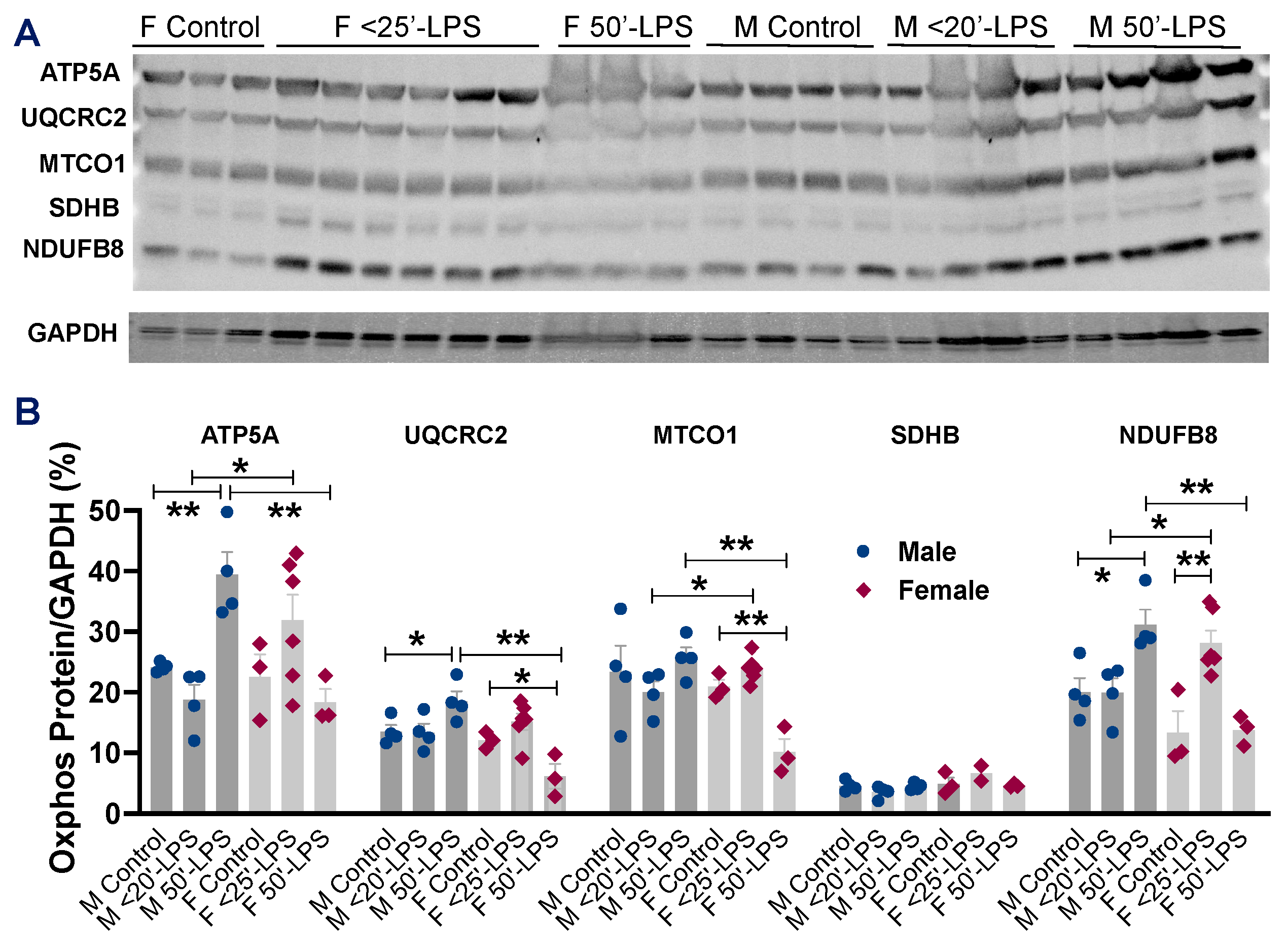
2.5. Direct Effects of LPS on Myocardial Mitochondrial OXPHOS Between Male and Female Hearts
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Assessment of Cardiac Function Using Langendorff Method
4.3. Experimental Protocols
4.4. Vaginal Smear
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| LVDP | left ventricular developed pressure |
| OXPHOS | oxidative phosphorylation |
| +/− dP/dt | positive and negative values of the first derivative of pressure |
| ROS | reactive oxygen species |
| TLR4 | Toll-like receptor 4 |
| TNFα | tumor necrosis factor α |
References
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Landesberg, G.; Benedetto, U.; Foex, P.; Cecconi, M. Diastolic dysfunction and mortality in septic patients: A systematic review and meta-analysis. Intensive Care Med. 2015, 41, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chiazza, F.; Collino, M.; Patel, N.S.; Coldewey, S.M.; Thiemermann, C. Gender dimorphism of the cardiac dysfunction in murine sepsis: Signalling mechanisms and age-dependency. PLoS ONE 2014, 9, e100631. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.P.; Guarido, K.L.; Assreuy, J.; da Silva-Santos, J.E. Gender-specific differences in the in situ cardiac function of endotoxemic rats detected by pressure-volume catheter. Shock 2014, 42, 415–423. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.; Tsai, B.; Wang, J.F.; Turrentine, M.; Brown, J.W.; Meldrum, D.R. Preconditioning up-regulates the soluble TNF receptor I response to endotoxin. J. Surg. Res. 2004, 121, 20–24. [Google Scholar] [CrossRef]
- El-Lakany, M.A.; Fouda, M.A.; El-Gowelli, H.M.; El-Gowilly, S.M.; El-Mas, M.M. Gonadal hormone receptors underlie the resistance of female rats to inflammatory and cardiovascular complications of endotoxemia. Eur. J. Pharmacol. 2018, 823, 41–48. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, L.; Peng, T. Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J. Mol. Cell. Cardiol. 2009, 47, 264–274. [Google Scholar] [CrossRef]
- Wang, M.; Tsai, B.M.; Crisostomo, P.R.; Meldrum, D.R. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation 2006, 114, I282–I289. [Google Scholar] [CrossRef]
- Sando, I.C.; Wang, Y.; Crisostomo, P.R.; Markel, T.A.; Sharma, R.; Erwin, G.S.; Guzman, M.J.; Meldrum, D.R.; Wang, M. Females exhibit relative resistance to depressive effects of tumor necrosis factor-alpha on the myocardium. J. Surg. Res. 2008, 150, 92–99. [Google Scholar] [CrossRef]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.E.; Wagner, J.E. The Biology and Medicine of Rabbits and Rodents, 3rd ed.; Lea & Febiger: Philadelphia, PA, USA, 1989; p. 230. [Google Scholar]
