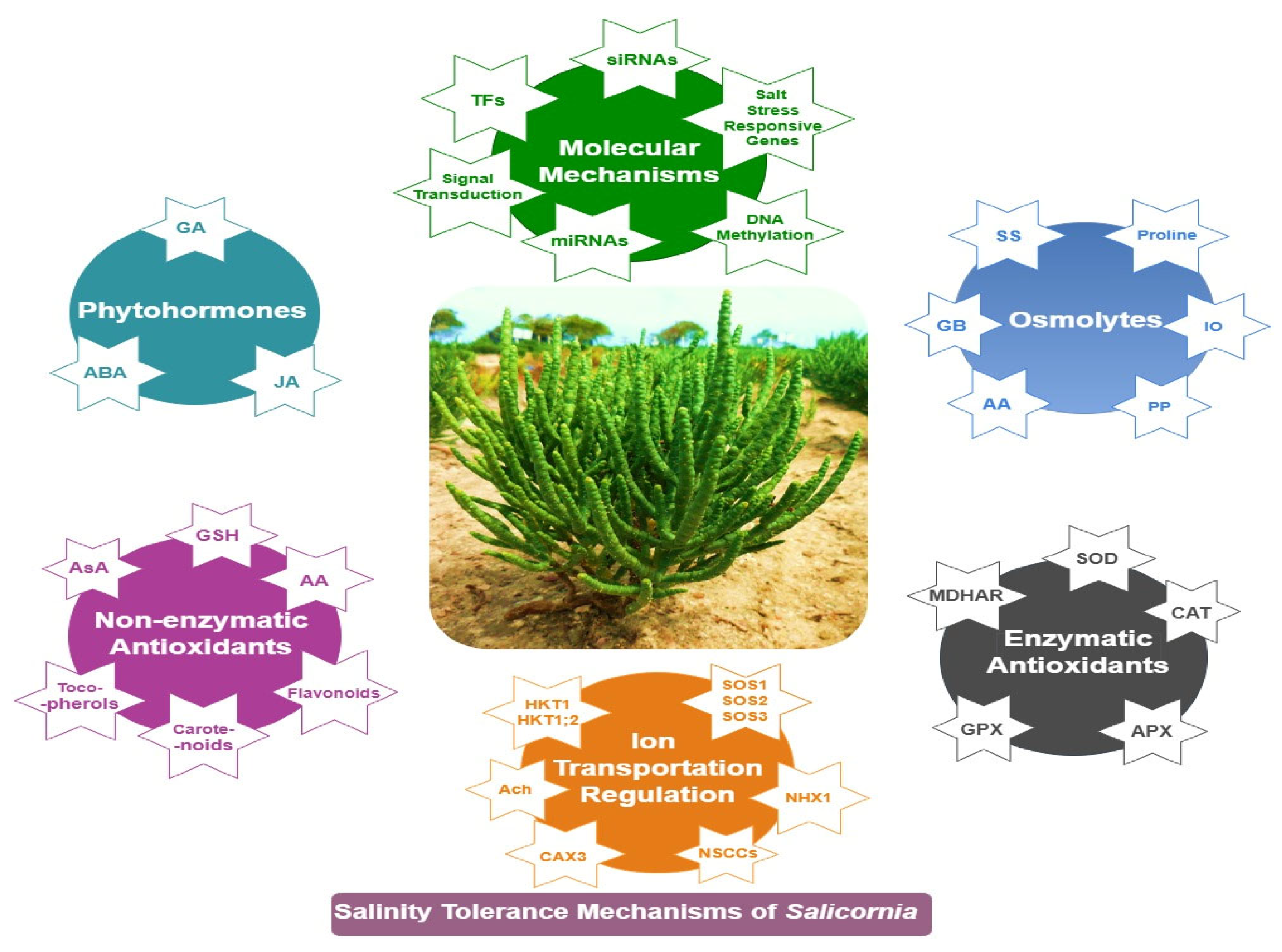
Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance
Abstract
1. Introduction
2. Cell Wall Nano-Mechanics
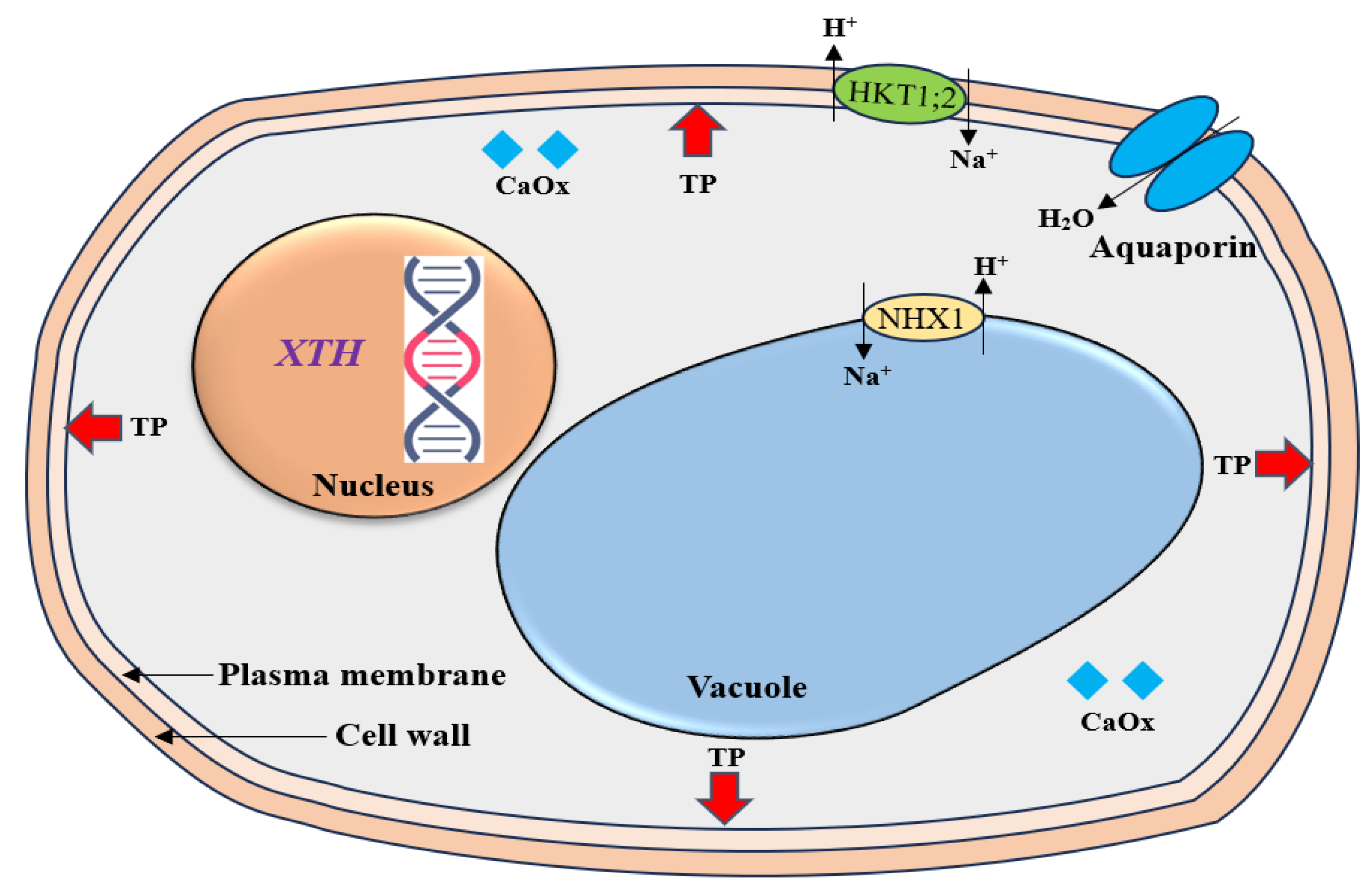
3. Ion Transport Regulation and Compartmentalization
4. Antioxidant Defense
4.1. Reactive Chemical Species and Their Crosstalk
4.2. Enzymatic Antioxidants
4.3. Non-Enzymatic Antioxidants
5. Maintenance of Osmotic Balance
6. Phytohormonal Regulation
7. Signal Transducing Cascades
8. Transcriptional and Post Transcriptional Regulation
9. Salt-Responsive Proteins
10. Crosstalk and Interactions Among Salinity Tolerance Mechanisms
11. Cross-Tolerance of Salicornia to Abiotic Stresses
12. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Gorokhova, I.N.; Pankova, E.I. Organizational Problems of Soil Salinization Monitoring on Irrigated Lands. Arid Ecosyst. 2024, 14, 17–24. [Google Scholar] [CrossRef]
- Ramos, T.B.; Gonçalves, M.C.; Van Genuchten, M.T. Soil Salinization in Portugal: An In-depth Exploration of Impact, Advancements, and Future Considerations. Vadose Zone J. 2024, 23, e20314. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Jiapaer, G.; Wu, G.; Li, Q.; Yang, J. Monitoring the Salinization of Agricultural Land and Assessing Its Drivers in the Altay Region. Ecol. Indic. 2024, 167, 112678. [Google Scholar] [CrossRef]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-Scale Soil Salinization Dynamics From Global to Pore Scale: A Review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Velilla, E.; Snethlage, J.; Poelman, M.; Van Der Meer, I.M.; Van Der Werf, A.; Deolu-Ajayi, A.O.; Van Belzen, J. Too Salty to Farm: Rethinking Coastal Land Use in Response to Soil Salinization. Restor. Ecol. 2025, 33, e70006. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Decsi, K. The Impact of Salinity on Crop Yields and the Confrontational Behavior of Transcriptional Regulators, Nanoparticles, and Antioxidant Defensive Mechanisms under Stressful Conditions: A Review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving Salinity Tolerance of Plants through Conventional Breeding and Genetic Engineering: An Analytical Comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamed, K.; Castagna, A.; Ranieri, A.; García-Caparrós, P.; Santin, M.; Hernandez, J.A.; Espin, G.B. Halophyte Based Mediterranean Agriculture in the Contexts of Food Insecurity and Global Climate Change. Environ. Exp. Bot. 2021, 191, 104601. [Google Scholar] [CrossRef]
- Robertson, S.M.; Lyra, D.A.; Mateo-Sagasta, J.; Ismail, S.; Akhtar, M.J.U. Financial Analysis of Halophyte Cultivation in a Desert Environment Using Different Saline Water Resources for Irrigation. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Singapore, 2019; pp. 347–364. [Google Scholar]
- Cheeseman, J.M. The Evolution of Halophytes. Glycophytes and Crops, and Its Implications for Food Security under Saline Conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Bostan, N.; Nabgha, A.; Maria, M.; Safdar, W. A Critical Review on Halophytes: Salt Tolerant Plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.d.M.; Bhuyan, M.H.M.B.; Oku, H.; Fujita, M. Exogenous Nitric Oxide Pretreatment Protects Brassica Napus L. Seedlings from Paraquat Toxicity through the Modulation of Antioxidant Defense and Glyoxalase Systems. Plant Physiol. Biochem. 2018, 126, 173–186. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Definition and Classification of Halophytes. In Anatomical Adaptations of Halophytes; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–28. ISBN 978-3-319-66479-8. [Google Scholar]
- Grigore, M.-N.; Toma, C. Morphological and Anatomical Adaptations of Halophytes: A Review. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–143. ISBN 978-3-030-17854-3. [Google Scholar]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wei, X.; Fan, H.; Wang, B. Adaptation Mechanism of Salt Excluders under Saline Conditions and Its Applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef]
- Lu, C.; Yuan, F.; Guo, J.; Han, G.; Wang, C.; Chen, M.; Wang, B. Current Understanding of Role of Vesicular Transport in Salt Secretion by Salt Glands in Recretohalophytes. Int. J. Mol. Sci. 2021, 22, 2203. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Patel, M.K.; Mishra, A.; Tiwari, V.; Jha, B. The SbMT-2 Gene from a Halophyte Confers Abiotic Stress Tolerance and Modulates ROS Scavenging in Transgenic Tobacco. PLoS ONE 2014, 9, e111379. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, N.S.; Tiwari, V.; Agarwal, P.K.; Jha, B. A SNARE-Like Superfamily Protein SbSLSP from the Halophyte Salicornia brachiata Confers Salt and Drought Tolerance by Maintaining Membrane Stability, K+/Na+ Ratio, and Antioxidant Machinery. Front. Plant Sci. 2016, 7, 737. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Jha, B. Engineering of a Novel Gene from a Halophyte: Potential for Agriculture in Degraded Coastal Saline Soil. Land Degrad. Dev. 2019, 30, 595–607. [Google Scholar] [CrossRef]
- Udawat, P.; Mishra, A.; Jha, B. Heterologous Expression of an Uncharacterized Universal Stress Protein Gene (SbUSP) from the Extreme Halophyte, Salicornia brachiata, Which Confers Salt and Osmotic Tolerance to E. Coli. Gene 2014, 536, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Pérez., S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Siridewa, K.; De Silva, W.; Ratnayake, R.M.C.S.; Wijesundara, S.; Perera, D.; Attanayake, R.N. Species Identification and Pollination Biology of an Economically Important True Halophyte, Salicornia Brachiata Roxb. Aquat. Bot. 2025, 196, 103827. [Google Scholar] [CrossRef]
- Rivers, W.G.; Weber, D.J. The Influence of Salinity and Temperature on Seed Germination in Salicornia Bigelovii. Physiol. Plant. 1971, 24, 73–75. [Google Scholar] [CrossRef]
- García-Galindo, E.; Nieto-Garibay, A.; Troyo-Diéguez, E.; Lucero-Vega, G.; Murillo-Amador, B.; Ruiz-Espinoza, F.H.; Fraga-Palomino, H.C. Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress. Agronomy 2021, 11, 424. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Effect of Salinity on the Growth and Ion Content of Salicornia Rubra. Commun. Soil Sci. Plant Anal. 2001, 32, 2965–2977. [Google Scholar] [CrossRef]
- Marco, P.; Carvajal, M.; Martínez-Ballesta, M.D.C. Efficient Leaf Solute Partioning in Salicornia fruticosa Allows Growth under Salinity. Environ. Exp. Bot. 2019, 157, 177–186. [Google Scholar] [CrossRef]
- Ekanayake, S.; Egodawatta, C.; Attanayake, R.N.; Perera, D. From Salt Pan to Saucepan: Salicornia, a Halophytic Vegetable with an Array of Potential Health Benefits. Food Front. 2023, 4, 641–676. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, T.; Dai, X.; Wei, Y.; Fang, Y.; Zhang, L.; Zhu, M.; Nawaz, G.; Cao, Q.; Xu, T. Comparative Analysis of Salt Responsive MicroRNAs in Two Sweetpotato [Ipomoea batatas (L.) Lam.] Cultivars With Different Salt Stress Resistance. Front. Plant Sci. 2022, 13, 879819. [Google Scholar] [CrossRef]
- Qarehkhani, M.; Soltanloo, H.; Mokhtarpour, H.; Khorasaninejad, S.; Boojar, M.M.A. Effects Foliar Spraying of Abscisic Acid and Melatonin on Salicornia europaea L. Morphological Characteristics and Yield Components under Salinity Stress. Iran. J. Field Crop Sci. 2021, 52, 175–188. [Google Scholar]
- Davy, A.J.; Bishop, G.F.; Costa, C.S.B. Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin and S. dolichostachya Moss). J. Ecol. 2001, 89, 681–707. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple Compartmentalization of Sodium Conferred Salt Tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Mian, S.; Christenhusz, M.J.M.; Leitch, I.J.; Leitch, A.R. The Genome Sequence of Purple Glasswort, Salicornia ramosissima Woods (Amaranthaceae). Wellcome Open Res. 2024, 9, 257. [Google Scholar] [CrossRef]
