Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle
Abstract
1. Introduction
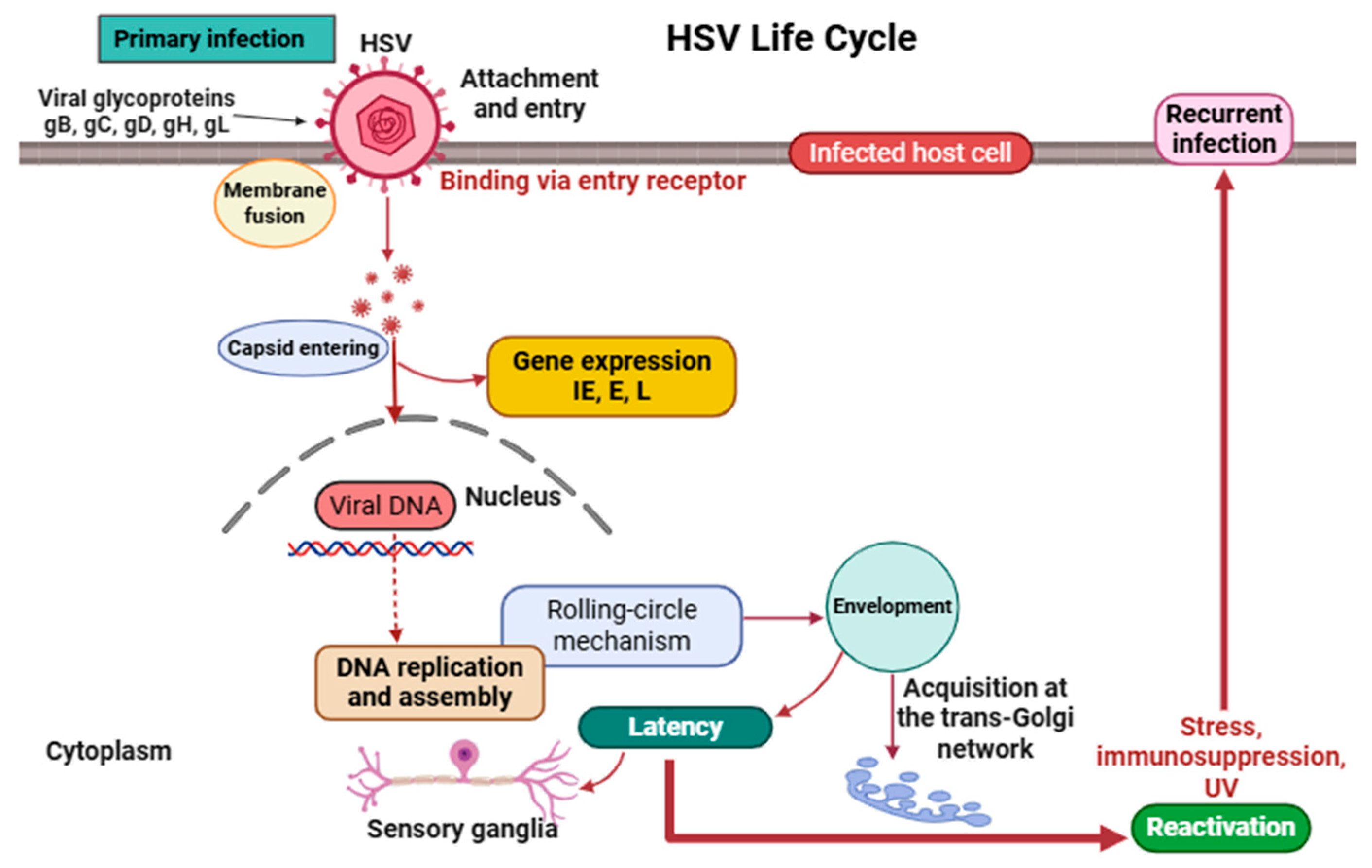
2. Virology and Life Cycle of HSV
2.1. Structure and Genomic Organization
2.2. Life Cycle
3. Overview of Phenolic Acids and Tannins: Chemistry and Antiviral Capacities
4. Anti-HSV Properties of Phenolic Acids and Their Mechanisms of Action
5. Anti-HSV Activities of Tannins and Their Mechanisms of Action
6. Structure–Activity Relationships of Phenolic Acids and Tannins
- Degree and position of hydroxylation, which affect redox activity and protein binding.
- Conjugation or esterification, influencing solubility and target affinity.
- Multivalency and molecular weight, particularly in tannins, enabling simultaneous interaction with multiple viral or host targets.
- Linkage type and core scaffold stability, which can affect intracellular delivery and resistance to metabolic degradation.
