Establishment of a Stable BK Polyomavirus-Secreting Cell Line: Characterization of Viral Genome Integration and Replication Dynamics Through Comprehensive Analysis
Abstract
1. Introduction
2. Results
2.1. Characterization of the COSSA Cell Line
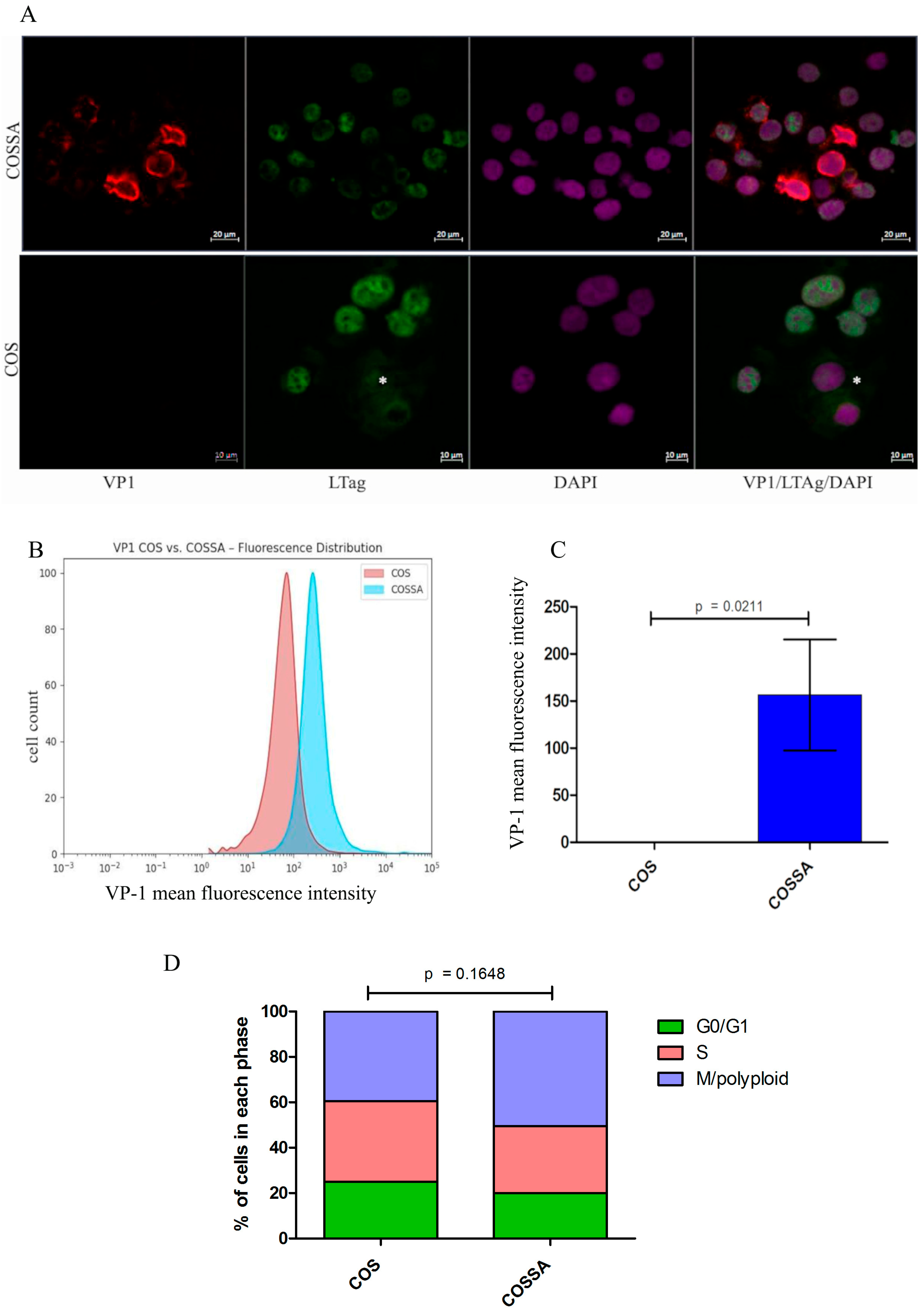
2.2. Polyomavirus Protein Confocal Immunofluorescence LTAg and VP1 in COSSA
2.3. BKPyV-VP1 Protein Measurement and Cell Cycle Analysis by Intracellular FACS in COSSA Versus COS-WT
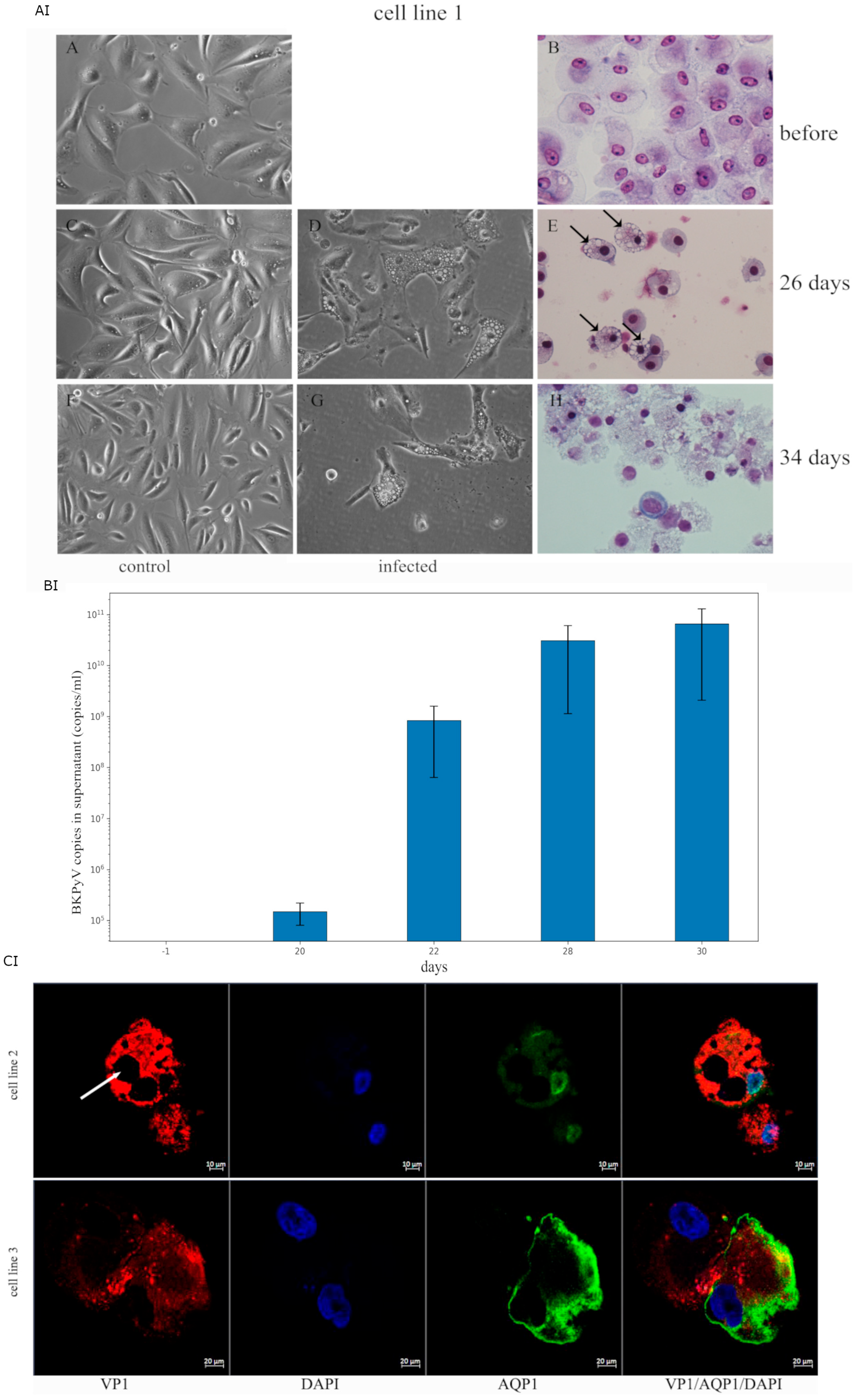
2.4. Infectivity Analysis of COSSA-Secreted BKPyV in Human Tubular Progenitor Cell Lines
2.5. BKPyV Protein Confocal Immunofluorescence VP1 and AQP1, a Membrane Marker Protein of Infected Tubular Progenitor Cell Lines #2 and #3
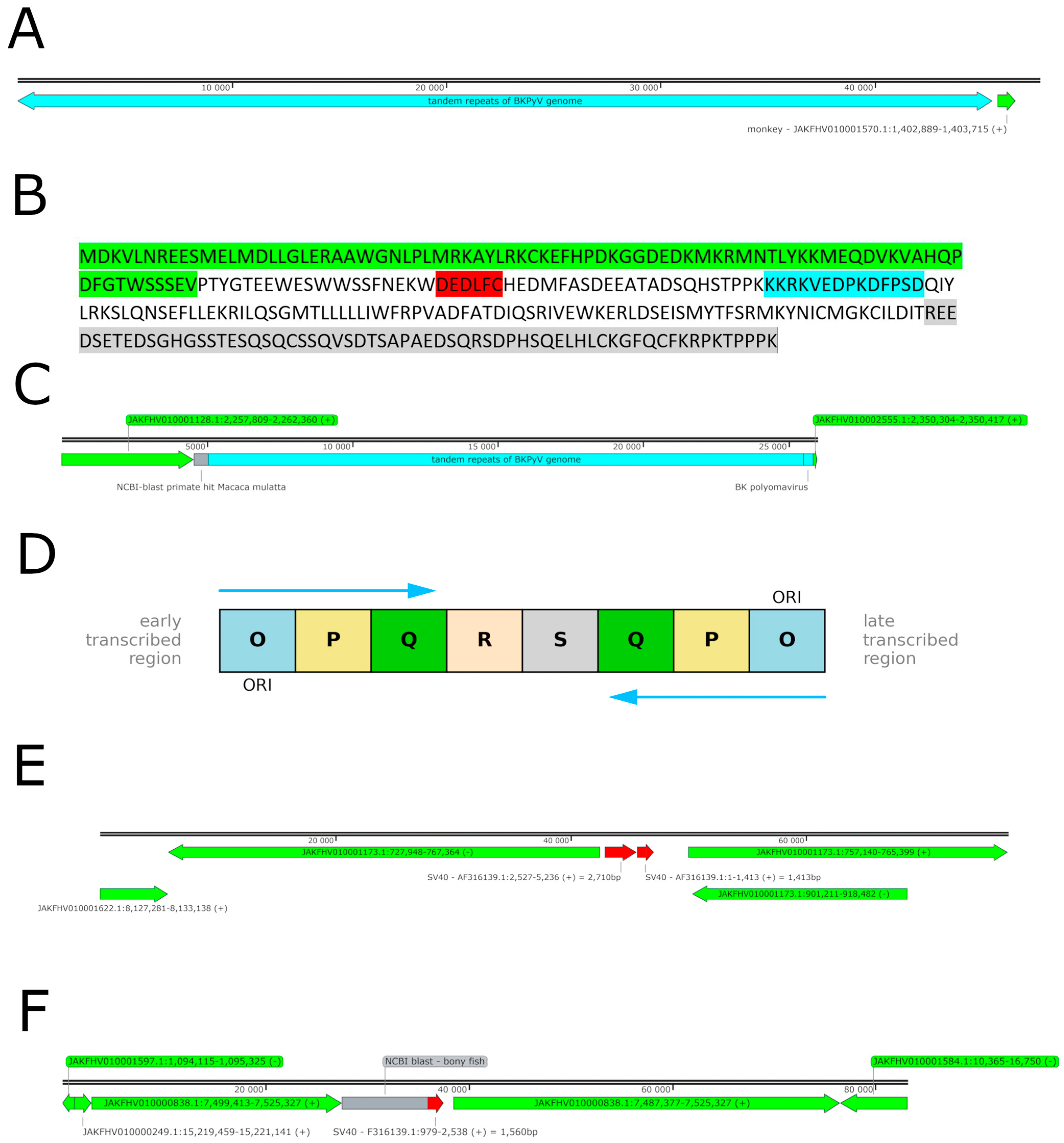
2.6. BKPyV Genome Integration into the Host Cell Genome
2.7. SV40 Genome Fragments Incorporated into the COS Cell Genome
2.8. Identification and Structural Analysis of Non-Monkey Mapped BKPyV-Specific Reads
2.9. Genomic Characterization of the BKPyV Clinical Isolate Used for COS-WT Cell Infection
3. Discussion
4. Methods
4.1. Tissue Culture of Human Proximal Tubular Epithelial Cell Lines and Monkey COS-1 Cell Line
4.2. Virus Production Rate in the Persistently BKPyV-Secreting COSSA Cell Line
4.3. DNA Extraction and Purification
4.4. Immunofluorescence
4.5. FACS Intracellular VP1 Staining
4.6. RNA and Protein Isolation
4.7. Immunoblotting
4.8. Testing the Infectivity of COSSA-Secreted Virus Particles
4.9. BKPyV Copy Number Evaluation
4.10. ONT Sequencing and Bioinformatic Analysis
4.11. Bioinformatical Workflow for Viral Genome Construct
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drachenberg, C.B.; Hirsch, H.H.; Papadimitriou, J.C.; Gosert, R.; Wali, R.K.; Munivenkatappa, R.; Nogueira, J.; Cangro, C.B.; Haririan, A.; Mendley, S.; et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: A prospective evaluation. Transplantation 2007, 84, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schrag, T.A.; Havel, E.F.; Kainz, A.; Omic, H.; Doberer, K.; Kozakowski, N.; Körmöczi, G.F.; Schönbacher, M.; Fischer, G.; et al. Polyomavirus Nephropathy in ABO Blood