- Sun, Y.; Yao, X.; Zhang, Q.J.; Zhu, M.; Liu, Z.P.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.A.; Sun, Y.; et al. Beclin-1-Dependent Autophagy Protects the Heart During Sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Zang, Q.S.; Martinez, B.; Yao, X.; Maass, D.L.; Ma, L.; Wolf, S.E.; Minei, J.P. Sepsis-induced cardiac mitochondrial dysfunction involves altered mitochondrial-localization of tyrosine kinase Src and tyrosine phosphatase SHP2. PLoS ONE 2012, 7, e43424. [Google Scholar] [CrossRef]
- Frantz, S.; Kobzik, L.; Kim, Y.D.; Fukazawa, R.; Medzhitov, R.; Lee, R.T.; Kelly, R.A. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Investig. 1999, 104, 271–280. [Google Scholar] [CrossRef]
- Avlas, O.; Fallach, R.; Shainberg, A.; Porat, E.; Hochhauser, E. Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid. Redox Signal. 2011, 15, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, M.; Zhou, F.; Cao, W.; Bi, L.; Xie, Y.; Yang, Q.; Wang, S. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: The regulation of autophagy and ROS production. J. Mol. Cell. Cardiol. 2016, 101, 11–24. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Jiang, X.; Lv, J.; Li, Y.; Ye, H.; Liu, W.; Wang, G.; Zhang, C.; Zheng, N.; et al. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca2+ leakage promote cardiac contractile dysfunction in sepsis. J. Biol. Chem. 2018, 293, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Tavener, S.A.; Long, E.M.; Robbins, S.M.; McRae, K.M.; Van Remmen, H.; Kubes, P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ. Res. 2004, 95, 700–707. [Google Scholar] [CrossRef]
- Fallach, R.; Shainberg, A.; Avlas, O.; Fainblut, M.; Chepurko, Y.; Porat, E.; Hochhauser, E. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J. Mol. Cell. Cardiol. 2010, 48, 1236–1244. [Google Scholar] [CrossRef]
- Wang, M.; Sankula, R.; Tsai, B.M.; Meldrum, K.K.; Turrentine, M.; March, K.L.; Brown, J.W.; Dinarello, C.A.; Meldrum, D.R. P38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock 2004, 21, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Brown, J.M.; Ao, L.; Shames, B.D.; Banerjee, A.; Harken, A.H. Reduction of infarct size in the rat heart by LPS preconditioning is associated with expression of angiogenic growth factors and increased capillary density. Shock 1999, 12, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Sato, C.; Mizoguchi, K.; Yamashita, Y.; Oe, M.; Maeta, H. Lipopolysaccharide triggers late preconditioning against myocardial infarction via inducible nitric oxide synthase. Cardiovasc. Res. 2002, 56, 33–42. [Google Scholar] [CrossRef]