- Fan, P.; Nie, L.; Jiang, P.; Feng, J.; Lv, S.; Chen, X.; Bao, H.; Guo, J.; Tai, F.; Wang, J.; et al. Transcriptome Analysis of Salicornia europaea under Saline Conditions Revealed the Adaptive Primary Metabolic Pathways as Early Events to Facilitate Salt Adaptation. PLoS ONE 2013, 8, e80595. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Fradera-Soler, M.; Leverett, A.; Mravec, J.; Jørgensen, B.; Borland, A.M.; Grace, O.M. Are Cell Wall Traits a Component of the Succulent Syndrome? Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Tai, F.; Wang, D.; Feng, J.; Fan, P.; Bao, H.; Li, Y. The V-ATPase Subunit A Is Essential for Salt Tolerance through Participating in Vacuolar Na+ Compartmentalization in Salicornia Europaea. Planta 2017, 246, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, J.; Bao, M.; Hasi, A.; Niu, Y. Cloning and Characterization of a Ca2+/H+ Exchanger from the Halophyte Salicornia europaea L. Plant Physiol. Biochem. 2015, 96, 321–328. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. Molecular Analysis of the Reactions in Salicornia europaea to Varying NaCl Concentrations at Various Stages of Development to Better Exploit Its Potential as a New Crop Plant. Front. Plant Sci. 2024, 15, 1454541. [Google Scholar] [CrossRef] [PubMed]
- Tiika, R.J.; Wei, J.; Cui, G.; Ma, Y.; Yang, H.; Duan, H. Transcriptome-Wide Characterization and Functional Analysis of Xyloglucan Endo-Transglycosylase/Hydrolase (XTH) Gene Family of Salicornia europaea L. under Salinity and Drought Stress. BMC Plant Biol. 2021, 21, 491. [Google Scholar] [CrossRef]
- Homayouni, H.; Razi, H.; Izadi, M.; Alemzadeh, A.; Kazemeini, S.A.; Niazi, A.; Vicente, O. Temporal Changes in Biochemical Responses to Salt Stress in Three Salicornia Species. Plants 2024, 13, 979. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of Drought Stress on the Osmotic Adjustment, Cell Wall Elasticity and Cell Volume of Six Cultivars of Common Beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Kishor, P.; Hong, Z.; Miao, G.H.; Hu, C.; Verma, D. Overexpression of [Delta]-Pyrroline-5-Carboxylate Synthetase Increases Proline Production and Confers Osmotolerance in Transgenic Plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef]
- Fradera-Soler, M.; Grace, O.M.; Jørgensen, B.; Mravec, J. Elastic and Collapsible: Current Understanding of Cell Walls in Succulent Plants. J. Exp. Bot. 2022, 73, 2290–2307. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of Salinity Stress on Crop Plants: Improving Salt Tolerance through Genetic and Molecular Dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Oelmüller, R.; Tseng, Y.-H.; Gandhi, A. Signals and Their Perception for Remodelling, Adjustment and Repair of the Plant Cell Wall. Int. J. Mol. Sci. 2023, 24, 7417. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.A.C.; Setter, T.L. Response of Cassava Leaf Area Expansion to Water Deficit: Cell Proliferation, Cell Expansion and Delayed Development. Ann. Bot. 2004, 94, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Boughalleb, F.; Denden, M.; Tiba, B.B. Anatomical Changes Induced by Increasing NaCl Salinity in Three Fodder Shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol. Plant. 2009, 31, 947–960. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Q.; Lei, Y.; Zou, J.; Li, Q. Adaptation of Cuticle Metabolism to Abiotic Stress in Plants. Crop Environ. 2025, 4, 38–44. [Google Scholar] [CrossRef]
- Cárdenas Pérez, S.; Strzelecki, J.; Piernik, A.; Rajabi Dehnavi, A.; Trzeciak, P.; Puchałka, R.; Mierek-Adamska, A.; Chanona Pérez, J.; Kačík, F.; Račko, V.; et al. Salinity-Driven Changes in Salicornia Cell Wall Nanomechanics and Lignin Composition. Environ. Exp. Bot. 2024, 218, 105606. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Are Calcium Oxalate Crystals a Dynamic Calcium Store in Plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Biosynthesis of the Plant Cell Wall Matrix Polysaccharide Xyloglucan. Annu. Rev. Plant Biol. 2016, 67, 235–259. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and Its Interactions with Other Components of the Growing Cell Wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Zhou, S. Overexpression of Phytoene Synthase Gene from Salicornia europaea Alters Response to Reactive Oxygen Species under Salt Stress in Transgenic Arabidopsis. Biotechnol. Lett. 2008, 30, 1501–1507. [Google Scholar] [CrossRef]
- Lou, T.; Lv, S.; Wang, J.; Wang, D.; Lin, K.; Zhang, X.; Zhang, B.; Guo, Z.; Yi, Z.; Li, Y. Cell Size and Xylem Differentiation Regulating Genes from Salicornia europaea Contribute to Plant Salt Tolerance. Plant Cell Environ. 2024, 47, 2638–2657. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A Thaumatin-like Protein of Ocimum Basilicum Confers Tolerance to Fungal Pathogen and Abiotic Stress in Transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef] [PubMed]
- Jesús-Pires, C.D.; Ferreira-Neto, J.R.C.; Oliveira-Silva, R.L.D.; Silva, J.B.D.; Silva, M.D.D.; Costa, A.F.D.; Benko-Iseppon, A.M. Genome-Wide Identification and Stress Responses of Cowpea Thaumatin-like Proteins: A Comprehensive Analysis. Plants 2024, 13, 3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.; Yin, W.; Xu, K.; Wang, S.; Shang, Q.; Sa, W.; Liang, J.; Wang, L. Genome-Wide Analysis of the Thaumatin-like Gene Family in Qingke (Hordeum vulgare L. Var. Nudum) Uncovers Candidates Involved in Plant Defense against Biotic and Abiotic Stresses. Front. Plant Sci. 2022, 13, 912296. [Google Scholar] [CrossRef]
- Nakahara, Y.; Sawabe, S.; Kainuma, K.; Katsuhara, M.; Shibasaka, M.; Suzuki, M.; Yamamoto, K.; Oguri, S.; Sakamoto, H. Yeast Functional Screen to Identify Genes Conferring Salt Stress Tolerance in Salicornia europaea. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Wynter, L. Analysis of Tobacco Resistance to Saline Conditions via Endogenous Expression of SeNN24 Gene from the Halophyte Plant Salicornia europaea. Master’s Thesis, Florida Atlantic University, Boca Raton, FL, USA, 2024. [Google Scholar]
- Singh, S.; Das, S.; Geeta, R. Role of Cuticular Wax in Adaptation to Abiotic Stress: A Molecular Perspective. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Zargar, S.M., Zargar, M.Y., Eds.; Springer: Singapore, 2018; pp. 155–182. ISBN 978-981-10-7478-3. [Google Scholar]
- Shepherd, T.; Wynne Griffiths, D. The Effects of Stress on Plant Cuticular Waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Tiika, R.J.; Yang, H.; Cui, G.; Ma, Y.; Boamah, S.; Li, Y.; Duan, H. Identification and Analysis of Cuticular Wax Biosynthesis Related Genes in Salicornia europaea Under NaCl Treatment. Int. J. Mol. Sci. 2025, 26, 2632. [Google Scholar] [CrossRef]
- Bhandal, I.S.; Malik, C.P. Potassium Estimation, Uptake, and Its Role in the Physiology and Metabolism of Flowering Plants. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 110, pp. 205–254. ISBN 978-0-12-364510-4. [Google Scholar]
- Wakeel, A. Potassium–Sodium Interactions in Soil and Plant under Saline-sodic Conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological Functions of Mineral Macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Katschnig, D.; Bliek, T.; Rozema, J.; Schat, H. Constitutive High-Level SOS1 Expression and Absence of HKT1;1 Expression in the Salt-Accumulating Halophyte Salicornia dolichostachya. Plant Sci. 2015, 234, 144–154. [Google Scholar] [CrossRef]
- Wu, S.; Su, Q.; An, L.; Ma, S. A Choline Monooxygenase Gene Promoter from Salicornia europaea Increases Expression of the Beta-Glucuronidase Gene under Abiotic Stresses in Tobacco (Nicotiana tabacum L.). Indian J. Biochem. Biophys. 2011, 48, 170–174. [Google Scholar] [PubMed]
- Liu, R.; Wang, T.; Li, Q.; Wang, L.; Song, J. The Role of Tissue Succulence in Plant Salt Tolerance: An Overview. Plant Growth Regul. 2024, 103, 283–292. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of Tolerance of Abiotic Stress by Metabolic Engineering of Betaines and Other Compatible Solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.S.; Tester, M. Salinity Tolerance of Arabidopsis: A Good Model for Cereals? Trends Plant Sci. 2007, 12, 534–540. [Google Scholar] [CrossRef]
- Møller, I.S.; Gilliham, M.; Jha, D.; Mayo, G.M.; Roy, S.J.; Coates, J.C.; Haseloff, J.; Tester, M. Shoot Na+ Exclusion and Increased Salinity Tolerance Engineered by Cell Type–Specific Alteration of Na+ Transport in Arabidopsis. Plant Cell 2009, 21, 2163–2178. [Google Scholar] [CrossRef]