7. Clinical Evidence and Translational Potential
8. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widener, R.W.; Whitley, R.J. Herpes Simplex Virus. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 251–263. ISBN 978-0-444-53488-0. [Google Scholar]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Lodi, G. The Controversial Natural History of Oral Herpes Simplex Virus Type 1 Infection. Oral Dis. 2019, 25, 1850–1865. [Google Scholar] [CrossRef]
- Kolokotronis, A.; Doumas, S. Herpes Simplex Virus Infection, with Particular Reference to the Progression and Complications of Primary Herpetic Gingivostomatitis. Clin. Microbiol. Infect. 2006, 12, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Gnann, J.W.; Whitley, R.J. Herpes Simplex Encephalitis: An Update. Curr. Infect. Dis. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Omarova, S.; Cannon, A.; Weiss, W.; Bruccoleri, A.; Puccio, J. Genital Herpes Simplex Virus—An Updated Review. Adv. Pediatr. 2022, 69, 149–162. [Google Scholar] [CrossRef]
- Harfouche, M.; Maalmi, H.; Abu-Raddad, L.J. Epidemiology of Herpes Simplex Virus Type 2 in Latin America and the Caribbean: Systematic Review, Meta-Analyses and Metaregressions. Sex. Transm. Infect. 2021, 97, 490–500. [Google Scholar] [CrossRef]
- Desai, D.V.; Kulkarni, S.S. Herpes Simplex Virus: The Interplay Between HSV, Host, and HIV-1. Viral Immunol. 2015, 28, 546–555. [Google Scholar] [CrossRef]
- Corey, L. Synergistic Copathogens—HIV-1 and HSV-2. N. Engl. J. Med. 2007, 356, 854–856. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human Herpes Simplex Virus Infections: Epidemiology, Pathogenesis, Symptomatology, Diagnosis, and Management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’Amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef]
- Pinninti, S.G.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Infections. Semin. Perinatol. 2018, 42, 168–175. [Google Scholar] [CrossRef]
- Hammad, W.A.B.; Konje, J.C. Herpes Simplex Virus Infection in Pregnancy—An Update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mindel, A. Psychological and Psychosexual Implications of Herpes Simplex Virus Infections. Scand. J. Infect. Dis. Suppl. 1996, 100, 27–32. [Google Scholar]
- Mindel, A.; Marks, C. Psychological Symptoms Associated with Genital Herpes Virus Infections: Epidemiology and Approaches to Management. CNS Drugs 2005, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir Resistance in Herpes Simplex Viruses: Prevalence and Therapeutic Alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, L.; Wu, J.; Cheng, J.; Duan, Y.; Qian, W. Anti-HSV-1 Agents: An Update. Front. Pharmacol. 2025, 15, 1451083. [Google Scholar] [CrossRef]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Denes, C.E.; Everett, R.D.; Diefenbach, R.J. Tour de Herpes: Cycling Through the Life and Biology of HSV-1. In Herpes Simplex Virus; Diefenbach, R.J., Fraefel, C., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2020; Volume 2060, pp. 1–30. ISBN 978-1-4939-9813-5. [Google Scholar]
