Decapeptide Inducer Promotes the Conidiation of Phytopathogenic Magnaporthe oryzae via the Mps1 MAPK Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Acid-Hydrolyzed Casein Significantly Promotes M. Oryzae Conidial Production
2.2. Decapeptide MCIDP from Acid-Hydrolyzed Casein Significantly Promotes Conidiation
2.3. MCIDP Significantly Induced Conidial Formation in Two Other Filamentous Phytopathogenic Ascomycetes
2.4. MCIDP Upregulates the Genes Involved in the Mps1 MAPK Signaling Pathway
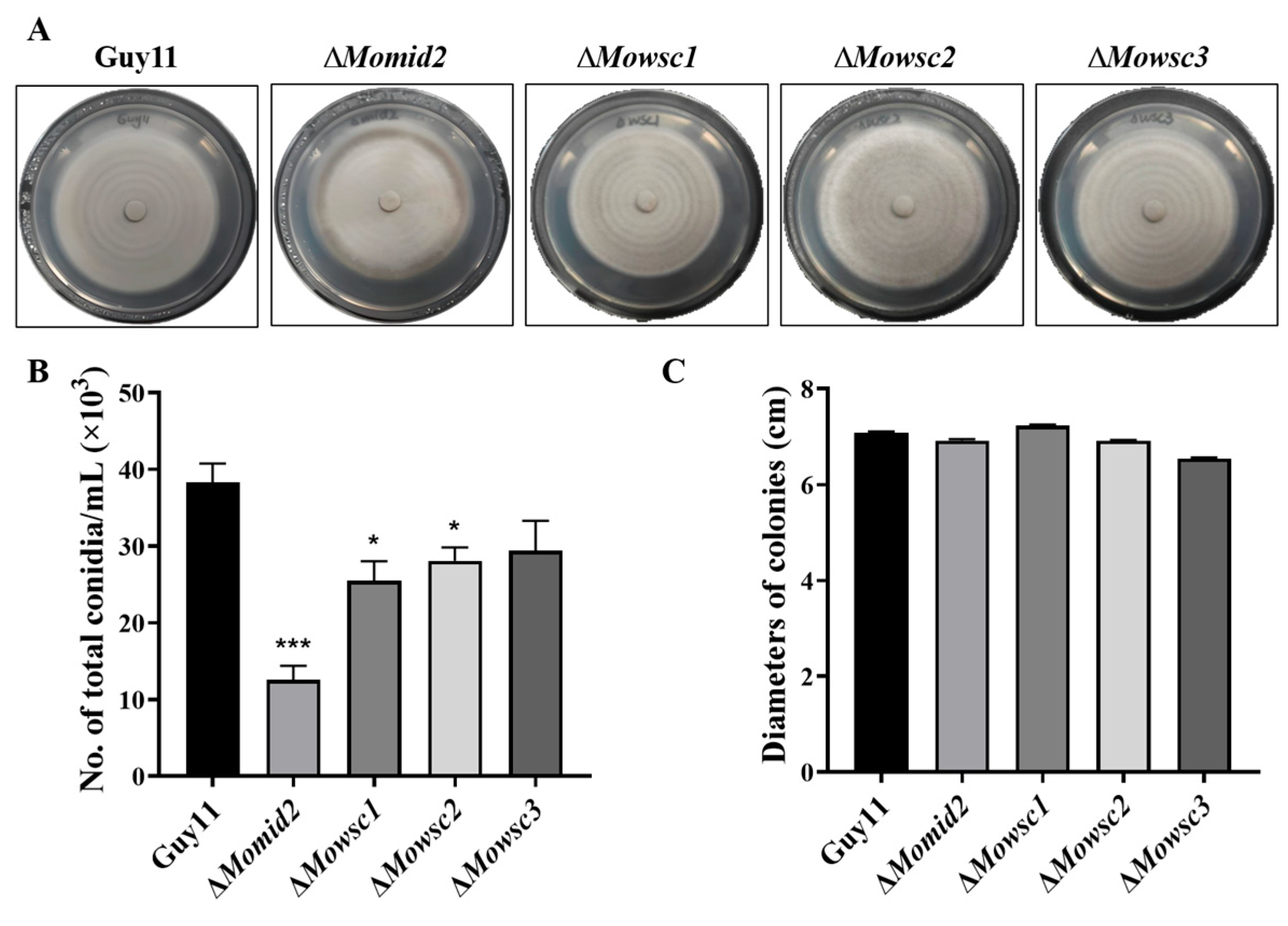
2.5. Conidial Production Decreases in ΔMomid2 and ΔMowsc1 Mutants
2.6. The Membrane Proteins, Wscs and Mid2, in the Mps1 MAPK Signaling Pathway Are Critical for MCIDP to Promote Conidiation and Hyphal Growth
3. Discussion
4. Materials and Methods
4.1. General
4.2. Strains and Culture Conditions
4.3. Preparation of MCIDP from AHC
4.4. Conidium Collection and CFW Staining
4.5. 24-Well Plate Liquid Culture Bioassay Method
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Generation of MoWsc1, MoWsc2, MoWsc3 and MoMid2 Gene Deletion Mutants
4.8. Phenotypic Analysis
4.8.1. Assessment of Mycelial Growth
4.8.2. Conidial Counting
4.8.3. Conidial Imaging
4.8.4. Lateral Imaging of Conidiophore and Hyphae
4.9. Data Processing and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M. oryzae | Magnaporthe oryzae |
| CM | Complete Medium |
| AHC | Acid Hydrolyzed Casein |
| HPLC | High-Performance Liquid Chromatography |
| MCIDP | M. oryzae Conidiation Inducer Decapeptide |
| FARI | Fusarium Asexual Reproduction Inducer |
| F. graminearum | Fusariumgraminearum |
| MAPK | Mitogen-Activated Protein Kinase |
| CFW | Calcofluor White |
| B. cinerea | Botrytis cinerea |
| P. capsici | Phytophthora capsici |
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.-R.; Pan, H.; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, Y.; Zhao, M.; Jing, M.; Liu, X.; Liu, M.; Guo, X.; Zhang, X.; Chen, Y.; Liu, Y.; et al. Global genome and transcriptome analyses of Magnaporthe oryzae Epidemic Isolate 98–06 uncover novel effectors and pathogenicity-related genes, revealing gene gain and lose dynamics in genome evolution. PLoS Pathog. 2015, 11, e1004801. [Google Scholar] [CrossRef]
- Wang, G.L.; Valent, B. Advances in Genetics, Genomics and Control of Rice Blast Disease; Springer: Dordrecht, The Netherlands, 2009; pp. 209–215. [Google Scholar]
- Younas, M.U.; Ahmad, I.; Qasim, M.; Ijaz, Z.; Rajput, N.; Memon, S.P.; Zaman, W.U.; Jiang, X.; Zhang, Y.; Zuo, S. Progress in the management of rice blast disease: The role of avirulence and resistance genes through gene-for-gene interactions. Agronomy 2024, 14, 163. [Google Scholar] [CrossRef]