- Salazar, O.R.; Chen, K.; Melino, V.J.; Reddy, M.P.; Hřibová, E.; Čížková, J.; Beránková, D.; Arciniegas Vega, J.P.; Cáceres Leal, L.M.; Aranda, M.; et al. SOS1 Tonoplast Neo-Localization and the RGG Protein SALTY Are Important in the Extreme Salinity Tolerance of Salicornia bigelovii. Nat. Commun. 2024, 15, 4279. [Google Scholar] [CrossRef]
- Katschnig, D.; Jaarsma, R.; Almeida, P.; Rozema, J.; Schat, H. Differences in Proton Pumping and Na+/H+ Exchange at the Leaf Cell Tonoplast between a Halophyte and a Glycophyte. AoB Plants 2014, 6, plu023. [Google Scholar] [CrossRef]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.-S.; Zhu, J.K. The Arabidopsis Thaliana SOS2 Gene Encodes a Protein Kinase That Is Required for Salt Tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. A Calcium Sensor Homolog Required for Plant Salt Tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Ali, A.; Petrov, V.; Yun, D.J.; Gechev, T. Revisiting Plant Salt Tolerance: Novel Components of the SOS Pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.S.; Shukla, P.S.; Jha, A.; Agarwal, P.K.; Jha, B. The SbSOS1 Gene from the Extreme Halophyte Salicornia brachiata Enhances Na+loading in Xylem and Confers Salt Tolerance in Transgenic Tobacco. BMC Plant Biol. 2012, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Goyal, E.; Singh, R.S.; Kanika, K. Isolation and Functional Characterization of Salt Overly Sensitive 1 (SOS1) Gene Promoter from Salicornia brachiata. Biol. Plant. 2013, 57, 465–473. [Google Scholar] [CrossRef]
- Yang, X. Over-Expressing Salicornia europaea (SeNHX1) Gene in Tobacco Improves Tolerance to Salt. Afr. J. Biotechnol. 2011, 10, 16452–16460. [Google Scholar] [CrossRef]
- Jha, A.; Joshi, M.; Yadav, N.S.; Agarwal, P.K.; Jha, B. Cloning and Characterization of the Salicornia brachiata Na+/H+ Antiporter Gene SbNHX1 and Its Expression by Abiotic Stress. Mol. Biol. Rep. 2011, 38, 1965–1973. [Google Scholar] [CrossRef]
- Bassil, E.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation Specificity of Vacuolar NHX-Type Cation/H+ Antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef]
- James, R.A.; Munns, R.; Von Caemmerer, S.; Trejo, C.; Miller, C.; Condon, T. Photosynthetic Capacity Is Related to the Cellular and Subcellular Partitioning of N+, K+ and Cl− in Salt-affected Barley and Durum Wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef]
- Blumwald, E.; Poole, R.J. Na+/H+ Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985, 78, 163–167. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, S.; Tao, S.; Feng, J.; Fan, X.; Xu, P.; Xu, Z.; Shen, X. Overexpression of a Samphire High-Affinity Potassium Transporter Gene SbHKT1 Enhances Salt Tolerance in Transgenic Cotton. Acta Physiol. Plant. 2020, 42, 36. [Google Scholar] [CrossRef]
- Haxim, Y.; Wang, L.; Pan, Z.; Fan, X.; Ma, J. A Novel High-Affinity Potassium Transporter SeHKT1;2 from Halophyte Salicornia europaea Shows Strong Selectivity for Na+ Rather than K+. Front. Plant Sci. 2023, 14, 1104070. [Google Scholar] [CrossRef]
- Almeida, P.; Katschnig, D.; De Boer, A. HKT Transporters—State of the Art. Int. J. Mol. Sci. 2013, 14, 20359–20385. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 Transporter Mediates Large Na+ Influx Component into K+-Starved Roots for Growth. EMBO J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Hosoo, Y.; Goshima, S.; Horie, T.; Eckelman, B.; Yamada, K.; Yoshida, K.; Bakker, E.P.; Shinmyo, A.; Oiki, S.; et al. Glycine Residues in Potassium Channel-like Selectivity Filters Determine Potassium Selectivity in Four-Loop-per-Subunit HKT Transporters from Plants. Proc. Natl. Acad. Sci. USA 2002, 99, 6428–6433. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Oguri, S.; Chiba, S.; Momonoki, Y.S. Molecular Cloning of Acetylcholinesterase Gene from Salicornia europaea L. Plant Signal. Behav. 2009, 4, 361–366. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium Homeostasis and Potential Roles in Combatting Environmental Stresses in Plants. S. Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium Signaling and Salt Tolerance Are Diversely Entwined in Plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Kaur, P.; Handa, N.; Verma, V.; Bakshi, P.; Kalia, R.; Sareen, S.; Nagpal, A.; Vig, A.P.; Mir, B.A.; Bhardwaj, R. Cross Talk Among Reactive Oxygen, Nitrogen and Sulfur During Abiotic Stress in Plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 857–871. ISBN 978-1-119-46869-1. [Google Scholar]
- Medrano-Macías, J.; Flores-Gallegos, A.C.; Nava-Reyna, E.; Morales, I.; Tortella, G.; Solís-Gaona, S.; Benavides-Mendoza, A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants 2022, 11, 3203. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Talaat, N.B. Role of Reactive Oxygen Species Signaling in Plant Growth and Development. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 225–266. ISBN 978-1-119-46869-1. [Google Scholar]
- Guo, W.; Xing, Y.; Luo, X.; Li, F.; Ren, M.; Liang, Y. Reactive Oxygen Species: A Crosslink between Plant and Human Eukaryotic Cell Systems. Int. J. Mol. Sci. 2023, 24, 13052. [Google Scholar] [CrossRef] [PubMed]
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive Oxygen Species Function as Signaling Molecules in Controlling Plant Development and Hormonal Responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Muday, G.K. Reactive Oxygen Species Are Signaling Molecules That Modulate Plant Reproduction. Plant Cell Environ. 2024, 47, 1592–1605. [Google Scholar] [CrossRef]
- Lal, M.; Kumari, A.; Pooja; Sheokand, S. Reactive Oxygen Species, Reactive Nitrogen Species and Oxidative Metabolism Under Waterlogging Stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 777–812. ISBN 978-1-119-46869-1. [Google Scholar]
- Kumar, A.; Guleria, S.; Ghosh, D.; Dogra, V.; Kumar, S. Managing Reactive Oxygen Species—Some Learnings from High Altitude Extremophytes. Environ. Exp. Bot. 2021, 189, 104525. [Google Scholar] [CrossRef]
- Bastas, K.K. Importance of Reactive Oxygen Species in Plants-Pathogens Interactions. Selcuk J. Agr. Food Sci. 2015, 28, 11–21. [Google Scholar]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules—Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Plant Adaptation and Tolerance to Heat Stress: Advance Approaches and Future Aspects. Comb. Chem. High Throughput Screen. 2024, 27, 1701–1715. [Google Scholar] [CrossRef]
- Sonmez, M.C.; Yirmibesoglu, S.S.S.; Ozgur, R.; Uzilday, B.; Turkan, I. Roles of Reactive Carbonyl Species (RCS) in Plant Response to Abiotic Stress. In ROS Signaling in Plants; Corpas, F.J., Palma, J.M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2024; Volume 2798, pp. 101–130. ISBN 978–1-0716-3825-5. [Google Scholar]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism. Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 1–23. ISBN 978-981-10-5253-8. [Google Scholar]
- Allen, R.D. Dissection of Oxidative Stress Tolerance Using Transgenic Plants. Plant Physiol. 1995, 107, 1049–1054. [Google Scholar] [CrossRef]
- Bor, M.; Özdemir, F.; Türkan, I. The Effect of Salt Stress on Lipid Peroxidation and Antioxidants in Leaves of Sugar Beet Beta vulgaris L. and Wild Beet Beta maritima L. Plant Sci. 2003, 164, 77–84. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants–Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Del Río, L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, A.; Zhang, J.; Jiang, M. Abscisic Acid Is a Key Inducer of Hydrogen Peroxide Production in Leaves of Maize Plants Exposed to Water Stress. Plant Cell Physiol. 2006, 47, 1484–1495. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Van Remmen, H.; Richardson, A.; Wehr, N.B.; Levine, R.L. Methionine Oxidation and Aging. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1703, 135–140. [Google Scholar] [CrossRef]
- Khator, K.; Parihar, S.; Jasik, J.; Shekhawat, G.S. Nitric Oxide in Plants: An Insight on Redox Activity and Responses toward Abiotic Stress Signaling. Plant Signal. Behav. 2024, 19, 2298053. [Google Scholar] [CrossRef]
