Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Systematic Identification of Radiation-Responsive lncRNAs via CRISPRa Screening in NSCLC
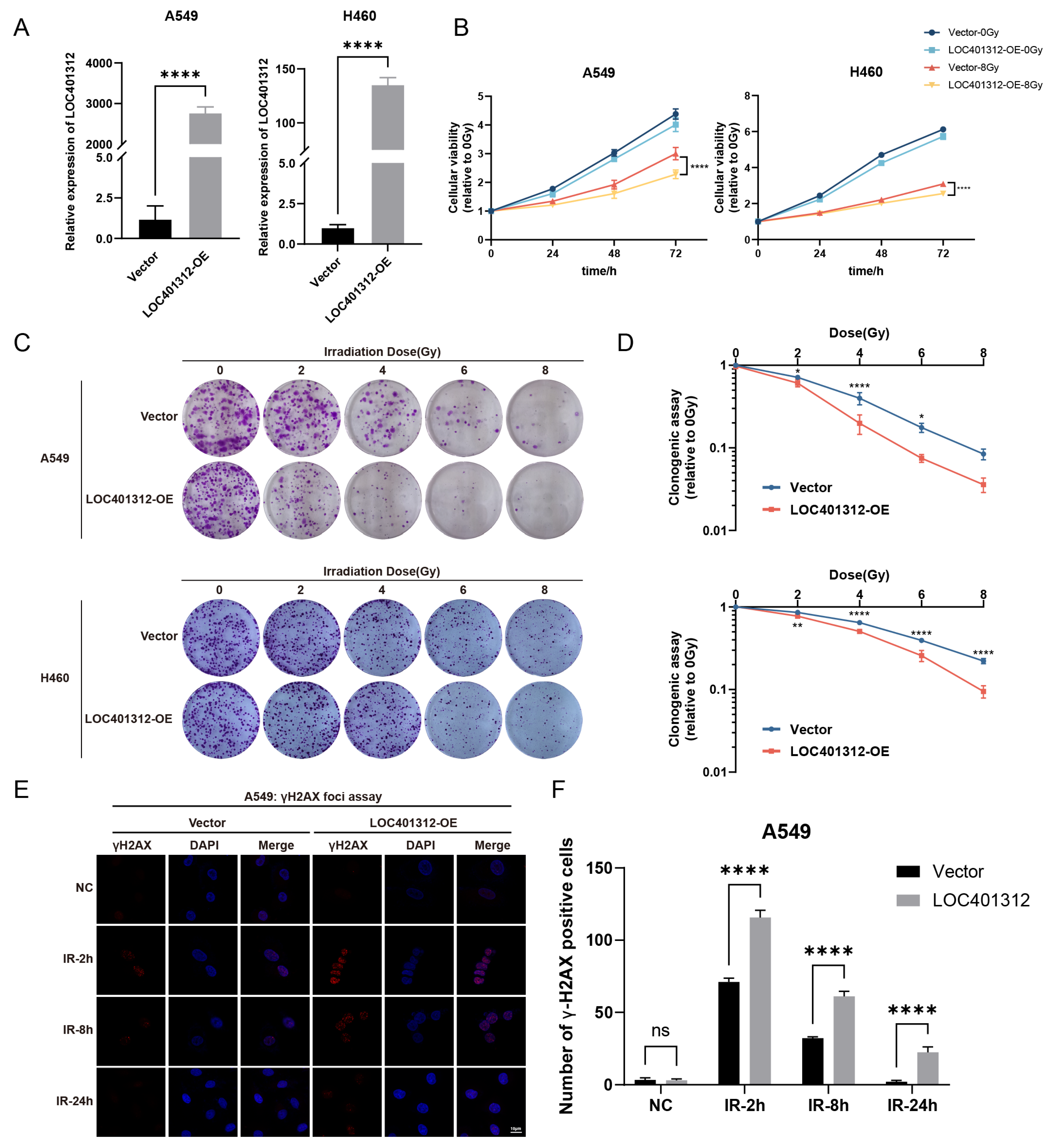
2.2. LOC401312 Validated as a Radiosensitizing lncRNA in Lung Cancer Cells
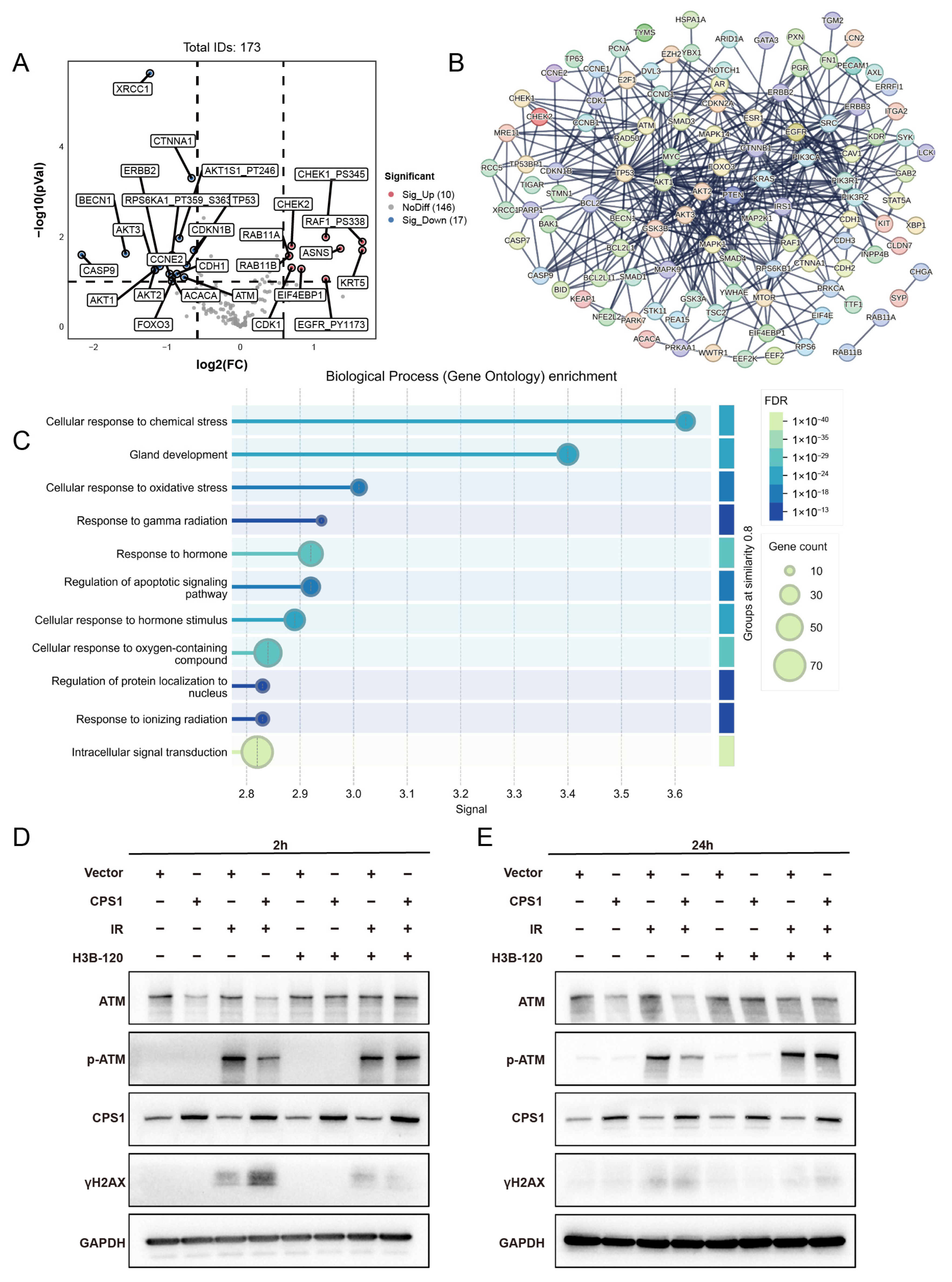
2.3. Transcriptomic Profiling of LOC401312-Overexpressing A549 Cells Identifies CPS1 as a Functional Mediator of Radiation Sensitivity
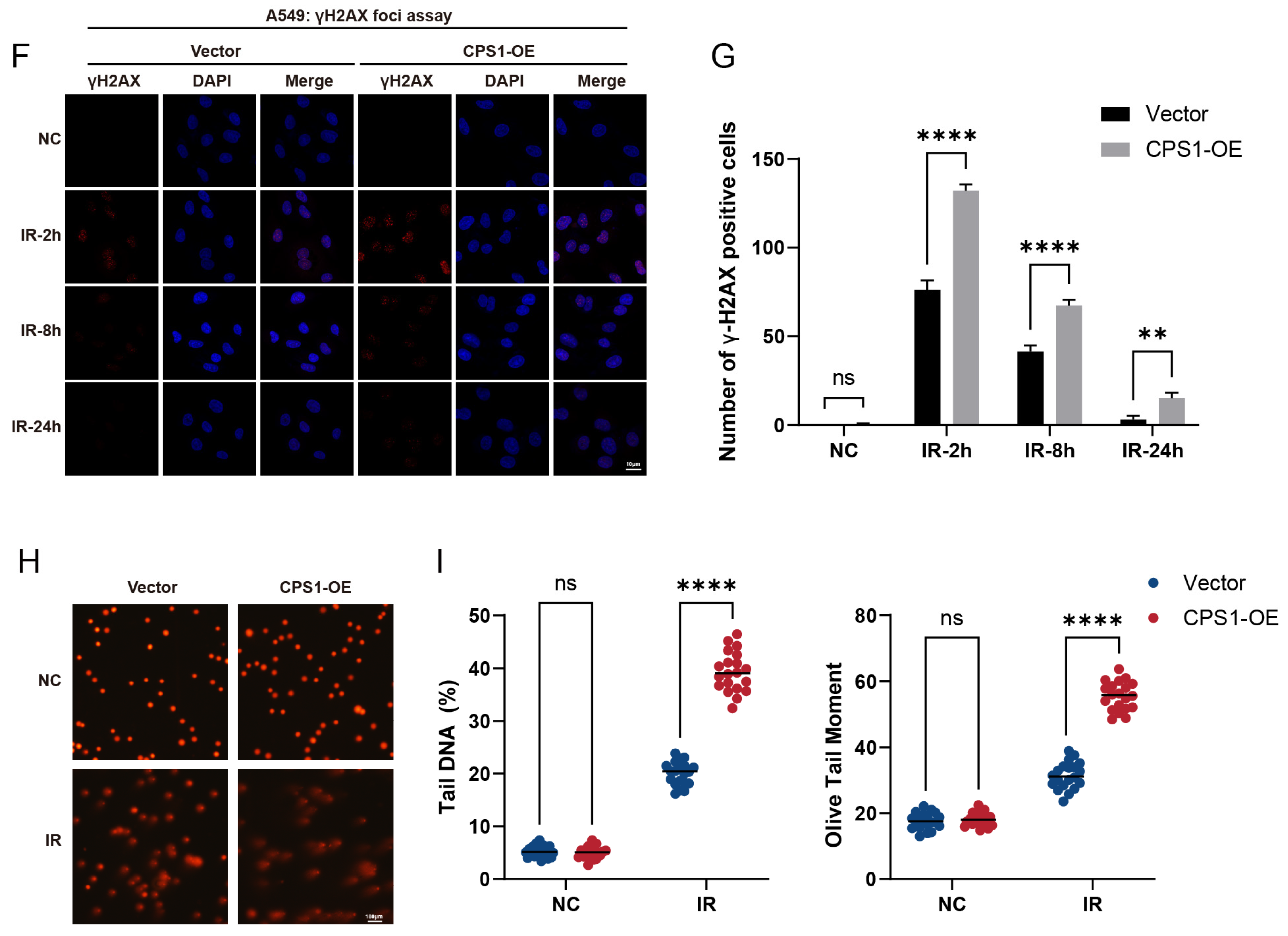
2.4. CPS1 Overexpression Enhances Radiosensitivity in Lung Carcinoma Cells
2.5. Co-Expression Network Analysis of CPS1 in Lung Cancer Identifies DNA Damage Repair Modulation as a Mechanism Governing Ionizing Radiation Sensitivity
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Infection of lncRNA Activation Libraries and Screening
4.3. Illumina Sequencing of sgRNAs
4.4. Construction of Overexpression Cell Lines
4.5. Transcriptome Sequencing
4.6. Colony Formation Assay
4.7. Cellular Viability Assay (CCK-8)
4.8. RNA Extraction and RT-qPCR
4.9. Western Blot Analysis
4.10. Immunofluorescence Assay
4.11. Comet Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Guan, L.L. Noncoding RNAs: Regulatory Molecules of Host-Microbiome Crosstalk. Trends Microbiol. 2021, 29, 713–724. [Google Scholar] [CrossRef]
- Geisler, S.; Coller, J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef]
- Lee, S.; Kopp, F.; Chang, T.C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef]
- Munschauer, M.; Nguyen, C.T.; Sirokman, K.; Hartigan, C.R.; Hogstrom, L.; Engreitz, J.M.; Ulirsch, J.C.; Fulco, C.P.; Subramanian, V.; Chen, J.; et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 2018, 561, 132–136. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Barlow, D.P.; Bartolomei, M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382. [Google Scholar] [CrossRef]