Group-Incompatible Kidney Transplantation: Torque Teno Virus and Immunosuppressive Burden as an Approximation to the Problem. Kidney Int. Rep. 2024, 9, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.; Bohl, D.; Brennan, D.; Ruppert, K.; Ramaswami, B.; Storch, G.; March, J.; Shapiro, R.; Viscidi, R. longitudinal analysis of levels of immunoglobulins against BK virus capsid proteins in kidney transplant recipients. Clin. Vaccine Immunol. 2008, 15, 1564–1571. [Google Scholar] [CrossRef]
- Nickeleit, V.; Davis, V.G.; Thompson, B.; Singh, H.K. The Urinary Polyomavirus-Haufen Test: A Highly Predictive Non-Invasive Biomarker to Distinguish “Presumptive” from “Definitive” Polyomavirus Nephropathy: How to Use It-When to Use It-How Does It Compare to PCR Based Assays? Viruses 2021, 13, 135. [Google Scholar] [CrossRef]
- Omić, H.; Kläger, J.P.; Herkner, H.; Aberle, S.W.; Regele, H.; Weseslindtner, L.; Schrag, T.A.; Bond, G.; Hohenstein, K.; Watschinger, B.; et al. Clinical Relevance of Absolute BK Polyoma Viral Load Kinetics in Patients With Biopsy Proven BK Polyomavirus Associated Nephropathy. Front. Med. 2021, 8, 791087. [Google Scholar] [CrossRef]
- Imperiale, M.J.; Jiang, M. Polyomavirus Persistence. Annu. Rev. Virol. 2016, 3, 517–532. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Deng, W.; Fu, F.; Yan, S.; Yang, H.; Liu, R.; Geng, J.; Xu, J.; Wu, Y.; et al. Viral integration in BK polyomavirus-associated urothelial carcinoma in renal transplant recipients: Multistage carcinogenesis revealed by next-generation virome capture sequencing. Oncogene 2020, 39, 5734–5742. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, Y.; Yan, Z.; Deng, W.; Geng, J.; Li, Z.; Xia, R.; Zeng, W.; Zhao, T.; et al. Dynamic viral integration patterns actively participate in the progression of BK polyomavirus-associated diseases after renal transplantation. Am. J. Transpl. 2023, 23, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.L.; Verhalen, B.; Jiang, M. Polyomavirus interaction with the DNA damage response. Virol. Sin. 2015, 30, 122–129. [Google Scholar] [CrossRef]