- Mariyam, S.; Bhardwaj, R.; Khan, N.A.; Sahi, S.V.; Seth, C.S. Review on Nitric Oxide at the Forefront of Rapid Systemic Signaling in Mitigation of Salinity Stress in Plants: Crosstalk with Calcium and Hydrogen Peroxide. Plant Sci. 2023, 336, 111835. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Wei, X.; Jahan, I.; Hasanuzzaman, M.; Sabuj, Z.H.; Zulfiqar, F.; Chen, J.; Iqbal, R.; Dastogeer, K.M.G.; Sohag, A.A.M.; et al. Exogenous Nitric Oxide Promotes Salinity Tolerance in Plants: A Meta-Analysis. Front. Plant Sci. 2022, 13, 957735. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.J. Regulation of Reactive Oxygen and Nitrogen Species by Salicylic Acid in Rice Plants under Salinity Stress Conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Siddique, K.H.M.; Schubert, S. Role of Nitric Oxide in Improving Plant Resistance Against Salt Stress. In Ecophysiology and Responses of Plants Under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 413–424. ISBN 978-1-4614-4746-7. [Google Scholar]
- Marques, I.C.D.S.; Silva, D.M.R.; Bispo, G.L.; Oliveira, F.D.A.D.; Ono, E.O.; Rodrigues, J.D. Nitric Oxide Modulates Salt Stress Tolerance in Lettuce. Stresses 2023, 3, 701–716. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel-Latef, A.A.; Hashem, A.; Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Moghaddam, A.; Larijani, H.R.; Oveysi, M.; Moghaddam, H.R.T.; Nasri, M. Alleviating the Adverse Effects of Salinity Stress on Salicornia persica Using Sodium Nitroprusside and Potassium Nitrate. BMC Plant Biol. 2023, 23, 166. [Google Scholar] [CrossRef]
- Mano, J.; Nagata, M.; Okamura, S.; Shiraya, T.; Mitsui, T. Identification of Oxidatively Modified Proteins in Salt-Stressed Arabidopsis: A Carbonyl-Targeted Proteomics Approach. Plant Cell Physiol. 2014, 55, 1233–1244. [Google Scholar] [CrossRef]
- Sultana, M.; Sakurai, C.; Biswas, M.S.; Szabados, L.; Mano, J. Accumulation of Reactive Carbonyl Species in Roots as the Primary Cause of Salt Stress-induced Growth Retardation of Arabidopsis thaliana. Physiol. Plant. 2024, 176, e14198. [Google Scholar] [CrossRef]
- Jha, B.; Sharma, A.; Mishra, A. Expression of SbGSTU (Tau Class Glutathione S-Transferase) Gene Isolated from Salicornia brachiata in Tobacco for Salt Tolerance. Mol. Biol. Rep. 2011, 38, 4823–4832. [Google Scholar] [CrossRef]
- Dhiman, S.; Ibrahim, M.; Khanna, K.; Bhardwaj, T.; Devi, K.; Sharma, I.; Arora, U.; Mir, B.A.; Bhardwaj, R. Cross-Talk between ROS, RNS, RCS, and RSS in Plants under Abiotic Stresses. In Nitric Oxide in Developing Plant Stress Resilience; Elsevier: Amsterdam, The Netherlands, 2023; pp. 305–326. ISBN 978-0-323-91209-9. [Google Scholar]
- Qiao, W.; Li, C.; Fan, L.M. Cross-Talk between Nitric Oxide and Hydrogen Peroxide in Plant Responses to Abiotic Stresses. Environ. Exp. Bot. 2014, 100, 84–93. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between Reactive Oxygen and Nitrogen Species in Living Organisms. Chem. Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I. The Roles of Reactive Carbonyl Species in Induction of Antioxidant Defence and ROS Signalling in Extreme Halophytic Model Eutrema parvulum and Glycophytic Model Arabidopsis thaliana. Environ. Exp. Bot. 2019, 160, 81–91. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Antioxidative Defense Potential to Salinity in the Euhalophyte Salicornia brachiata. J. Plant Growth Regul. 2010, 29, 137–148. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of Superoxide Dismutase Protects Plants from Oxidative Stress (Induction of Ascorbate Peroxidase in Superoxide Dismutase-Overexpressing Plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Kim, W. Cobalt-Induced Oxidative Stress Causes Growth Inhibition Associated with Enhanced Lipid Peroxidation and Activates Antioxidant Responses in Indian Mustard (Brassica juncea L.) Leaves. Acta Physiol. Plant. 2013, 35, 2429–2443. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Effect of Salt Stress on Physiological and Antioxidative Responses in Two Species of Salicornia (S. persica and S. europaea). Acta Physiol. Plant. 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Loewen, P.C.; Klotz, M.G.; Hassett, D.J. Catalase—An “Old” Enzyme That Continues to Surprise Us. ASM News 2000, 66, 76–80. [Google Scholar]
- Andre, C.; Kim, S.W.; Yu, X.-H.; Shanklin, J. Fusing Catalase to an Alkane-Producing Enzyme Maintains Enzymatic Activity by Converting the Inhibitory Byproduct H2O2 to the Cosubstrate O2. Proc. Natl. Acad. Sci. USA 2013, 110, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, W.; Capiau, K.; Van Montagu, M.; Inze, D.; Slooten, L. Enhancement of Oxidative Stress Tolerance in Transgenic Tobacco Plants Overproducing Fe-Superoxide Dismutase in Chloroplasts. Plant Physiol. 1996, 112, 1703–1714. [Google Scholar] [CrossRef]
- Mano, J.; Hideg, É.; Asada, K. Ascorbate in Thylakoid Lumen Functions as an Alternative Electron Donor to Photosystem II and Photosystem I. Arch. Biochem. Biophys. 2004, 429, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutothione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Smirnoff, N.; Pallanca, J.E. Ascorbate Metabolism in Relation to Oxidative Stress. Biochem. Soc. Trans. 1996, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Zámocký, M.; Furtmüller, P.G.; Obinger, C. Evolution of Structure and Function of Class I Peroxidases. Arch. Biochem. Biophys. 2010, 500, 45–57. [Google Scholar] [CrossRef]
- Singh, N.; Mishra, A.; Jha, B. Over-Expression of the Peroxisomal Ascorbate Peroxidase (SbpAPX) Gene Cloned from Halophyte Salicornia brachiata Confers Salt and Drought Stress Tolerance in Transgenic Tobacco. Mar. Biotechnol. 2014, 16, 321–332. [Google Scholar] [CrossRef]
- Wu, X.; Huang, H.; Liu, X.; Chen, L.; Liu, C.; Su, M.; Hong, F. Oxidative Stress Induced by Lead in Chloroplast of Spinach. Biol. Trace Elem. Res. 2008, 126, 257–268. [Google Scholar] [CrossRef][Green Version]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional Characterization of the Tau Class Glutathione-S-Transferases Gene (SbGSTU) Promoter of Salicornia brachiata under Salinity and Osmotic Stress. PLoS ONE 2016, 11, e0148494. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Rausch, T. Glutathione, Photosynthesis and the Redox Regulation of Stress-Responsive Gene Expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Mano, J.; Kanameda, S.; Kuramitsu, R.; Matsuura, N.; Yamauchi, Y. Detoxification of Reactive Carbonyl Species by Glutathione Transferase Tau Isozymes. Front. Plant Sci. 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione Transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Glutathione Transferases in Bacteria. FEBS J. 2009, 276, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Frova, C. Glutathione Transferases in the Genomics Era: New Insights and Perspectives. Biomol. Eng. 2006, 23, 149–169. [Google Scholar] [CrossRef]
- Lo Cicero, L.; Madesis, P.; Tsaftaris, A.; Lo-Piero, A.R. Tobacco Plants Over-Expressing the Sweet Orange Tau Glutathione Transferases (CsGSTUs) Acquire Tolerance to the Diphenyl Ether Herbicide Fluorodifen and to Salt and Drought Stresses. Phytochemistry 2015, 116, 69–77. [Google Scholar] [CrossRef]
- Mannervik, B.; Board, P.G.; Hayes, J.D.; Listowsky, I.; Pearson, W.R. Nomenclature for Mammalian Soluble Glutathione Transferases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 401, pp. 1–8. ISBN 978-0-12-182806-6. [Google Scholar]
- Axarli, I.; Dhavala, P.; Papageorgiou, A.C.; Labrou, N.E. Crystallographic and Functional Characterization of the Fluorodifen-Inducible Glutathione Transferase from Glycine Max Reveals an Active Site Topography Suited for Diphenylether Herbicides and a Novel L-Site. J. Mol. Biol. 2009, 385, 984–1002. [Google Scholar] [CrossRef]
- Droog, F. Plant Glutathione S-Transferases, a Tale of Theta and Tau. J. Plant Growth Regul. 1997, 16, 95–107. [Google Scholar] [CrossRef]