- Yan, B.X.; Ma, J.X. Promoter-associated RNAs and promoter-targeted RNAs. Cell Mol. Life Sci. 2012, 69, 2833–2842. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, Y.; Kang, J.; Hu, M.; Li, N.; Sun, K.; Zhao, Y. Enhancer RNAs in transcriptional regulation: Recent insights. Front. Cell Dev. Biol. 2023, 11, 1205540. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, Y.; Liu, C.; Su, Z.; Ren, S.; Wang, Y.; Deng, T.; Huang, D.; Tian, Y.; Qiu, Y. Genome-wide analyses of long noncoding RNA expression profiles correlated with radioresistance in nasopharyngeal carcinoma via next-generation deep sequencing. BMC Cancer 2016, 16, 719. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pinera, P.; Kocak, D.D.; Vockley, C.M.; Adler, A.F.; Kabadi, A.M.; Polstein, L.R.; Thakore, P.I.; Glass, K.A.; Ousterout, D.G.; Leong, K.W.; et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 2013, 10, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.I.; Mikkelsen, N.S.; Gao, Z.; Foßelteder, J.; Pabst, G.; Axelgaard, E.; Laustsen, A.; König, S.; Reinisch, A.; Bak, R.O. Targeted regulation of transcription in primary cells using CRISPRa and CRISPRi. Genome Res. 2021, 31, 2120–2130. [Google Scholar] [CrossRef]
- Chardon, F.M.; McDiarmid, T.A.; Page, N.F.; Daza, R.M.; Martin, B.K.; Domcke, S.; Regalado, S.G.; Lalanne, J.B.; Calderon, D.; Li, X.; et al. Multiplex, single-cell CRISPRa screening for cell type specific regulatory elements. Nat. Commun. 2024, 15, 8209. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Li, W.; Yau, E.; Hui, H.; Singh, P.K.; Achuthan, V.; Young Karris, M.A.; Engelman, A.N.; Rana, T.M. Genome-wide CRISPR/Cas9 transcriptional activation screen identifies a histone acetyltransferase inhibitor complex as a regulator of HIV-1 integration. Nucleic Acids Res. 2022, 50, 6687–6701. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Tang, Z.; Li, M.; Ling, X.; Liao, J.; Zhou, X.; Fang, S.; Zhao, H.; Zhong, W.; et al. Genome-Scale CRISPR-Cas9 Transcriptional Activation Screening in Metformin Resistance Related Gene of Prostate Cancer. Front. Cell Dev. Biol. 2020, 8, 616332. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wang, X.J.; Wu, Q.J.; Liu, C.; Fan, C.D. Induction of G1 Cell Cycle Arrest in Human Glioma Cells by Salinomycin Through Triggering ROS-Mediated DNA Damage In Vitro and In Vivo. Neurochem. Res. 2016, 42, 997–1005. [Google Scholar] [CrossRef]
- Yao, M.; Rogers, L.; Suchowerska, N.; Choe, D.; Al-Dabbas, M.A.; Narula, R.S.; Lyons, J.G.; Sved, P.; Li, Z.; Dong, Q. Sensitization of prostate cancer to radiation therapy: Molecules and pathways to target. Radiother. Oncol. 2018, 128, 283–300. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Zeng, Z.; Wu, Q.; Jiang, X.; Li, S.; Sun, W.; Zhang, J.; Li, Y.; Li, J.; et al. LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e792. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Shankavaram, U.; Bylicky, M.; Dalo, J.; Scott, K.; Aryankalayil, M.J.; Coleman, C.N. Profiling mRNA, miRNA and lncRNA expression changes in endothelial cells in response to increasing doses of ionizing radiation. Sci. Rep. 2022, 12, 19941. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Bylicky, M.; Chopra, S.; Coleman, C.N.; Aryankalayil, M.J. Long and short non-coding RNA and radiation response: A review. Transl. Res. 2021, 233, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, Y.; Hu, W.; Zhang, J.; Shi, Y.; Kong, X.; Jiang, J. A novel long noncoding RNA SP100-AS1 induces radioresistance of colorectal cancer via sponging miR-622 and stabilizing ATG3. Cell Death Differ. 2023, 30, 111–124. [Google Scholar] [CrossRef]