- Nesselhauf, N.; Strutt, J.; Bastani, B. Evaluation of leflunomide for the treatment of BK viremia and biopsy proven BK nephropathy; a single center experience. J. Nephropathol. 2016, 5, 34–37. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Chanda, R.; Chandran, S.; Lee, B.; Webber, A.; Macaraig, M.; Tomlanovich, S.; Vincenti, F. Ciprofloxacin prophylaxis in kidney transplant recipients reduces BK virus infection at 3 months but not at 1 year. Transplantation 2012, 94, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Shirai, H.; Tojimbara, T. Use of Leflunomide as an Antiviral Agent with Everolimus for BK Virus Nephropathy Patients After Kidney Transplantation: A Case Series. Am. J. Case Rep. 2020, 21, e927367. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Bueno, M.; Camachos, C.J.; Ramaswami, B.; Luo, C.; Randhawa, P. Validation of BKV large T-antigen ATP-binding site as a target for drug discovery. Antivir. Res. 2009, 81, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Asseri, A.H.; Alam, M.J.; Alzahrani, F.; Khames, A.; Pathan, M.T.; Abourehab, M.A.S.; Hosawi, S.; Ahmed, R.; Sultana, S.A.; Alam, N.F.; et al. Toward the Identification of Natural Antiviral Drug Candidates against Merkel Cell Polyomavirus: Computational Drug Design Approaches. Pharmaceuticals 2022, 15, 501. [Google Scholar] [CrossRef]
- O’Hara, B.A.; Rupasinghe, C.; Yatawara, A.; Gaidos, G.; Mierke, D.F.; Atwood, W.J. Gallic acid-based small-molecule inhibitors of JC and BK polyomaviral infection. Virus Res. 2014, 189, 280–285. [Google Scholar] [CrossRef]
- Onwubiko, N.O.; Diaz, S.A.; Krečmerová, M.; Nasheuer, H.P. Alkoxylalkyl Esters of Nucleotide Analogs Inhibit Polyomavirus DNA Replication and Large T Antigen Activities. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Wu, Z.; Graf, F.E.; Hirsch, H.H. Antivirals against human polyomaviruses: Leaving no stone unturned. Rev. Med. Virol. 2021, 31, e2220. [Google Scholar] [CrossRef]
- An, P.; Cantalupo, P.G.; Zheng, W.; Saenz-Robles, M.T.; Duray, A.M.; Weitz, D.; Pipas, J.M. Single-Cell Transcriptomics Reveals a Heterogeneous Cellular Response to BK Virus Infection. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- An, P.; Sáenz Robles, M.T.; Cantalupo, P.G.; Naik, A.S.; Sealfon, R.; Imperiale, M.J.; Pipas, J.M. Cultured Renal Proximal Tubular Epithelial Cells Resemble a Stressed/Damaged Kidney While Supporting BK Virus Infection. J. Virol. 2023, 97, e0034323. [Google Scholar] [CrossRef]