- Ritika; Rizwana; Shukla, S.; Sondhi, A.; Tripathi, A.D.; Lee, J.-K.; Patel, S.K.S.; Agarwal, A. Valorisation of Fruit Waste for Harnessing the Bioactive Compounds and Its Therapeutic Application. Trends Food Sci. Technol. 2024, 144, 104302. [Google Scholar] [CrossRef]
- Larson, R.A. The Antioxidants of Higher Plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Riddick, E.W. Evaluating the Effects of Flavonoids on Insects: Implications for Managing Pests Without Harming Beneficials. Insects 2024, 15, 956. [Google Scholar] [CrossRef]
- Terzaghi, M.; De Tullio, M.C. Ascorbic Acid in Seeds, Priming and Beyond. Seeds 2023, 2, 421–435. [Google Scholar] [CrossRef]
- Somai-Jemmali, L.; Magnin-Robert, M.; Randoux, B.; Siah, A.; Tisserant, B.; Halama, P.; Reignault, P.; Hamada, W. Ascorbic Acid Controls Mycosphaerella graminicola in Bread and Durum Wheat through Direct Effect on The Pathogen and Indirect Action via Plant Defence. Commun. Agric. Appl. Biol. Sci. 2015, 80, 477–490. [Google Scholar] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Miyahara, K.; Tabata, K.; Esaka, M. Generation and Properties of Ascorbic Acid-Overproducing Transgenic Tobacco Cells Expressing Sense RNA for l-Galactono-1,4-Lactone Dehydrogenase. Planta 2005, 220, 854–863. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A.; Ouchi, S.; Tsukatani, H. Antioxidant Activities of Tocopherols on Fe2+-ascorbate-induced Lipid Peroxidation in Lecithin Liposomes. Lipids 1982, 17, 511–513. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M.I.; Nasir, M.K.A.; Ismail, S. Analytical Characterization of Salicornia bigelovii Seed Oil Cultivated in Pakistan. J. Agric. Food Chem. 2002, 50, 4210–4214. [Google Scholar] [CrossRef]
- Magni, N.N.; Veríssimo, A.C.S.; Silva, H.; Pinto, D.C.G.A. Metabolomic Profile of Salicornia perennis Plant’s Organs under Diverse In Situ Stress: The Ria de Aveiro Salt Marshes Case. Metabolites 2023, 13, 280. [Google Scholar] [CrossRef]
- Heber, D.; Lu, Q.Y. Overview of Mechanisms of Action of Lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Arslansoy, N.; Fidan, O. Carotenoids and Their Antioxidant Power. In Biochemistry; Novo Barros, A., Cristina Santos Abraão, A., Eds.; IntechOpen: Rijeka, Croatia, 2024; Volume 60, ISBN 978-0-85466-206-7. [Google Scholar]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as Modulators of Lipid Membrane Physical Properties. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.M.; Mendes, C.R.; Doncato, K.B.; Badiale-Furlong, E.; Costa, C.S.B. Growth, Phenolics, Photosynthetic Pigments, and Antioxidant Response of Two New Genotypes of Sea Asparagus (Salicornia neei Lag.) to Salinity under Greenhouse and Field Conditions. Agriculture 2018, 8, 115. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. Genes and Enzymes of Carotenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Brunetti, C.; Di-Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-Induced Flavonoid Biosynthesis and the Antioxidant Machinery of Plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and Iron-Chelating Activities of the Flavonoids Catechin, Quercetin and Diosmetin on Iron-Loaded Rat Hepatocyte Cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Duan, H.; Tiika, R.J.; Tian, F.; Lu, Y.; Zhang, Q.; Hu, Y.; Cui, G.; Yang, H. Metabolomics Analysis Unveils Important Changes Involved in the Salt Tolerance of Salicornia europaea. Front. Plant Sci. 2023, 13, 1097076. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic Implications of Stress-Induced Proline Accumulation in Plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hua, X.J.; May, M.; Van Montagu, M. Environmental and Developmental Signals Modulate Proline Homeostasis: Evidence for a Negative Transcriptional Regulator. Proc. Natl. Acad. Sci. USA 1996, 93, 8787–8791. [Google Scholar] [CrossRef] [PubMed]
- Kemble, A.R.; Macpherson, H.T. Liberation of Amino Acids in Perennial Rye Grass during Wilting. Biochem. J. 1954, 58, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Matsushima, D.; Rhaman, M.S.; Okuma, E.; Nakamura, T.; Nakamura, Y.; Munemasa, S.; Murata, Y. Exogenous Proline Enhances Antioxidant Enzyme Activities but Does Not Mitigate Growth Inhibition by Selenate Stress in Tobacco BY-2 Cells. Biosci. Biotechnol. Biochem. 2020, 84, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Sima, N.A.K.K.; Mirzaei, H.H. Effects of Sodium Chloride on Physiological Aspects of Salicornia persica Growth. J. Plant Nutr. 2013, 36, 401–414. [Google Scholar] [CrossRef]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Introgression of the SbASR-1 Gene Cloned from a Halophyte Salicornia brachiata Enhances Salinity and Drought Endurance in Transgenic Groundnut (Arachis hypogaea) and Acts as a Transcription Factor. PLoS ONE 2015, 10, e0131567. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Haque, I.; Jha, B. A Novel Transcription Factor-like Gene SbSDR1 Acts as a Molecular Switch and Confers Salt and Osmotic Endurance to Transgenic Tobacco. Sci. Rep. 2016, 6, 31686. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium Chloride Toxicity and the Cellular Basis of Salt Tolerance in Halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Betzen, B.M.; Smart, C.M.; Maricle, K.L.; MariCle, B.R. Effects of Increasing Salinity on Photosynthesis and Plant Water Potential in Kansas Salt Marsh Species. Trans. Kans. Acad. Sci. 2019, 122, 49. [Google Scholar] [CrossRef]
- Zhifang, G.; Loescher, W.H. Expression of a Celery Mannose 6-phosphate Reductase in Arabidopsis thaliana Enhances Salt Tolerance and Induces Biosynthesis of Both Mannitol and a Glucosyl-mannitol Dimer. Plant Cell Environ. 2003, 26, 275–283. [Google Scholar] [CrossRef]
- Kumari, A.; Rathore, A.P.; Sutariya, J.A.; Chaudhary, D.R.; Rathore, M.S. Defense Enzyme Encoding Gene and Metabolite Expression Profiling in Salicornia brachiata Roxb. under Different Salinity. Biocatal. Agric. Biotechnol. 2025, 66, 103605. [Google Scholar] [CrossRef]
- Takabe, T.; Rai, V.; Hibino, T. Metabolic Engineering of Glycinebetaine. In Abiotic Stress Tolerance in Plants; Rai, A.K., Takabe, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 137–151. ISBN 978-1-4020-4388-8. [Google Scholar]
- Ahmad, R.; Lim, C.J.; Kwon, S.-Y. Glycine Betaine: A Versatile Compound with Great Potential for Gene Pyramiding to Improve Crop Plant Performance against Environmental Stresses. Plant Biotechnol. Rep. 2013, 7, 49–57. [Google Scholar] [CrossRef]
- Wu, S.; Su, Q.; An, L.J. Isolation of Choline Monooxygenase (CMO) Gene from Salicornia europaea and Enhanced Salt Tolerance of Transgenic Tobacco with CMO Genes. Indian J. Biochem. Biophys. 2010, 47, 298–305. [Google Scholar] [PubMed]
- Sekhar, P.N.; Amrutha, R.N.; Sangam, S.; Verma, D.P.S.; Kishor, P.B.K. Biochemical Characterization, Homology Modeling and Docking Studies of Ornithine δ-Aminotransferase—An Important Enzyme in Proline Biosynthesis of Plants. J. Mol. Graph. Model. 2007, 26, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Nuclear Gene Encoding Mitochondrial Proline Dehydrogenase, an Enzyme Involved in Proline Metabolism, Is Upregulated by Proline but Downregulated by Dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar] [CrossRef]
- Ribarits, A.; Abdullaev, A.; Tashpulatov, A.; Richter, A.; Heberle-Bors, E.; Touraev, A. Two Tobacco Proline Dehydrogenases Are Differentially Regulated and Play a Role in Early Plant Development. Planta 2007, 225, 1313–1324. [Google Scholar] [CrossRef]
- Wang, P.; Ma, C.; Zhao, K.; Zhao, Y.; Zhang, H. Isolation and Characterizing of a DELTA(1)-Pyrroline-5-Carboxylate Synthase Gene in Suaeda salsa under Salinity Stress. J. Shandong Norm. Univ. Nat. Sci. 2002, 17, 59–62. [Google Scholar]