- Couch, B.C.; Fudal, I.; Lebrun, M.H.; Tharreau, D.; Valent, B.; van Kim, P.; Nottéghem, J.-L.; Kohn, L.M. Origins of host-specific populations of the blast pathogen Magnaporthe oryzae in crop domestication with subsequent expansion of pandemic clones on rice and weeds of rice. Genetics 2005, 170, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Ashu, E.E.; Xu, J. The roles of sexual and asexual reproduction in the origin and dissemination of strains causing fungal infectious disease outbreaks. Infect. Genet. Evol. 2015, 36, 199–209. [Google Scholar] [CrossRef]
- Yajima, A.; Qin, Y.; Zhou, X.; Kawanishi, N.; Xiao, X.; Wang, J.; Zhang, D.; Wu, Y.; Nukada, T.; Yabuta, G.; et al. Synthesis and absolute configuration of hormone alpha1. Nat. Chem. Biol. 2008, 4, 235–237. [Google Scholar] [CrossRef]
- Ojika, M.; Molli, S.D.; Kanazawa, H.; Yajima, A.; Toda, K.; Nukada, T.; Mao, H.; Murata, R.; Asano, T.; Qi, J.; et al. The second phytophthora mating hormone defines interspecies biosynthetic crosstalk. Nat. Chem. Biol. 2011, 7, 591. [Google Scholar] [CrossRef]
- Qi, J.; Asano, T.; Jinno, M.; Matsui, K.; Atsumi, K.; Sakagami, Y.; Ojika, M. Characterization of a phytophthora mating hormone. Science 2005, 309, 1828. [Google Scholar] [CrossRef]
- Qi, J.; Cheng, L.; Sun, Y.; Hirata, Y.; Ushida, N.; Ma, Z.; Osada, H.; Nishikawa, T.; Xiang, L. Identification of an asexual reproduction inducer of phytopathogenic and toxigenic Fusarium. Angew. Chem.-Int. Edit. 2018, 57, 8100–8104. [Google Scholar] [CrossRef]
- Molinari, C.; Talbot, N.J. A basic guide to the growth and manipulation of the blast fungus, Magnaporthe oryzae. Curr. Protoc. 2022, 2, e523. [Google Scholar] [CrossRef] [PubMed]
- Iwai, R.; Han, C.; Govindam, S.V.S.; Ojika, M. Lycosides, unusual carotenoid-derived terpenoid glycosides from a vegetable juice, inhibit asexual reproduction of the plant pathogen Phytophthora. J. Agric. Food Chem. 2017, 66, 163–169. [Google Scholar] [CrossRef]
- Hamel, L.P.; Nicole, M.C.; Duplessis, S.; Ellis, B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: Distinct messages from conserved messengers. Plant Cell 2012, 24, 1327–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Jiang, C.; Xu, J.R. Regulation of biotic interactions and responses to abiotic stresses by MAP kinase pathways in plant pathogenic fungi. Stress Biol. 2021, 1, 5. [Google Scholar] [CrossRef]
- Rispail, N.; Soanes, D.M.; Ant, C.; Czajkowski, R.; Grünler, A.; Huguet, R.; Perez-Nadales, E.; Poli, A.; Sartorel, E.; Valiante, V.; et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 2009, 46, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chen, R.; Yang, Y.; Xu, H.; Jiang, J.; Li, L. Involvement of the cell wall-integrity pathway in signal recognition, cell-wall biosynthesis, and virulence in Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2023, 36, 608–622. [Google Scholar] [CrossRef]
- Penn, T.J.; Wood, M.E.; Soanes, D.M.; Csukai, M.; Corran, A.J.; Talbot, N.J. Protein kinase C is essential for viability of the rice blast fungus Magnaporthe oryzae. Mol. Microbiol. 2015, 98, 403–419. [Google Scholar] [CrossRef]