- Fan, M.H.; Wong, K.L.; Wu, S.; Leung, W.K.; Yam, W.C.; Wong, T.M. Preconditioning with Porphyromonas gingivalis lipopolysaccharide may confer cardioprotection and improve recovery of the electrically induced intracellular calcium transient during ischemia and reperfusion. J. Periodontal Res. 2010, 45, 100–108. [Google Scholar] [CrossRef][Green Version]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Dombrovskiy, V.Y.; Martin, A.A.; Sunderram, J.; Paz, H.L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit. Care Med. 2007, 35, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Elia, C.; Mascia, L.; Barberis, B.; Cardellino, S.; Livigni, S.; Fiore, G.; Filippini, C.; Ranieri, V.M. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit. Care 2013, 17, R50. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- El-Lakany, M.A.; Fouda, M.A.; El-Gowelli, H.M.; El-Mas, M.M. Ovariectomy provokes inflammatory and cardiovascular effects of endotoxemia in rats: Dissimilar benefits of hormonal supplements. Toxicol. Appl. Pharmacol. 2020, 393, 114928. [Google Scholar] [CrossRef]
- Farrell, S.R.; Ross, J.L.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H36–H45. [Google Scholar] [CrossRef]
- Trexler, C.L.; Odell, A.T.; Jeong, M.Y.; Dowell, R.D.; Leinwand, L.A. Transcriptome and Functional Profile of Cardiac Myocytes Is Influenced by Biological Sex. Circ. Cardiovasc. Genet. 2017, 10, e001770. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, G.; Duchen, M.R.; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 759–773. [Google Scholar] [CrossRef]
- Fukumoto, K.; Pierro, A.; Spitz, L.; Eaton, S. Cardiac and renal mitochondria respond differently to hydrogen peroxide in suckling rats. J. Surg. Res. 2003, 113, 146–150. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Staniek, K.; Haindl, S.; Piskernik, C.; Ohlinger, W.; Gille, L.; Nohl, H.; Bahrami, S.; Redl, H. Different effects of endotoxic shock on the respiratory function of liver and heart mitochondria in rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G543–G549. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Opalka, J.R.; Neuhof, C.; Zierz, S.; Gellerich, F.N. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur. J. Biochem. 2001, 268, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Lagranha, C.J.; Deschamps, A.; Aponte, A.; Steenbergen, C.; Murphy, E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010, 106, 1681–1691. [Google Scholar] [CrossRef]
- Colom, B.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc. Res. 2007, 74, 456–465. [Google Scholar] [CrossRef]
- Khalifa, A.R.; Abdel-Rahman, E.A.; Mahmoud, A.M.; Ali, M.H.; Noureldin, M.; Saber, S.H.; Mohsen, M.; Ali, S.S. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol. Rep. 2017, 5, e13125. [Google Scholar] [CrossRef]