- Moghaieb, R.E.A.; Saneoka, H.; Fujita, K. Effect of Salinity on Osmotic Adjustment, Glycinebetaine Accumulation and the Betaine Aldehyde Dehydrogenase Gene Expression in Two Halophytic Plants, Salicornia europaea and Suaeda maritima. Plant Sci. 2004, 166, 1345–1349. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan Nanoparticles Effectively Combat Salinity Stress by Enhancing Antioxidant Activity and Alkaloid Biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; Ali, E.F. Effect of Jasmonic Acid on Alkaloids Content and Salinity Tolerance of Catharanthus roseus Based on Morpho-Physiological Evaluation. S. Afr. J. Bot. 2021, 141, 440–446. [Google Scholar] [CrossRef]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidón, A.; Vicente, O. Soluble Carbohydrates as Osmolytes in Several Halophytes from a Mediterranean Salt Marsh. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 9. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt Stress Effects on Growth, Pigments, Proteins and Lipid Peroxidation in Salicornia persica and S. europaea. Biol. Plant. 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Haque, M.I.; Rathore, M.S.; Gupta, H.; Jha, B. Inorganic Solutes Contribute More than Organic Solutes to the Osmotic Adjustment in Salicornia brachiata (Roxb.) under Natural Saline Conditions. Aquat. Bot. 2017, 142, 78–86. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the Roles of Osmolytes for Acclimatizing Plants to Changing Environment: A Review of Potential Mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Dai, L.; Sun, H.; Chen, M.; Sun, Y. Effects of Moderate Soil Salinity on Osmotic Adjustment and Energy Strategy in Soybean under Drought Stress. Plant Physiol. Biochem. 2019, 139, 307–313. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Llanes, A.; Masciarelli, O.; Ordóñez, R.; Isla, M.I.; Luna, V. Differential Growth Responses to Sodium Salts Involve Different Abscisic Acid Metabolism and Transport in Prosopis strombulifera. Biol. Plant. 2014, 58, 80–88. [Google Scholar] [CrossRef]
- Yang, G.; Yu, Z.; Gao, L.; Zheng, C. SnRK2s at the Crossroads of Growth and Stress Responses. Trends Plant Sci. 2019, 24, 672–676. [Google Scholar] [CrossRef]
- Cornforth, J.W.; Milborrow, B.V.; Ryback, G. Synthesis of (±)-Abscisin II. Nature 1965, 206, 715. [Google Scholar] [CrossRef]
- Neuman, H.; Galpaz, N.; Cunningham, F.X.; Zamir, D.; Hirschberg, J. The Tomato Mutation Nxd1 Reveals a Gene Necessary for Neoxanthin Biosynthesis and Demonstrates That Violaxanthin Is a Sufficient Precursor for Abscisic Acid Biosynthesis. Plant J. 2014, 78, 80–93. [Google Scholar] [CrossRef]
- Fernando, V.C.D.; Schroeder, D.F. Role of ABA in Arabidopsis Salt, Drought, and Desiccation Tolerance. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; InTech: Vienna, Austria, 2016; ISBN 978-953-51-2250-0. [Google Scholar]
- Nojavan-Asghari, M.; Ishizawa, K. Inhibitory Effects of Methyl Jasmonate on the Germination and Ethylene Production in Cocklebur Seeds. J. Plant Growth Regul. 1998, 17, 13–18. [Google Scholar] [CrossRef]
- Staswick, P.E.; Su, W.; Howell, S.H. Methyl Jasmonate Inhibition of Root Growth and Induction of a Leaf Protein are Decreased in an Arabidopsis thaliana Mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Jia, A.; Xu, G.; Hu, H.; Hu, X.; Hu, L. Jasmonate Mediates Salt-Induced Nicotine Biosynthesis in Tobacco (Nicotiana tabacum L.). Plant Divers. 2016, 38, 118–123. [Google Scholar] [CrossRef]
- Valenzuela, C.E.; Acevedo-Acevedo, O.; Miranda, G.S.; Vergara-Barros, P.; Holuigue, L.; Figueroa, C.R.; Figueroa, P.M. Salt Stress Response Triggers Activation of the Jasmonate Signaling Pathway Leading to Inhibition of Cell Elongation in Arabidopsis Primary Root. J. Exp. Bot. 2016, 67, 4209–4220. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; Elgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Jośko, I.; et al. Association of Jasmonic Acid Priming with Multiple Defense Mechanisms in Wheat Plants under High Salt Stress. Front. Plant Sci. 2022, 13, 886862. [Google Scholar] [CrossRef]
- Chauhan, A.; Abu-Amarah, B.A.; Kumar, A.; Verma, J.S.; Ghramh, H.A.; Khan, K.A.; Ansari, M.J. Influence of Gibberellic Acid and Different Salt Concentrations on Germination Percentage and Physiological Parameters of Oat Cultivars. Saudi J. Biol. Sci. 2019, 26, 1298–1304. [Google Scholar] [CrossRef]
- Pearce, S.; Huttly, A.K.; Prosser, I.M.; Li, Y.; Vaughan, S.P.; Gallova, B.; Patil, A.; Coghill, J.A.; Dubcovsky, J.; Hedden, P.; et al. Heterologous Expression and Transcript Analysis of Gibberellin Biosynthetic Genes of Grasses Reveals Novel Functionality in the GA3ox Family. BMC Plant Biol. 2015, 15, 130. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Dong, C.-H. Molecular Regulations of Ethylene Signaling in Plant Salt Stress Responses. Plant Stress 2024, 14, 100583. [Google Scholar] [CrossRef]
- Gul, B.; Khan, M.A. Effect of Growth Regulators and Osmotica in Alleviating Salinity Effects on the Germination of Salicornia utahensis. Pak. J. Bot. 2004, 35, 885–894. [Google Scholar]
- Khan, M.A.; Gul, B.; Weber, D.J. Improving Seed Germination of Salicornia rubra (Chenopodiaceae) under Saline Conditions Using Germination-Regulating Chemicals. West. N. Am. Nat. 2002, 62, 11. [Google Scholar]
- Gupta, K.; Agarwal, P.K.; Reddy, M.K.; Jha, B. SbDREB2A, an A-2 Type DREB Transcription Factor from Extreme Halophyte Salicornia brachiata Confers Abiotic Stress Tolerance in Escherichia coli. Plant Cell Rep. 2010, 29, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jha, B.; Agarwal, P.K. A Dehydration-Responsive Element Binding (DREB) Transcription Factor from the Succulent Halophyte Salicornia brachiata Enhances Abiotic Stress Tolerance in Transgenic Tobacco. Mar. Biotechnol. 2014, 16, 657–673. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA Methylation and Human Disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a Key Regulator in Seed Germination under Abiotic Stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Li, J.; Teng, H. Melatonin—Angel of Plant Growth Regulation and Protection. Adv. Agrochem. 2025, S2773237125000012. [Google Scholar] [CrossRef]
- Giménez, A.; Gallegos-Cedillo, V.M.; Benaissa, R.R.; Egea-Gilabert, C.; Signore, A.; Ochoa, J.; Gruda, N.S.; Arnao, M.B.; Fernández, J.A. Enhancing the Cultivation of Salicornia fruticosa with Agroindustrial Compost Leachates in a Cascade Cropping System: Evaluating the Impact of Melatonin Application. Front. Plant Sci. 2024, 15, 1441884. [Google Scholar] [CrossRef]
- Rehman, N.; Khan, M.R.; Abbas, Z.; Rafique, R.S.; Zaynab, M.; Qasim, M.; Noor, S.; Inam, S.; Ali, G.M. Functional Characterization of Mitogen-Activated Protein Kinase Kinase (MAPKK) Gene in Halophytic Salicornia europaea against Salt Stress. Environ. Exp. Bot. 2020, 171, 103934. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK Machinery in Plants: Recognition and Response to Different Stresses through Multiple Signal Transduction Pathways. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C.; et al. Mitogen-Activated Protein Kinase Cascades in Plants: A New Nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Tatebayashi, K. A Docking Site Determining Specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the Yeast HOG Pathway. EMBO J. 2003, 22, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Gupta, K.; Jha, B. Molecular Characterization of the Salicornia brachiata SbMAPKK Gene and Its Expression by Abiotic Stress. Mol. Biol. Rep. 2010, 37, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Bhatt, I.D.; Nandi, S.K. Role and Regulation of Auxin Signaling in Abiotic Stress Tolerance. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–331. ISBN 978-0-12-816451-8. [Google Scholar]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin Response Factors in Plant Adaptation to Drought and Salinity Stress. Physiol. Plant. 2022, 174, e13714. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Q.; Hu, Z.; Sun, X.; Fan, S.; Zhang, H. Function of the Auxin-Responsive Gene TaSAUR75 under Salt and Drought Stress. Crop J. 2018, 6, 181–190. [Google Scholar] [CrossRef]
- Katschnig, D. On the Physiology and Molecular Genetics of Salt Tolerance in Salicornia. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2015. [Google Scholar]