- Jeon, J.; Goh, J.; Yoo, S.; Chi, M.H.; Choi, J.; Rho, H.S.; Park, J.; Han, S.-S.; Kim, B.R.; Park, S.-Y.; et al. A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2008, 21, 525–534. [Google Scholar] [CrossRef]
- Yin, Z.; Feng, W.; Chen, C.; Xu, J.; Li, Y.; Yang, L.; Wang, J.; Liu, X.; Wang, W.; Gao, C.; et al. Shedding light on autophagy coordinating with cell wall integrity signaling to govern pathogenicity of Magnaporthe oryzae. Autophagy 2020, 16, 900–916. [Google Scholar] [CrossRef]
- Xu, J.R.; Staiger, C.J.; Hamer, J.E. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 1998, 95, 12713–12718. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, R.; Ding, S.; Xu, J.R. MADS-box transcription factor mig1 is required for infectious growth in Magnaporthe grisea. Eukaryot. Cell. 2008, 7, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhai, S.; Zhang, H.; Zuo, R.; Wang, J.; Guo, M.; Zheng, X.; Wang, P.; Zhang, Z. Shared and distinct functions of two Gti1/Pac2 family proteins in growth, morphogenesis and pathogenicity of Magnaporthe oryzae. Environ. Microbiol. 2014, 16, 788–801. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Q.; Dou, X.; Wang, W.; Zhao, Q.; LV, R.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef]
- Geng, Z.; Zhu, W.; Su, H.; Zhao, Y.; Zhang, K.Q.; Yang, J. Recent advances in genes involved in secondary metabolite synthesis, hyphal development, energy metabolism and pathogenicity in Fusarium graminearum (teleomorph Gibberella zeae). Biotechnol. Adv. 2014, 32, 390–402. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Scle rotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Englebretsen, D.R.; Harding, D.R.K. Fmoe SPPS using Perloza™ beaded cellulose. Int. J. Peptide Protein Res. 1994, 43, 546–554. [Google Scholar] [CrossRef]
- Vitale, S.; Di Pietro, A.; Turrà, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Liu, Y.; Qi, J. Decapeptide Inducer Promotes the Conidiation of Phytopathogenic Magnaporthe oryzae via the Mps1 MAPK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 5880. https://doi.org/10.3390/ijms26125880
Yang M, Liu Y, Qi J. Decapeptide Inducer Promotes the Conidiation of Phytopathogenic Magnaporthe oryzae via the Mps1 MAPK Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(12):5880. https://doi.org/10.3390/ijms26125880
Chicago/Turabian StyleYang, Mengya, Yanan Liu, and Jianhua Qi. 2025. "Decapeptide Inducer Promotes the Conidiation of Phytopathogenic Magnaporthe oryzae via the Mps1 MAPK Signaling Pathway" International Journal of Molecular Sciences 26, no. 12: 5880. https://doi.org/10.3390/ijms26125880
APA StyleYang, M., Liu, Y., & Qi, J. (2025). Decapeptide Inducer Promotes the Conidiation of Phytopathogenic Magnaporthe oryzae via the Mps1 MAPK Signaling Pathway. International Journal of Molecular Sciences, 26(12), 5880. https://doi.org/10.3390/ijms26125880


_Kim.png)