- Šudomová, M.; Hassan, S.T.S. Flavonoids with Anti-Herpes Simplex Virus Properties: Deciphering Their Mechanisms in Disrupting the Viral Life Cycle. Viruses 2023, 15, 2340. [Google Scholar] [CrossRef]
- Patel, A.; Patel, R. Recent Insights into HSV Infection and Disease: Results of Wider Genome Analysis. Curr. Opin. Infect. Dis. 2019, 32, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Furlong, D.; Swift, H.; Roizman, B. Arrangement of Herpesvirus Deoxyribonucleic Acid in the Core. J. Virol. 1972, 10, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Kuny, C.V.; Szpara, M.L. Alphaherpesvirus Genomics: Past, Present and Future. Curr. Issues Mol. Biol. 2022, 42, 41–80. [Google Scholar] [CrossRef]
- Bhowmik, D.; Zhu, F. Evasion of Intracellular DNA Sensing by Human Herpesviruses. Front. Cell. Infect. Microbiol. 2021, 11, 647992. [Google Scholar] [CrossRef] [PubMed]
- Homa, F.L.; Brown, J.C. Capsid Assembly and DNA Packaging in Herpes Simplex Virus. Rev. Med. Virol. 1997, 7, 107–122. [Google Scholar] [CrossRef]
- Goshima, F.; Watanabe, D.; Takakuwa, H.; Wada, K.; Daikoku, T.; Yamada, M.; Nishiyama, Y. Herpes Simplex Virus UL17 Protein Is Associated with B Capsids and Colocalizes with ICP35 and VP5 in Infected Cells. Arch. Virol. 2000, 145, 417–426. [Google Scholar] [CrossRef]
- Ding, X.; Neumann, D.M.; Zhu, L. Host Factors Associated with Either VP16 or VP16-induced Complex Differentially Affect HSV-1 Lytic Infection. Rev. Med. Virol. 2022, 32, e2394. [Google Scholar] [CrossRef]
- Shiflett, L.A.; Read, G.S. mRNA Decay during Herpes Simplex Virus (HSV) Infections: Mutations That Affect Translation of an mRNA Influence the Sites at Which It Is Cleaved by the HSV Virion Host Shutoff (Vhs) Protein. J. Virol. 2013, 87, 94–109. [Google Scholar] [CrossRef]
- DuRaine, G.; Wisner, T.W.; Johnson, D.C. Characterization of the Herpes Simplex Virus (HSV) Tegument Proteins That Bind to gE/gI and US9, Which Promote Assembly of HSV and Transport into Neuronal Axons. J. Virol. 2020, 94, e01113-20. [Google Scholar] [CrossRef]
- Owen, D.; Crump, C.; Graham, S. Tegument Assembly and Secondary Envelopment of Alphaherpesviruses. Viruses 2015, 7, 5084–5114. [Google Scholar] [CrossRef]
- Frost, T.C.; Salnikov, M.; Rice, S.A. Enhancement of HSV-1 Cell-Free Virion Release by the Envelope Protein gC. Virology 2024, 596, 110120. [Google Scholar] [CrossRef]
- Vallbracht, M.; Backovic, M.; Klupp, B.G.; Rey, F.A.; Mettenleiter, T.C. Common Characteristics and Unique Features: A Comparison of the Fusion Machinery of the Alphaherpesviruses Pseudorabies Virus and Herpes Simplex Virus. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 104, pp. 225–281. ISBN 978-0-12-818394-6. [Google Scholar]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human Herpes Simplex Virus: Life Cycle and Development of Inhibitors. Biochem. Mosc. 2014, 79, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Zachariah, S.; Phillips, A.T.; Tscharke, D. Lytic Promoter Activity during Herpes Simplex Virus Latency Is Dependent on Genome Location. J. Virol. 2024, 98, e01258-24. [Google Scholar] [CrossRef] [PubMed]