- Zhao, L.; Imperiale, M.J. A Cell Culture Model of BK Polyomavirus Persistence, Genome Recombination, and Reactivation. MBio 2021, 12, e0235621. [Google Scholar] [CrossRef]
- Tomlinson, S.M.; Malmstrom, R.D.; Russo, A.; Mueller, N.; Pang, Y.-P.; Watowich, S.J. Structure-based discovery of dengue virus protease inhibitors. Antivir. Res. 2009, 82, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Teimourpour, R.; Meshkat, Z.; Gholoubi, A.; Nomani, H.; Rostami, S. Viral Load Analysis of Hepatitis C Virus in Huh7.5 Cell Culture System. Jundishapur. J. Microbiol. 2015, 8, e19279. [Google Scholar] [CrossRef]
- Gluzman, Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell 1981, 23, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Gerges, D.; Abd El-Ghany, K.; Hevesi, Z.; Aiad, M.; Omic, H.; Baumgartner, C.; Winnicki, W.; Eder, M.; Schmidt, A.; Eskandary, F.; et al. Shedding Light on Viral Shedding: Novel Insights into Nuclear Assembly, Cytoplasmic Transformation and Extracellular Vesicle Release of the BK Virus. Int. J. Mol. Sci. 2024, 25, 9130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-D.; Gao, R.; Hou, X.-T.; Zhang, H.; Chen, X.-T.; Luo, J.-Q.; Yang, H.-F.; Chen, T.; Shen, X.; Yang, S.-C.; et al. Endoplasmic Reticulum Stress Mediates Renal Tubular Vacuolation in BK Polyomavirus-Associated Nephropathy. Front. Endocrinol. 2022, 13, 834187. [Google Scholar] [CrossRef]
- Bruštíková, K.; Ryabchenko, B.; Liebl, D.; Horníková, L.; Forstová, J.; Huérfano, S. BK Polyomavirus Infection of Bladder Microvascular Endothelial Cells Leads to the Activation of the cGAS-STING Pathway. J. Med. Virol. 2024, 96, e70038. [Google Scholar] [CrossRef]
- Broekema, N.M.; Abend, J.R.; Bennett, S.M.; Butel, J.S.; Vanchiere, J.A.; Imperiale, M.J. A system for the analysis of BKV non-coding control regions: Application to clinical isolates from an HIV/AIDS patient. Virology 2010, 407, 368–373. [Google Scholar] [CrossRef]