- Scott, S.R.; Singh, K.; Yu, Q.; Sen, C.K.; Wang, M. Sex as Biological Variable in Cardiac Mitochondrial Bioenergetic Responses to Acute Stress. Int. J. Mol. Sci. 2022, 23, 9312. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Losantos-Ramos, K.; Gonzales, M.; Furrer, D.; Soliz, J. NADH-linked mitochondrial respiration in the developing mouse brain is sex-, age- and tissue-dependent. Respir. Physiol. Neurobiol. 2019, 266, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, I.; Yoval-Sanchez, B.; Konrad, C.; Manfredi, G.; Wittig, I.; Galkin, A. Sex-dependent differences in macaque brain mitochondria. Biochim. Biophys. Acta Bioenerg. 2024, 1865, 149494. [Google Scholar] [CrossRef] [PubMed]
- Guevara, R.; Santandreu, F.M.; Valle, A.; Gianotti, M.; Oliver, J.; Roca, P. Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free. Radic. Biol. Med. 2009, 46, 169–175. [Google Scholar] [CrossRef]
- Odendaal-Gambrell, C.P.; O’Brien, C.; Cairns, M.; Maarman, G.J.; Joseph, D.E.; Smith, C.; Rautenbach, F.; Marnewick, J.L.; Essop, M.F. Chronic stress elicits sex-specific mitochondrial respiratory functional changes in the rat heart. Physiol. Rep. 2025, 13, e70371. [Google Scholar] [CrossRef]
- Colom, B.; Alcolea, M.P.; Valle, A.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell. Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [CrossRef]
- Wang, L.; Gu, H.; Turrentine, M.; Wang, M. Estradiol treatment promotes cardiac stem cell (CSC)-derived growth factors, thus improving CSC-mediated cardioprotection after acute ischemia/reperfusion. Surgery 2014, 156, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Smith, K.; Yu, Q.; Miller, C.; Singh, K.; Sen, C.K. Mitochondrial connexin 43 in sex-dependent myocardial responses and estrogen-mediated cardiac protection following acute ischemia/reperfusion injury. Basic Res. Cardiol. 2019, 115, 1. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, B.I.; Yoniles, A.M.; Monsivais, A.; Du, J.; Zadorozny, L.; Yu, Q.; Wang, M. Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction. Int. J. Mol. Sci. 2025, 26, 5963. https://doi.org/10.3390/ijms26135963
Harvey BI, Yoniles AM, Monsivais A, Du J, Zadorozny L, Yu Q, Wang M. Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction. International Journal of Molecular Sciences. 2025; 26(13):5963. https://doi.org/10.3390/ijms26135963
Chicago/Turabian StyleHarvey, Brianna I., Arris M. Yoniles, Andrea Monsivais, Jiayue Du, Lauren Zadorozny, Qing Yu, and Meijing Wang. 2025. "Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction" International Journal of Molecular Sciences 26, no. 13: 5963. https://doi.org/10.3390/ijms26135963
APA StyleHarvey, B. I., Yoniles, A. M., Monsivais, A., Du, J., Zadorozny, L., Yu, Q., & Wang, M. (2025). Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction. International Journal of Molecular Sciences, 26(13), 5963. https://doi.org/10.3390/ijms26135963