- Azab, W.; Osterrieder, K. Initial Contact: The First Steps in Herpesvirus Entry. In Cell Biology of Herpes Viruses; Advances in Anatomy, Embryology and Cell Biology; Osterrieder, K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 223, pp. 1–27. ISBN 978-3-319-53167-0. [Google Scholar]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The Structural Basis of Herpesvirus Entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef]
- Arii, J.; Kawaguchi, Y. The Role of HSV Glycoproteins in Mediating Cell Entry. In Human Herpesviruses; Advances in Experimental Medicine and Biology; Kawaguchi, Y., Mori, Y., Kimura, H., Eds.; Springer: Singapore, 2018; Volume 1045, pp. 3–21. ISBN 978-981-10-7229-1. [Google Scholar]
- Pertel, P.E.; Fridberg, A.; Parish, M.L.; Spear, P.G. Cell Fusion Induced by Herpes Simplex Virus Glycoproteins gB, gD, and gH-gL Requires a gD Receptor but Not Necessarily Heparan Sulfate. Virology 2001, 279, 313–324. [Google Scholar] [CrossRef]
- Heming, J.D.; Conway, J.F.; Homa, F.L. Herpesvirus Capsid Assembly and DNA Packaging. In Cell Biology of Herpes Viruses; Advances in Anatomy, Embryology and Cell Biology; Osterrieder, K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 223, pp. 119–142. ISBN 978-3-319-53167-0. [Google Scholar]
- Adlakha, M.; Livingston, C.M.; Bezsonova, I.; Weller, S.K. The Herpes Simplex Virus 1 Immediate Early Protein ICP22 Is a Functional Mimic of a Cellular J Protein. J. Virol. 2020, 94, e01564-19. [Google Scholar] [CrossRef] [PubMed]
- Baines, J.D. Herpes Simplex Virus Capsid Assembly and DNA Packaging: A Present and Future Antiviral Drug Target. Trends Microbiol. 2011, 19, 606–613. [Google Scholar] [CrossRef]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA Polymerases: Structures, Functions and Inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef]
- Skaliter, R.; Lehman, I.R. Rolling Circle DNA Replication in Vitro by a Complex of Herpes Simplex Virus Type 1-Encoded Enzymes. Proc. Natl. Acad. Sci. USA 1994, 91, 10665–10669. [Google Scholar] [CrossRef]
- Weller, S.K.; Coen, D.M. Herpes Simplex Viruses: Mechanisms of DNA Replication. Cold Spring Harb. Perspect. Biol. 2012, 4, a013011. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Badani, H.; Bos, N.; Scianna, C.; Hoskins, I.; Baird, N.L.; Gilden, D. Alphaherpesvirus DNA Replication in Dissociated Human Trigeminal Ganglia. J. Neurovirol. 2016, 22, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, I.; Tang, K.-W.; Elias, P. Replication and Recombination of Herpes Simplex Virus DNA. J. Biol. Chem. 2011, 286, 15619–15624. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.E.; Mossman, K.L. Cellular Protein WDR11 Interacts with Specific Herpes Simplex Virus Proteins at the Trans-Golgi Network To Promote Virus Replication. J. Virol. 2015, 89, 9841–9852. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Lomonte, P. Herpesvirus Latency: On the Importance of Positioning Oneself. In Cell Biology of Herpes Viruses; Advances in Anatomy, Embryology and Cell Biology; Osterrieder, K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 223, pp. 95–117. ISBN 978-3-319-53167-0. [Google Scholar]