- Liu, Y.; Qiao, Y.; Liao, W. Calmodulin-Binding Transcription Factors: Roles in Plant Response to Abiotic Stresses. Plants 2025, 14, 532. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, H.; Zhang, Y.; Zhao, Y.; Zhang, Y.; Feng, X.; Lin, H. Diverse Roles of MYB Transcription Factors in Plants. J. Integr. Plant Biol. 2025, 67, 539–562. [Google Scholar] [CrossRef]
- Thilakarathne, A.S.; Liu, F.; Zou, Z. Plant Signaling Hormones and Transcription Factors: Key Regulators of Plant Responses to Growth, Development, and Stress. Plants 2025, 14, 1070. [Google Scholar] [CrossRef]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A Comprehensive Review of Transcription Factor-Mediated Regulation of Secondary Metabolites in Plants under Environmental Stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Shukla, P.S.; Gupta, K.; Agarwal, P.; Jha, B.; Agarwal, P.K. Overexpression of a Novel SbMYB15 from Salicornia brachiata Confers Salinity and Dehydration Tolerance by Reduced Oxidative Damage and Improved Photosynthesis in Transgenic Tobacco. Planta 2015, 242, 1291–1308. [Google Scholar] [CrossRef]
- Adcock, I.M.; Caramori, G. Transcription Factors. In Asthma and COPD; Elsevier: Amsterdam, The Netherlands, 2009; pp. 373–380. ISBN 978-0-12-374001-4. [Google Scholar]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB Transcription Factors in Abiotic and Biotic Stress Tolerance in Plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lü, S.; Tran, L.P.; Xu, J. The R2R3-MYB Transcription Factor ATMYB49 Modulates Salt Tolerance in Arabidopsis by Modulating the Cuticle Formation and Antioxidant Defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB Transcription Factor Genes as Regulators for Plant Responses: An Overview. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Camargo, E.L.O.; Carocha, V.; Cassan-Wang, H.; San Clemente, H.; Savelli, B.; Hefer, C.A.; Paiva, J.A.P.; Myburg, A.A.; Grima-Pettenati, J. The Eucalyptus grandis R2R3- MYB Transcription Factor Family: Evidence for Woody Growth-related Evolution and Function. New Phytol. 2015, 206, 1364–1377. [Google Scholar] [CrossRef]
- Meissner, R.C.; Jin, H.; Cominelli, E.; Denekamp, M.; Fuertes, A.; Greco, R.; Kranz, H.D.; Penfield, S.; Petroni, K.; Urzainqui, A.; et al. Function Search in a Large Transcription Factor Gene Family in Arabidopsis: Assessing the Potential of Reverse Genetics to Identify Insertional Mutations in R2R3 MYB Genes. Plant Cell 1999, 11, 1827–1840. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.; Gupta, K.; Agarwal, P.K. Molecular Characterization of an MYB Transcription Factor from a Succulent Halophyte Involved in Stress Tolerance. AoB Plants 2015, 7, plv054. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef]
- Li, C.; Ng, C.K.-Y.; Fan, L.M. MYB Transcription Factors, Active Players in Abiotic Stress Signaling. Environ. Exp. Bot. 2015, 114, 80–91. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, H.; Xin, Z.; Zhou, C.; Li, H.; Li, T.; Zhang, A.; Cheng, M.; Li, X.; Li, G.; et al. Functional Characterization of TaWRKY254 in Salt Tolerance Based on Genome-Wide Analysis of the WRKY Gene Family in Wheat Core Parent Zhou8425B. Plant Sci. 2025, 357, 112540. [Google Scholar] [CrossRef]
- Price, L.; Han, Y.; Angessa, T.; Li, C. Molecular Pathways of WRKY Genes in Regulating Plant Salinity Tolerance. Int. J. Mol. Sci. 2022, 23, 10947. [Google Scholar] [CrossRef] [PubMed]
- Chanwala, J.; Kumari, K.; Jha, D.K.; Giri, M.K.; Dey, N. Pearl Millet WRKY Transcription Factor PgWRKY52 Positively Regulates Salt Stress Tolerance through ABA-MeJA Mediated Transcriptional Regulation. Plant Stress 2025, 16, 100814. [Google Scholar] [CrossRef]
- Yang, L.; Fang, S.; Liu, L.; Zhao, L.; Chen, W.; Li, X.; Xu, Z.; Chen, S.; Wang, H.; Yu, D. WRKY Transcription Factors: Hubs for Regulating Plant Growth and Stress Responses. J. Integr. Plant Biol. 2025, 67, 488–509. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.; Baig, A.; Mehbood, I.; Maroof, M. Analysis of SOS1 Promoter in Various Plant Species for WRKY Transcription Activation. Agric. Res. Technol. Open Access J. 2019, 22, 209–212. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Qu, C.; Mo, X.; Zhang, X. Genome-Wide Identification of WRKY in Suaeda australis against Salt Stress. Forests 2024, 15, 1297. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC Transcription Factors in the Regulation of Biotic and Abiotic Stress Responses in Plants. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC Transcription Factors in Plant Abiotic Stress Responses. Biochim. Biophys. Acta (BBA)—GeneRegul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef]
- Islam, W.; Waheed, A.; Naveed, H.; Zeng, F. MicroRNAs Mediated Plant Responses to Salt Stress. Cells 2022, 11, 2806. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Fan, P.; Jia, W.; Nie, L.; Jiang, P.; Chen, X.; Lv, S.; Wan, L.; Chang, S.; et al. High-Throughput Deep Sequencing Reveals That microRNAs Play Important Roles in Salt Tolerance of Euhalophyte Salicornia europaea. BMC Plant Biol. 2015, 15, 63. [Google Scholar] [CrossRef]
- Udawat, P.; Jha, R.K.; Mishra, A.; Jha, B. Overexpression of a Plasma Membrane-Localized SbSRP-Like Protein Enhances Salinity and Osmotic Stress Tolerance in Transgenic Tobacco. Front. Plant Sci. 2017, 8, 582. [Google Scholar] [CrossRef]
- Dubey, A.K.; Khatri, K.; Jha, B.; Rathore, M.S. The Novel Galactosyl Transferase-like (SbGalT) Gene from Salicornia brachiata Maintains Photosynthesis and Enhances Abiotic Stress Tolerance in Transgenic Tobacco. Gene 2021, 786, 145597. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.H.; Koo, S.S.; Oh, H.T.; Lee, E.S.; Park, J.H.; Phan, K.A.T.; Wi, S.D.; Bae, S.B.; Paeng, S.K.; Chae, H.B.; et al. The Physiological Functions of Universal Stress Proteins and Their Molecular Mechanism to Protect Plants From Environmental Stresses. Front. Plant Sci. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Udawat, P.; Dubey, A.K.; Haque, M.I.; Rathore, M.S.; Jha, B. Overexpression of SbSI-1, A Nuclear Protein from Salicornia brachiata Confers Drought and Salt Stress Tolerance and Maintains Photosynthetic Efficiency in Transgenic Tobacco. Front. Plant Sci. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Singhal, R.K.; Saha, D.; Skalicky, M.; Mishra, U.N.; Chauhan, J.; Behera, L.P.; Lenka, D.; Chand, S.; Kumar, V.; Dey, P.; et al. Crucial Cell Signaling Compounds Crosstalk and Integrative Multi-Omics Techniques for Salinity Stress Tolerance in Plants. Front. Plant Sci. 2021, 12, 670369. [Google Scholar] [CrossRef] [PubMed]
- Hurrah, I.M.; Mohiuddin, T.; Mandal, S.; Kumar, V.; Gupta, A. Crosstalk and Interaction among Salt Stress Tolerance Pathways. In Exogenous Priming and Engineering of Plant Metabolic and Regulatory Genes; Elsevier: Amsterdam, The Netherlands, 2025; pp. 513–529. ISBN 978-0-443-13490-6. [Google Scholar]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of Irrigation Salinity and Ecotype on the Growth, Physiological Indicators and Seed Yield and Quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated Protein Kinase Cascades in Plant Signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Santin, M.; Parichanon, P.; Sciampagna, M.C.; Ranieri, A.; Castagna, A. Enhancing Tomato Productivity and Quality in Moderately Saline Soils through Salicornia-Assisted Cultivation Methods: A Comparative Study. Horticulturae 2024, 10, 655. [Google Scholar] [CrossRef]
- AlYammahi, J.; Chelaifa, H.; Hasan, A.; Darwish, A.S.; Lemaoui, T.; Hernandez, H.H.; Rios-Galvan, A. Salicornia Seed Oil: A High-Yielding and Sustainable Halophytic Feedstock for Biodiesel and Energy in Underutilized Hypersaline Coastal Deserts. Energy Convers. Manag. 2024, 318, 118914. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Grigore, M.N.; Piernik, A. Prediction of Salicornia europaea L. Biomass Using a Computer Vision System to Distinguish Different Salt-Tolerant Populations. BMC Plant Biol. 2024, 24, 1086. [Google Scholar] [CrossRef]
- Wynter, L.; Suissa, A.; Michnik, M.; Cremona, L.B.; Jin, X.-L.; Zhang, X.-H. Ectopic Expression of a Thaumatin-like Gene from the Halophyte Plant Salicornia europaea Enhances Salt Tolerance in Glycophyte Plants. J. Plant Biochem. Biotechnol. 2025, 1–11. [Google Scholar] [CrossRef]