- Moens, U.; Van Ghelue, M. Polymorphism in the genome of non-passaged human polyomavirus BK: Implications for cell tropism and the pathological role of the virus. Virology 2005, 331, 209–231. [Google Scholar] [CrossRef]
- Bethge, T.; Hachemi Helen, A.; Manzetti, J.; Gosert, R.; Schaffner, W.; Hirsch Hans, H. Sp1 Sites in the Noncoding Control Region of BK Polyomavirus Are Key Regulators of Bidirectional Viral Early and Late Gene Expression. J. Virol. 2015, 89, 3396–3411. [Google Scholar] [CrossRef]
- Gosert, R.; Rinaldo, C.H.; Funk, G.A.; Egli, A.; Ramos, E.; Drachenberg, C.B.; Hirsch, H.H. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J. Exp. Med. 2008, 205, 841–852. [Google Scholar] [CrossRef]
- Pajenda, S.; Hevesi, Z.; Eder, M.; Gerges, D.; Aiad, M.; Koldyka, O.; Winnicki, W.; Wagner, L.; Eskandary, F.; Schmidt, A. Lessons from Polyomavirus Immunofluorescence Staining of Urinary Decoy Cells. Life 2023, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.H.; Muller, Y.D.; Dietrich, P.Y.; Tille, J.C.; Nikolaev, S.; Sartori, A.; Labidi-Galy, I.; Ernandez, T.; Kaur, A.; Hirsch, H.H.; et al. Immunologic Clearance of a BK Virus-associated Metastatic Renal Allograft Carcinoma. Transplantation 2021, 105, 423–429. [Google Scholar] [CrossRef]
- Mineeva-Sangwo, O.; Van Loon, E.; Andrei, G.; Kuypers, D.; Naesens, M.; Snoeck, R. Time-dependent variations in BK polyomavirus genome from kidney transplant recipients with persistent viremia. Sci. Rep. 2023, 13, 13534. [Google Scholar] [CrossRef]
- Rosenquist, Å.; Samuelsson, B.; Johansson, P.-O.; Cummings, M.D.; Lenz, O.; Raboisson, P.; Simmen, K.; Vendeville, S.; de Kock, H.; Nilsson, M.; et al. Discovery and Development of Simeprevir (TMC435), a HCV NS3/4A Protease Inhibitor. J. Med. Chem. 2014, 57, 1673–1693. [Google Scholar] [CrossRef]
- Ciardi, M.R.; Zingaropoli, M.A.; Iannetta, M.; Prezioso, C.; Perri, V.; Pasculli, P.; Lichtner, M.; d’Ettorre, G.; Altieri, M.; Conte, A.; et al. JCPyV NCCR analysis in PML patients with different risk factors: Exploring common rearrangements as essential changes for neuropathogenesis. Virol. J. 2020, 17, 23. [Google Scholar] [CrossRef]