- Wechsler, S.L.; Nesburn, A.B.; Watson, R.; Slanina, S.M.; Ghiasi, H. Fine Mapping of the Latency-Related Gene of Herpes Simplex Virus Type 1: Alternative Splicing Produces Distinct Latency-Related RNAs Containing Open Reading Frames. J. Virol. 1988, 62, 4051–4058. [Google Scholar] [CrossRef]
- Efstathiou, S.; Preston, C.M. Towards an Understanding of the Molecular Basis of Herpes Simplex Virus Latency. Virus Res. 2005, 111, 108–119. [Google Scholar] [CrossRef]
- Bloom, D.C. HSV LAT and neuronal survival. Int. Rev. Immunol. 2004, 23, 187–198. [Google Scholar] [CrossRef]
- Harrison, K.S.; Jones, C. Regulation of Herpes Simplex Virus Type 1 Latency-Reactivation Cycle and Ocular Disease by Cellular Signaling Pathways. Exp. Eye Res. 2022, 218, 109017. [Google Scholar] [CrossRef]
- Roizman, B.; Whitley, R.J. An Inquiry into the Molecular Basis of HSV Latency and Reactivation. Annu. Rev. Microbiol. 2013, 67, 355–374. [Google Scholar] [CrossRef]
- Ho, D.Y.; Enriquez, K.; Multani, A. Herpesvirus Infections Potentiated by Biologics. Infect. Dis. Clin. N. Am. 2020, 34, 311–339. [Google Scholar] [CrossRef]
- Turuvekere Vittala Murthy, N.; Agrahari, V.; Chauhan, H. Polyphenols against Infectious Diseases: Controlled Release Nano-Formulations. Eur. J. Pharm. Biopharm. 2021, 161, 66–79. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M.; Mazurakova, A.; Kubatka, P. Insights into Antiviral Properties and Molecular Mechanisms of Non-Flavonoid Polyphenols against Human Herpesviruses. Int. J. Mol. Sci. 2022, 23, 13891. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive Natural Products with Anti-Herpes Simplex Virus Properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why Is It Important to Understand the Nature and Chemistry of Tannins to Exploit Their Potential as Nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in Foods: Classification, Methods of Identification, and Nutritional Aspects in Human Health. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 98, pp. 1–33. ISBN 978-0-12-822506-6. [Google Scholar]
- Seidi, F.; Liu, Y.; Huang, Y.; Xiao, H.; Crespy, D. Chemistry of Lignin and Condensed Tannins as Aromatic Biopolymers. Chem. Soc. Rev. 2025, 54, 3140–3232. [Google Scholar] [CrossRef]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Lin, L.-T.; Chen, T.-Y.; Chung, C.-Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.-C.; Wang, G.-H.; Lin, C.-C.; Richardson, C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) Target Viral Glycoprotein-Glycosaminoglycan Interactions To Inhibit Herpes Simplex Virus 1 Entry and Cell-to-Cell Spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, L.; Ju, F. In Vitro and Silico Studies of Geraniin Interfering with HSV-2 Replication by Targeting Glycoprotein D. Nat. Prod. Res. 2024, 38, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus Sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-Enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L.; et al. Ginkgolic Acid Inhibits Fusion of Enveloped Viruses. Sci. Rep. 2020, 10, 4746. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Sobczyński, M.; Ochnik, M.; Zwolińska, K.; Leszek, J. Hampering Herpesviruses HHV-1 and HHV-2 Infection by Extract of Ginkgo Biloba (EGb) and Its Phytochemical Constituents. Front. Microbiol. 2019, 10, 2367. [Google Scholar] [CrossRef]