- Orzoł, A.; Głowacka, K.; Pätsch, R.; Piernik, A.; Gallegos-Cerda, S.D.; Cárdenas-Pérez, S. The Local Environment Influences Salt Tolerance Differently in Four Salicornia europaea L. Inland Populations. Sci. Rep. 2025, 15, 13128. [Google Scholar] [CrossRef] [PubMed]
- Shiri, M.; Rabhi, M.; El Amrani, A.; Abdelly, C. Cross-Tolerance to Abiotic Stresses in Halophytes: Application for Phytoremediation of Organic Pollutants. Acta Physiol. Plant. 2015, 37, 209. [Google Scholar] [CrossRef]
- Jiang, Z.; Van Zanten, M.; Sasidharan, R. Mechanisms of Plant Acclimation to Multiple Abiotic Stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Jordine, A.; Retzlaff, J.; Gens, L.; Ehrt, B.; Fürtauer, L.; Van Dongen, J.T. Introducing the Halophyte Salicornia europaea to Investigate Combined Impact of Salt and Tidal Submergence Conditions. Funct. Plant Biol. 2024, 51, FP23228. [Google Scholar] [CrossRef]
- Jha, R.K.; Mishra, A. Introgression of SbERD4 Gene Encodes an Early-Responsive Dehydration-Stress Protein That Confers Tolerance against Different Types of Abiotic Stresses in Transgenic Tobacco. Cells 2021, 11, 62. [Google Scholar] [CrossRef]
- Chen, X.; Han, H.; Jiang, P.; Nie, L.; Bao, H.; Fan, P.; Lv, S.; Feng, J.; Li, Y. Transformation of β-Lycopene Cyclase Genes from Salicornia europaea and Arabidopsis Conferred Salt Tolerance in Arabidopsis and Tobacco. Plant Cell Physiol. 2011, 52, 909–921. [Google Scholar] [CrossRef]
- Sun, X.; Deng, Y.; Liang, L.; Jia, X.; Xiao, Z.; Su, J. Overexpression of a PIP1 Gene from Salicornia bigelovii in Tobacco Plants Improves Their Drought Tolerance. J. Am. Soc. Hortic. Sci. 2017, 142, 235–245. [Google Scholar] [CrossRef]
- Ermawati, N.; Liang, Y.S.; Cha, J.-Y.; Shin, D.; Jung, M.H.; Lee, J.J.; Lee, B.-H.; Han, C.D.; Lee, K.H.; Son, D. A New Tip Homolog, ShTIP, from Salicornia Shows a Different Involvement in Salt Stress Compared to That of TIP from Arabidopsis. Biol. Plant. 2009, 53, 271–277. [Google Scholar] [CrossRef]

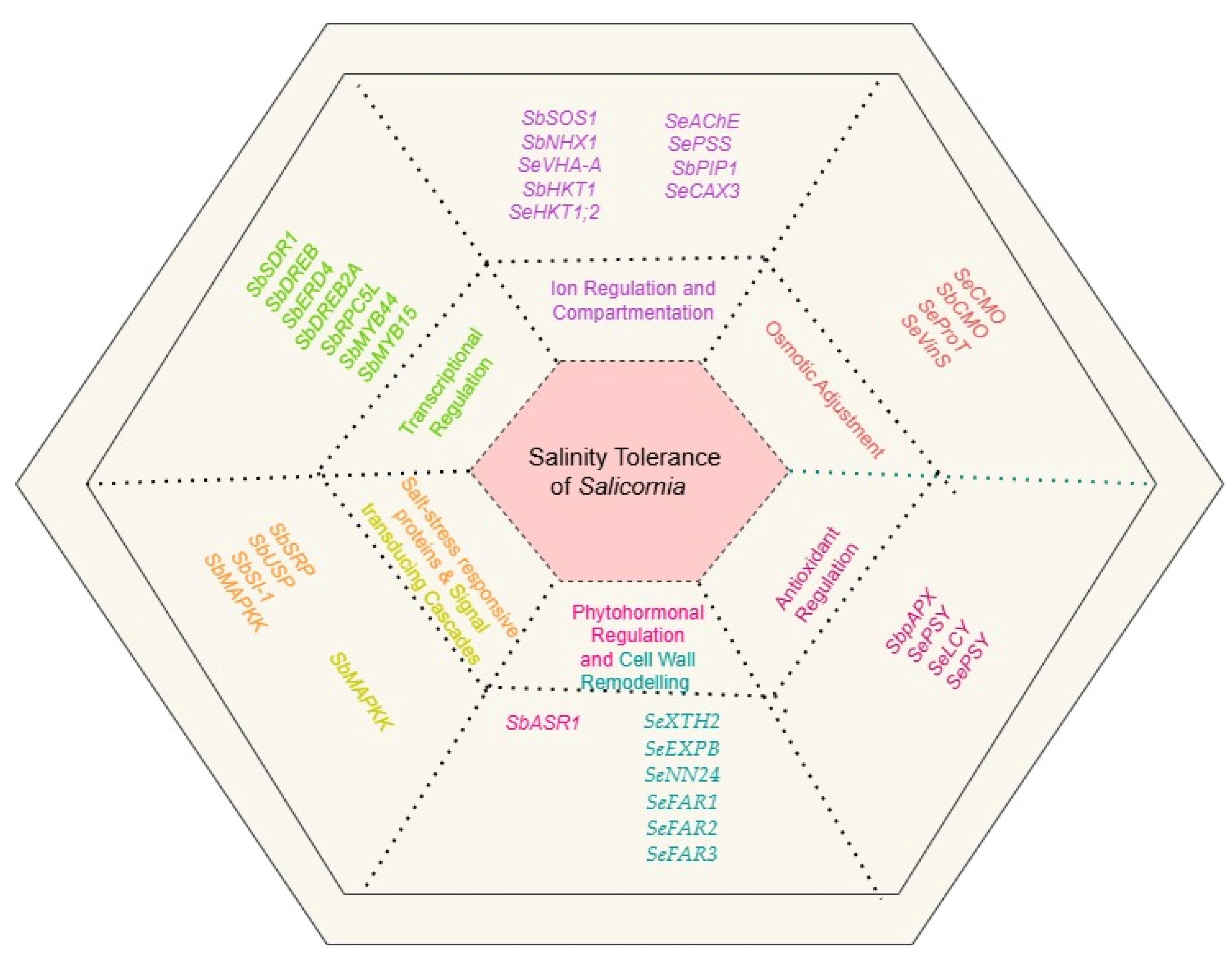
| Salicornia Species | Gene | Function | Source |
|---|---|---|---|
| Salicornia brachiata | SbGSTU | Reduces secondary noxious by-products generated during oxidative stress and exhibited potential signaling functions | [142,162] |
| Salicornia brachiata Salicornia europaea | SbNHX1 SeNHX1 | Maintains ion homeostasis by regulating the sequestration of Na+ into vacuoles | [90,91] |
| Salicornia bigelovi | SbHKT1 | Assists in maintaining K+/Na+ homeostasis by increasing the capacity of K+ uptake | [95] |
| Salicornia europaea | SeHKT1;2 | Reduces Na+ retrieval from the xylem and enhances Na+ transport into shoot tissues | [96] |
| Salicornia brachiata | SbMAPKK | Phosphorylates proteins and other cellular substrates to regulate them over abiotic stress | [250] |
| Salicornia brachiata | SbDREB2A | Serves as a transcription factor (TF) by regulating the expression of stress- responsive genes | [240,241] |
| Salicornia brachiata | SbMT-2 | Modulates the ROS scavenging and confers abiotic stress tolerance tolerance to plants | [23] |
| Salicornia brachiata | SbSLSP | Enhances ROS scavenging, efficiency of transporters and the stability of cell membrane, and improves clathrin-coated vesicle-mediated endocytosis, leading to efficient the stress signaling | [24] |
| Salicornia brachiata | SbpAPX | Involved in scavenging ROS and protecting cells against their toxic effects under salt and drought stress conditions | [160] |
| Salicornia brachiata | SbSOS1 | Encodes a Na+/H+ antiporter located in plasma membrane that plays an important role in imparting salt stress tolerance to plants | [88] |
| Salicornia brachiata | SbASR1 | Encodes stress-responsive nuclear protein functioning as a transcription factor which regulates expression of stress responsive genes | [200] |
| Salicornia brachiata | SbUSP | Encodes a membrane-bound cytosolic protein, regulates ROS accumulation, and is involved in maintaining ion homeostasis | [26] |
| Salicornia brachiata | SbSRP | Encodes transporter protein to transmit the environmental stimuli downward through the plasma membrane improving the abiotic stress tolerance | [280] |
| Salicornia brachiata | SbSI-1 | Encodes a salt-responsive nuclear protein which enhances the antioxidant activity and maintains osmotic homeostasis | [283] |
| Salicornia brachiata | SbRPC5L | Regulates expression of many stress-responsive genes and transcription factors | [25] |
| Salicornia brachiata | SbGalT | Minimizes the buildup of reactive oxygen species (ROS) and maintains the membrane integrity | [281] |
| Salicornia brachiata | SbSDR1 | Functions as a molecular switch and contributes to salt and osmotic tolerance | [201] |
| Salicornia brachiata | SbERD4 | Encodes a plasma-membrane-bound protein which alleviates osmotic and salt stresses by moderating physio-biochemical processes | [296] |
| Salicornia brachiata | SbMYB44 SbMYB15 | Act as transcription factors which regulate a range of genes crucil for abiotic stress tolerance | [259,267] |
| Salicornia europaea | SeXTH | Encodes a cell wall manipulating enzyme, which improves cellular anatomy and physiology to mitigate abiotic stresses | [47] |
| Salicornia europaea | SeXTH2 | Involved in cell wall remodelling by producing enzyme under abiotic stress | [64] |
| Salicornia europaea | SeEXPB | Encodes an expansin protein, which assists in cell wall remodelling via enhancing the expansion properties | [64] |
| Salicornia europaea | SeNN24 | Encodes a TPL-like protein which is involved in cell wall modifications to alleviate salt stress | [68,69] |
| Salicornia europaea | SeFAR1 SeFAR2 SeFAR3 | Involved in cuticular wax biosynthesis to enhance defense gainst abiotic and biotic stresses | [72] |
| Salicornia europaea | SeCAX3 | Encodes a putative Ca2+/H+ antiporter which modulate ionic homeostasis under salt stress | [45] |
| Salicornia europaea | SeAChE | Believed to be involved in ion transport through channels by a similar way in animal systems | [100] |
| Salicornia europaea | SeVHA-A | Regulates the proton pumping reaction by stimulating the hydrolysis of PPi to energize the antiporters | [44] |
| Salicornia europaea | SePSY | Involved in carotenoid biosynthesis, which detoxifies ROS effectively | [63] |
| Salicornia europaea | SeLCY | Regulates the carotenoid biosynthesis and improve ROS scavenging potential | [297] |
| Salicornia europaea | SeVinS | Encodes a vinorine synthase, which is crucial for alkaloid biosynthesis to maintain osmotic balance | [46] |
| Salicornia europaea | SeProT | Regulates proline accumulation in response to salinity stress by encoding a proline transporter | [46] |
| Salicornia bigelovii | SbPIP | Serves as an aquaporin in plants which facilitates the water and ion transportation | [298] |
| Salicornia herbacea | ShTIP | Modulates a type of aquaporins in vacuoles required for ionic and osmotic stress adaptation | [299] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendis, C.L.; Padmathilake, R.E.; Attanayake, R.N.; Perera, D. Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance. Int. J. Mol. Sci. 2025, 26, 5936. https://doi.org/10.3390/ijms26135936
Mendis CL, Padmathilake RE, Attanayake RN, Perera D. Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance. International Journal of Molecular Sciences. 2025; 26(13):5936. https://doi.org/10.3390/ijms26135936
Chicago/Turabian StyleMendis, Chamara L., Rasanie E. Padmathilake, Renuka N. Attanayake, and Dinum Perera. 2025. "Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance" International Journal of Molecular Sciences 26, no. 13: 5936. https://doi.org/10.3390/ijms26135936
APA StyleMendis, C. L., Padmathilake, R. E., Attanayake, R. N., & Perera, D. (2025). Learning from Salicornia: Physiological, Biochemical, and Molecular Mechanisms of Salinity Tolerance. International Journal of Molecular Sciences, 26(13), 5936. https://doi.org/10.3390/ijms26135936