- Perets Tsachi, T.; Silberstein, I.; Rubinov, J.; Sarid, R.; Mendelson, E.; Shulman Lester, M. High Frequency and Diversity of Rearrangements in Polyomavirus BK Noncoding Regulatory Regions Cloned from Urine and Plasma of Israeli Renal Transplant Patients and Evidence for a New Genetic Subtype. J. Clin. Microbiol. 2009, 47, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.M.; Gupta, G.; Vats, A.; Shapiro, R.; Randhawa, P.S. Polyomavirus BK non-coding control region rearrangements in health and disease. J. Med. Virol. 2007, 79, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles To Infect Target Cells. MBio 2019, 10. [Google Scholar] [CrossRef]
- Morris-Love, J.; O’Hara, B.A.; Gee, G.V.; Dugan, A.S.; O’Rourke, R.S.; Armstead, B.E.; Assetta, B.; Haley, S.A.; Atwood, W.J. Biogenesis of JC polyomavirus associated extracellular vesicles. J. Extracell. Biol. 2022, 1, e43. [Google Scholar] [CrossRef]
- Handala, L.; Blanchard, E.; Raynal, P.I.; Roingeard, P.; Morel, V.; Descamps, V.; Castelain, S.; Francois, C.; Duverlie, G.; Brochot, E.; et al. BK Polyomavirus Hijacks Extracellular Vesicles for En Bloc Transmission. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Helle, F.; Handala, L.; Bentz, M.; Duverlie, G.; Brochot, E. Intercellular Transmission of Naked Viruses through Extracellular Vesicles: Focus on Polyomaviruses. Viruses 2020, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kerviel, A.; Zhang, M.; Altan-Bonnet, N. A New Infectious Unit: Extracellular Vesicles Carrying Virus Populations. Annu. Rev. Cell Dev. Biol. 2021, 37, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Zhu, T.; Hou, Y.; Shi, Y.; Sun, J.; Wu, N. High-sensitivity BK virus detection system using viewRNA in situ hybridization. Diagn. Microbiol. Infect. Dis. 2025, 112, 116790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Imperiale, M.J. Establishing Renal Proximal Tubule Epithelial-Derived Cell Lines Expressing Human Telomerase Reverse Transcriptase for Studying BK Polyomavirus. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef]