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice. Viruses 2021, 13, 86. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum Melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef] [PubMed]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- AbouAitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13, 2174. [Google Scholar] [CrossRef]
- Todorova, N.; Rangelov, M.; Dincheva, I.; Badjakov, I.; Enchev, V.; Markova, N. Potential of Hydroxybenzoic Acids from Graptopetalum Paraguayense for Inhibiting of Herpes Simplex Virus DNA Polymerase—Metabolome Profiling, Molecular Docking and Quantum-Chemical Analysis. Pharmacia 2022, 69, 113–123. [Google Scholar] [CrossRef]
- EL-Aguel, A.; Pennisi, R.; Smeriglio, A.; Kallel, I.; Tamburello, M.P.; D’Arrigo, M.; Barreca, D.; Gargouri, A.; Trombetta, D.; Mandalari, G.; et al. Punica granatum Peel and Leaf Extracts as Promising Strategies for HSV-1 Treatment. Viruses 2022, 14, 2639. [Google Scholar] [CrossRef]
- Aljohani, A.K.; Maghrabi, N.A.; Alrehili, O.M.; Alharbi, A.S.; Alsihli, R.S.; Alharthe, A.M.; Albladi, R.S.; Alosaimi, K.A.; Albadrani, B.M.; Miski, S.F.; et al. Ajwa Date Extract (Phoenix Dactylifera L.): Phytochemical Analysis, Antiviral Activity against Herpes Simplex Virus-I and Coxsackie B4 Virus, and in Silico Study. Saudi Med. J. 2025, 46, 26–35. [Google Scholar] [CrossRef]
- Siqueira, E.M.d.S.; Lima, T.L.C.; Boff, L.; Lima, S.G.M.; Lourenço, E.M.G.; Ferreira, É.G.; Barbosa, E.G.; Machado, P.R.L.; Farias, K.J.S.; Ferreira, L.d.S.; et al. Antiviral Potential of Spondias Mombin L. Leaves Extract Against Herpes Simplex Virus Type-1 Replication Using In Vitro and In Silico Approaches. Planta Med. 2020, 86, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 Activity of Terminalia Chebula Retz Extract and Its Constituents, Chebulagic and Chebulinic Acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Deffieux, D.; Pouységu, L.; Sylla, T.; Chassaing, S.; Nikolova, I.; Quideau, S.; Galabov, A.S. Anti-Herpes Simplex Virus Type 1 Activity of Specially Selected Groups of Tannins. Drug Res. 2019, 69, 373–374. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A.S. Ellagitannins as Synergists of ACV on the Replication of ACV-Resistant Strains of HSV 1 and 2. Antivir. Res. 2014, 110, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.; Popatanasov, A.; Rashev, V.; Tancheva, L.; Quideau, S.; Galabov, A.S. Effect of Castalagin against HSV-1 Infection in Newborn Mice. Nat. Prod. Res. 2023, 37, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Luo, T.; Wu, F.; Liu, H.; Li, H.-R.; Mei, Y.-W.; Zhang, S.-L.; Tao, J.-Y.; Dong, J.-H.; Fang, Y.; et al. Corilagin Protects Against HSV1 Encephalitis Through Inhibiting the TLR2 Signaling Pathways In Vivo and In Vitro. Mol. Neurobiol. 2015, 52, 1547–1560. [Google Scholar] [CrossRef]