- Fan, L.; Huang, C.; Li, J.; Gao, T.; Lin, Z.; Yao, T. Long non-coding RNA urothelial cancer associated 1 regulates radioresistance via the hexokinase 2/glycolytic pathway in cervical cancer. Int. J. Mol. Med. 2018, 42, 2247–2259. [Google Scholar] [CrossRef]
- Chen, L.; Ren, P.; Zhang, Y.; Gong, B.; Yu, D.; Sun, X. Long non-coding RNA GAS5 increases the radiosensitivity of A549 cells through interaction with the miR-21/PTEN/Akt axis. Oncol. Rep. 2020, 43, 897–907. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Rong, Z.; Dai, L.; Qin, C.; Wang, S.; Geng, W. LncRNA LINC01134 Contributes to Radioresistance in Hepatocellular Carcinoma by Regulating DNA Damage Response via MAPK Signaling Pathway. Front. Pharmacol. 2021, 12, 791889. [Google Scholar] [CrossRef]
- Nitzahn, M.; Lipshutz, G.S. CPS1: Looking at an ancient enzyme in a modern light. Mol. Genet. Metab. 2020, 131, 289–298. [Google Scholar] [CrossRef]
- Çeliktas, M.; Tanaka, I.; Tripathi, S.C.; Fahrmann, J.F.; Aguilar-Bonavides, C.; Villalobos, P.; Delgado, O.; Dhillon, D.; Dennison, J.B.; Ostrin, E.J.; et al. Role of CPS1 in Cell Growth, Metabolism and Prognosis in LKB1-Inactivated Lung Adenocarcinoma. J. Natl. Cancer Inst. 2017, 109, djw231. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, L.; Guo, J.; Niu, Y.; Wu, Y.; Li, H.; Zhao, L.; Li, X.; Teng, X.; Sun, X.; et al. NONCODEV5: A comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018, 46, D308–D314. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.P.; Sinha, R.; Mukhtar, M.S.; Athar, M. Epigenetic regulation in the pathogenesis of non-melanoma skin cancer. Semin. Cancer Biol. 2022, 83, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Khoja, S.; Nitzahn, M.; Truong, B.; Lambert, J.; Willis, B.; Allegri, G.; Rüfenacht, V.; Häberle, J.; Lipshutz, G.S. A constitutive knockout of murine carbamoyl phosphate synthetase 1 results in death with marked hyperglutaminemia and hyperammonemia. J. Inherit. Metab. Dis. 2019, 42, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, R.M.; Mansy, S.S.; Nessim, I.G.; Hosni, H.N.; El Hindawi, A.; Hassanein, M.H.; AbdelFattah, A.S. Carbamoyl phosphate synthetase 1 (CPS1) as a prognostic marker in chronic hepatitis C infection. Apmis 2019, 127, 93–105. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Li, C.F.; Lin, C.Y.; Lee, S.W.; Sheu, M.J.; Lin, L.C.; Chen, T.J.; Wu, T.F.; Hsing, C.H. Overexpression of CPS1 is an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. Tumor Biol. 2014, 35, 11097–11105. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Y.; Wu, Z.; Zhou, X.; Wu, T.; Li, P.; Lian, Q.; Xu, S.; Gu, J.; Chen, L.; et al. Deficiency of Carbamoyl Phosphate Synthetase 1 Engenders Radioresistance in Hepatocellular Carcinoma via Deubiquitinating c-Myc. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1244–1256. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 796–814. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Zhu, L.; Xie, S.; Tu, L.; Yang, Y.; Wu, K.; Zhao, Y.; Wang, Y.; Xu, Y.; et al. SERPINE2/PN-1 regulates the DNA damage response and radioresistance by activating ATM in lung cancer. Cancer Lett. 2022, 524, 268–283. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Lin, M.; Li, Z.; Shen, Z.; Yin, S.; Zheng, Y.; Zou, Y.; Zhang, Y.; Zhan, Y.; et al. Targeting ATM enhances radiation sensitivity of colorectal cancer by potentiating radiation-induced cell death and antitumor immunity. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Pham-Danis, C.; Gehrke, S.; Danis, E.; Rozhok, A.I.; Daniels, M.W.; Gao, D.; Collins, C.; Paola, J.T.D.; D’Alessandro, A.; DeGregori, J. Urea Cycle Sustains Cellular Energetics upon EGFR Inhibition in EGFR-Mutant NSCLC. Mol. Cancer Res. 2019, 17, 1351–1364. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, Z.; Yan, Y.; Zhou, Y.; Wei, J.; Chen, X.; Lin, W.; Ou, C.; Li, J.; Wang, X.; et al. CPS1 expression and its prognostic significance in lung adenocarcinoma. Ann. Transl. Med. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Li, X.; Huang, H.; Zhou, Y.; Zhang, C.; Wang, S.; Bohnenberger, H.; Gao, Y. Identification of a novel prognostic signature based on vitamin metabolism clustering-related genes in lung adenocarcinoma. Transl. Lung Cancer Res. 2024, 13, 1084–1100. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Wang, T.; Tai, F.; Zhai, R.; Li, H.; Li, J.; Xiang, S.; Gao, H.; Zheng, X.; Li, C. Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 5865. https://doi.org/10.3390/ijms26125865
Cao Z, Wang T, Tai F, Zhai R, Li H, Li J, Xiang S, Gao H, Zheng X, Li C. Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2025; 26(12):5865. https://doi.org/10.3390/ijms26125865
Chicago/Turabian StyleCao, Zhengyue, Tiantian Wang, Fumin Tai, Rui Zhai, Hujie Li, Jingjing Li, Shensi Xiang, Huiying Gao, Xiaofei Zheng, and Changyan Li. 2025. "Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 26, no. 12: 5865. https://doi.org/10.3390/ijms26125865
APA StyleCao, Z., Wang, T., Tai, F., Zhai, R., Li, H., Li, J., Xiang, S., Gao, H., Zheng, X., & Li, C. (2025). Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 26(12), 5865. https://doi.org/10.3390/ijms26125865