- Pajenda, S.; Gerges, D.A.; Freire, R.; Wagner, L.; Hevesi, Z.; Aiad, M.; Eder, M.; Schmidt, A.; Winnicki, W.; Eskandary, F.A. Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells. Int. J. Mol. Sci. 2023, 24, 17511. [Google Scholar] [CrossRef]

| Contig | Total Length | Proportion PyV | Coverage of Reads | Type |
|---|---|---|---|---|
| 1 * | 19,291 | 1200 | 4 | BKPyV |
| 2 | 14,584 | 2944 | 20 | BKPyV |
| 3 | 4155 | 1668 | 20 | BKPyV |
| 4 | 47,651 | 45,481 | 22 | BKPyV |
| 5 | 25,964 | 20,460 | 109 | BKPyV |
| A | 77,115 | 4124 | 25 | SV40 |
| B | 83,050 | 1560 | 39 | SV40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löwenstern, T.; Vecsei, D.; Horner, D.; Strassl, R.; Bozdogan, A.; Eder, M.; Laccone, F.; Hengstschläger, M.; Eskandary, F.; Wagner, L. Establishment of a Stable BK Polyomavirus-Secreting Cell Line: Characterization of Viral Genome Integration and Replication Dynamics Through Comprehensive Analysis. Int. J. Mol. Sci. 2025, 26, 5745. https://doi.org/10.3390/ijms26125745
Löwenstern T, Vecsei D, Horner D, Strassl R, Bozdogan A, Eder M, Laccone F, Hengstschläger M, Eskandary F, Wagner L. Establishment of a Stable BK Polyomavirus-Secreting Cell Line: Characterization of Viral Genome Integration and Replication Dynamics Through Comprehensive Analysis. International Journal of Molecular Sciences. 2025; 26(12):5745. https://doi.org/10.3390/ijms26125745
Chicago/Turabian StyleLöwenstern, Tamara, David Vecsei, David Horner, Robert Strassl, Anil Bozdogan, Michael Eder, Franco Laccone, Markus Hengstschläger, Farsad Eskandary, and Ludwig Wagner. 2025. "Establishment of a Stable BK Polyomavirus-Secreting Cell Line: Characterization of Viral Genome Integration and Replication Dynamics Through Comprehensive Analysis" International Journal of Molecular Sciences 26, no. 12: 5745. https://doi.org/10.3390/ijms26125745
APA StyleLöwenstern, T., Vecsei, D., Horner, D., Strassl, R., Bozdogan, A., Eder, M., Laccone, F., Hengstschläger, M., Eskandary, F., & Wagner, L. (2025). Establishment of a Stable BK Polyomavirus-Secreting Cell Line: Characterization of Viral Genome Integration and Replication Dynamics Through Comprehensive Analysis. International Journal of Molecular Sciences, 26(12), 5745. https://doi.org/10.3390/ijms26125745