- Jin, F.; Ma, K.; Chen, M.; Zou, M.; Wu, Y.; Li, F.; Wang, Y. Pentagalloylglucose Blocks the Nuclear Transport and the Process of Nucleocapsid Egress to Inhibit HSV-1 Infection. Jpn. J. Infect. Dis. 2016, 69, 135–142. [Google Scholar] [CrossRef]
- Arunkumar, J.; Rajarajan, S. Study on Antiviral Activities, Drug-Likeness and Molecular Docking of Bioactive Compounds of Punica granatum L. to Herpes Simplex Virus-2 (HSV-2). Microb. Pathog. 2018, 118, 301–309. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated Virucidal Activity of Pomegranate Rind Extract (PRE) and Punicalagin against Herpes Simplex Virus (HSV) When Co-Administered with Zinc (II) Ions, and Antiviral Activity of PRE against HSV and Aciclovir-Resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.S.; Brawner, T.A.; Hall, D.G. New Treatment for Herpes Simplex Virus Type 2 [Ultrasound and Zinc, Urea and Tannic Acid Ointment]. Part II: Female Patients. J. Med. 1980, 11, 143–167. [Google Scholar] [PubMed]
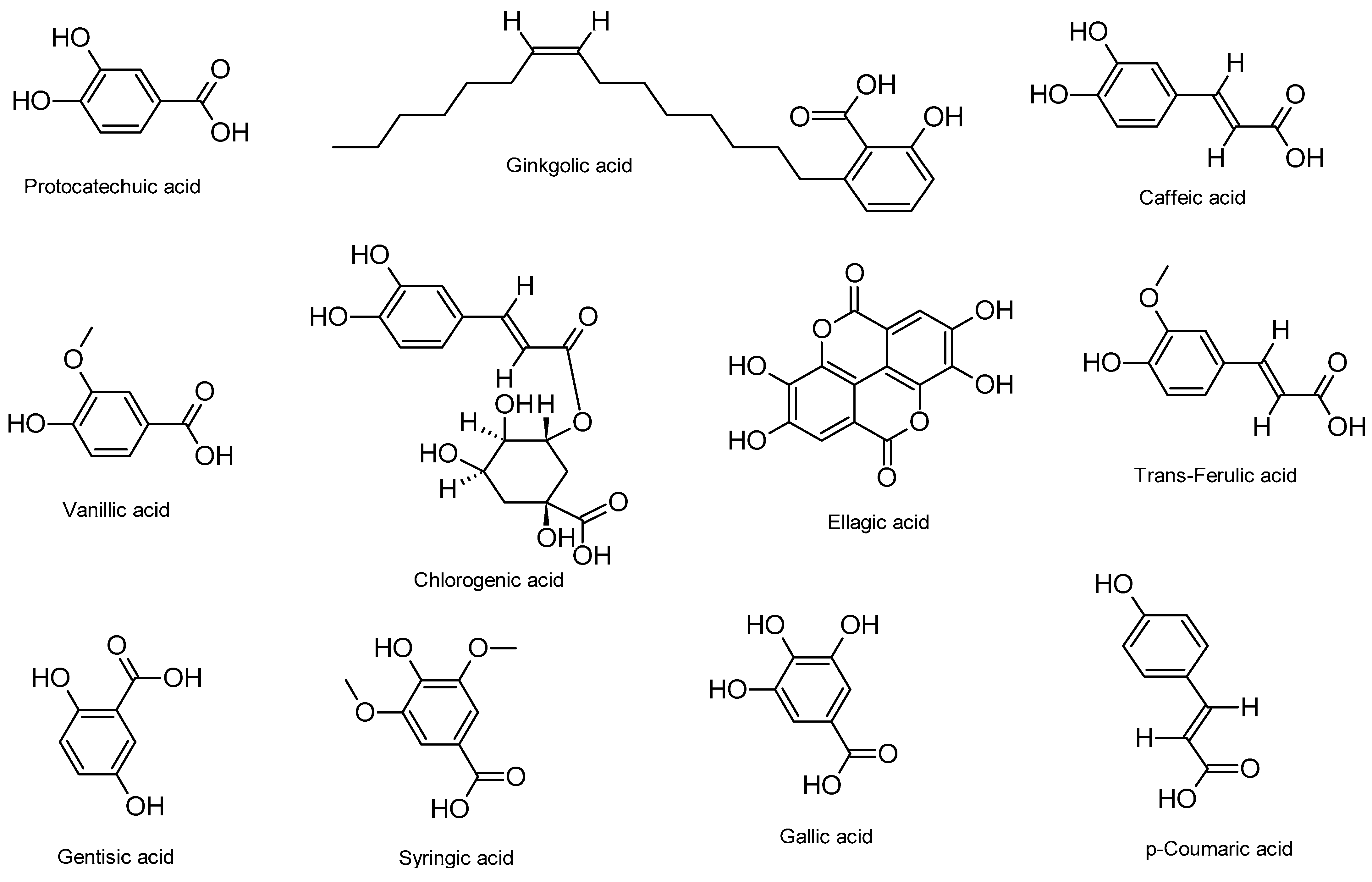
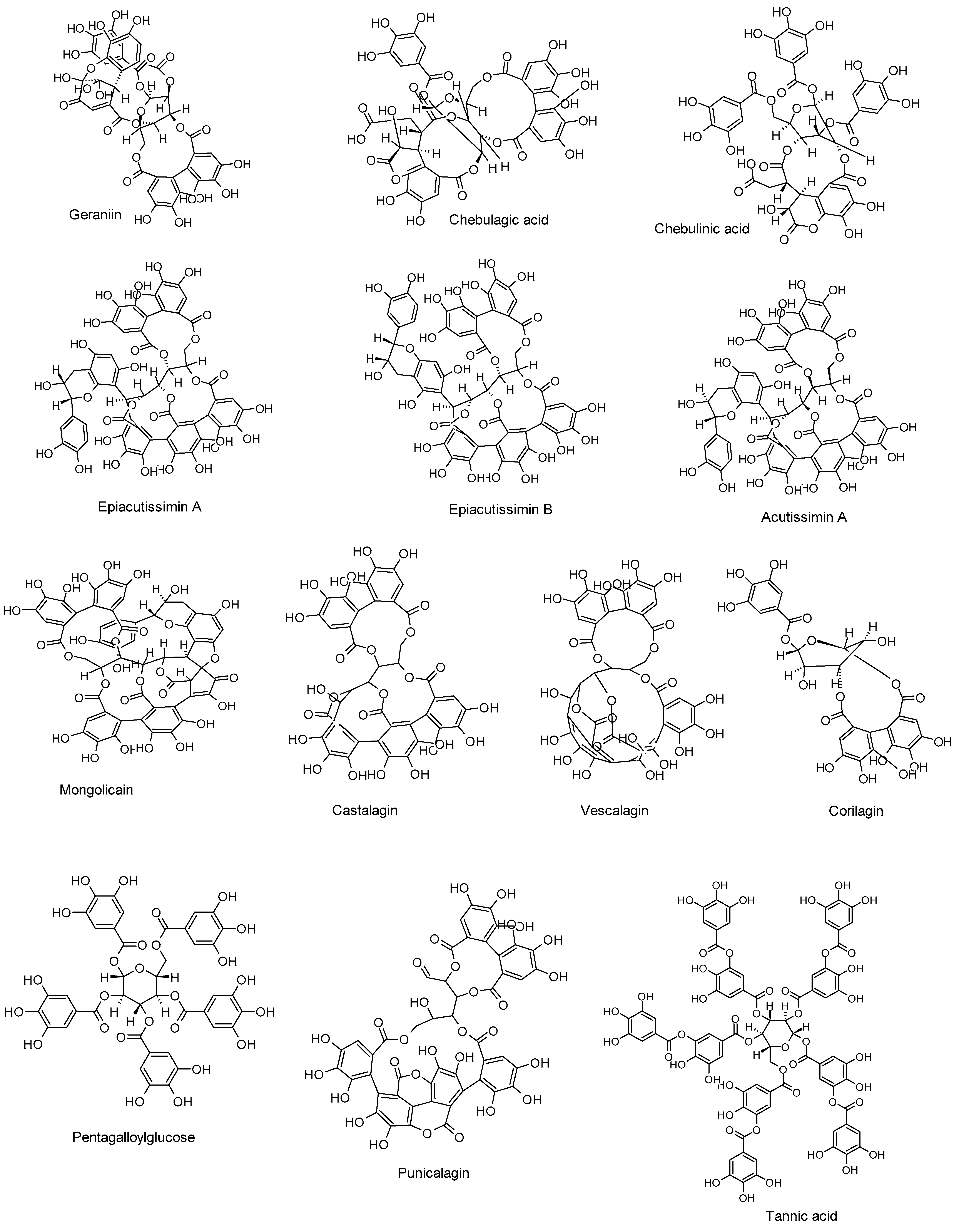
| Compound and Botanical Source | Study Type, HSV Strains, Cells, and Animal Models | Mechanisms of Action (Inhibition) | Effective Concentration/Dose | Refs. |
|---|---|---|---|---|
| Protocatechuic acid Hibiscus sabdariffa L. | In vitro HSV-2 Vero cells | DNA replication | 0.9 µg/mL | [79] |
| Ginkgolic acid Ginkgo biloba | In vitro HSV-1 HEp-2, 293T, and Vero cells | DNA replication ICP27, ICP8, US11 protein expressions, viral particles, and post-entry | 2.5–50 µM | [80] |
| In vitro HSV-1 and HSV-2 A549 cells | Viral attachment, entry, and DNA replication | <5 ppm | [81] | |
| In vitro and in vivo ACV-resistant HSV-1 Vero cells BALB/cJ mice | Viral particles DNA replication | 10 µM (in vitro) 10 mM in 2.5% HEC gel (twice daily for 14 days; in vivo) | [82] | |
| Caffeic acid, vanillic acid, and chlorogenic acid Solanum melongena L. | In vitro HSV-1 Vero cells | Viral attachment, entry, DNA replication, and gB expression | IC50 = 83.4 µg/mL (the extract) | [83] |
| Caffeic acid with metal chelates Various plants | In vitro HSV-1 and HSV-2 Vero cells | Viral attachment, entry, and DNA replication | EC50 = 27.2 µM (HSV-1) EC50 = 17.2 µM (HSV-2) | [84] |
| Ellagic acid and ellagic acid nano-formulated with ZnO NPs Different medicinal and edible plants | In vitro HSV-2 Vero cells | DNA replication and viral particles | IC50 = 4 µg/mL (ellagic acid) and IC50 = 3.6 µg/mL (ellagic acid nano-formulation) | [85] |
| Trans-ferulic acid, gentisic acid, vanillic acid, syringic acid, and gallic acid (Graptopetalum paraguayense E. Walther) Punica granatum (gallic acid) | In silico HSV-1 | Viral replication and DNA polymerase | Binding affinities (118.5–163.4 kcal/mol) | [86] |
| In vitro HSV-1 Vero cells | DNA replication | EC50 = 10.9 µg/mL (gallic acid) | [87] | |
| p-Coumaric acid Phoenix dactylifera L. | In silico HSV-1 | gD, viral entry | Binding affinity (not determined) | [88] |
| Compound and Botanical Source | Study Type, HSV Strains, Cells, and Animal Models | Mechanisms of Action (Inhibition) | Effective Concentration/Dose | Refs. |
|---|---|---|---|---|
| Geraniin Spondias mombin L | In vitro and in silico HSV-1 Vero cells | Viral attachment and DNA replication (in vitro) gB expression (in silico) | 20.4 µg/mL (in vitro) | [89] |
| Chebulagic and chebulinic acids Terminalia chebula Retz | In vitro HSV-2 Vero cells | Viral attachment, entry, and DNA replication | IC50 values of 31.8 and 8.7 µg/mL, respectively | [90] |
| Epiacutissimin A, epiacutissimin B, acutissimin A, and mongolicain Various medicinal plants | In vitro HSV-1 MDBK cells | DNA replication Viral glycoproteins | 16.5–19.7 µM | [91] |
| Castalagin and vescalagin Quercus robur | In vitro HSV-1, HSV-2 (wild types), ACV-resistant HSV-1, and ACV-resistant HSV-2 Vero cells | DNA replication | IC50 values ranging from 0.04 to 0.46 µM. | [92] |
| In vivo HSV-1 Newborn mice | DNA replication Viral titers | 0.02 mL of castalagin (at doses of 7.5 and 10 mg/kg, administered over a 7-day course) | [93] | |
| Corilagin The genus Phyllanthus | In vitro and in vivo HSV-1 Vero and BV2 microglia cells Balb/c male mice | DNA replication, TLR2, TNF-α, and IL-6 (in vitro and in vivo) | 100 ng/mL (in vitro) 0.4 mg/mouse/day for 5 days (in vivo) | [94] |
| Pentagalloylglucose Various medicinal plants | In vitro HSV-1 (wild type) and ACV-resistant HSV-1 Vero cells | DNA replication, nuclear transport and nucleocapsid egress, and dynein expression | 3.1–10 µM | [95] |
| Punicalagin Punica granatum | In vitro and in silico HSV-2 Vero cells | DNA replication (in vitro) HSV-2 protease (in silico) | 31.2 µg/mL (in vitro) | [96] |
| In vitro HSV-1 Vero cells | DNA replication | 0.05 mg/mL | [97] | |
| Tannic acid with AgNPs Numerous plant sources | In vitro and in vivo HSV-1 and HSV-2 Immortal human keratinocyte cells Murine models | Viral attachment, gB, gC expressions, and DNA replication (HSV-1; in vitro) Viral attachment, entry, and DNA replication (HSV-2; in vitro) HSV-2 vaginal transmission (in vivo) | 25 and 50 ppm (in vitro) 25 ppm (in vivo) | [98] |
| In vivo HSV-2 Mouse models | DNA replication, viral particles, and viral transmission | 5 µg/mouse (administered after 6, 24, and 48 h of infection) | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.T.S. Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle. Int. J. Mol. Sci. 2025, 26, 5932. https://doi.org/10.3390/ijms26135932
Hassan STS. Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle. International Journal of Molecular Sciences. 2025; 26(13):5932. https://doi.org/10.3390/ijms26135932
Chicago/Turabian StyleHassan, Sherif T. S. 2025. "Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle" International Journal of Molecular Sciences 26, no. 13: 5932. https://doi.org/10.3390/ijms26135932
APA StyleHassan, S. T. S. (2025). Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle. International Journal of Molecular Sciences, 26(13), 5932. https://doi.org/10.3390/ijms26135932






