Yamabushitake Mushroom (Hericium erinaceus (Bull.) Pers. 1797) Mycelium Improves Reproductive System Dysfunction in Male Rats Induced by Polystyrene Microplastics
Abstract
1. Introduction
2. Results
2.1. Effects of Erinacine A-Enriched H. erinaceus Mycelium (HE) on the Organ Weights of the Rats
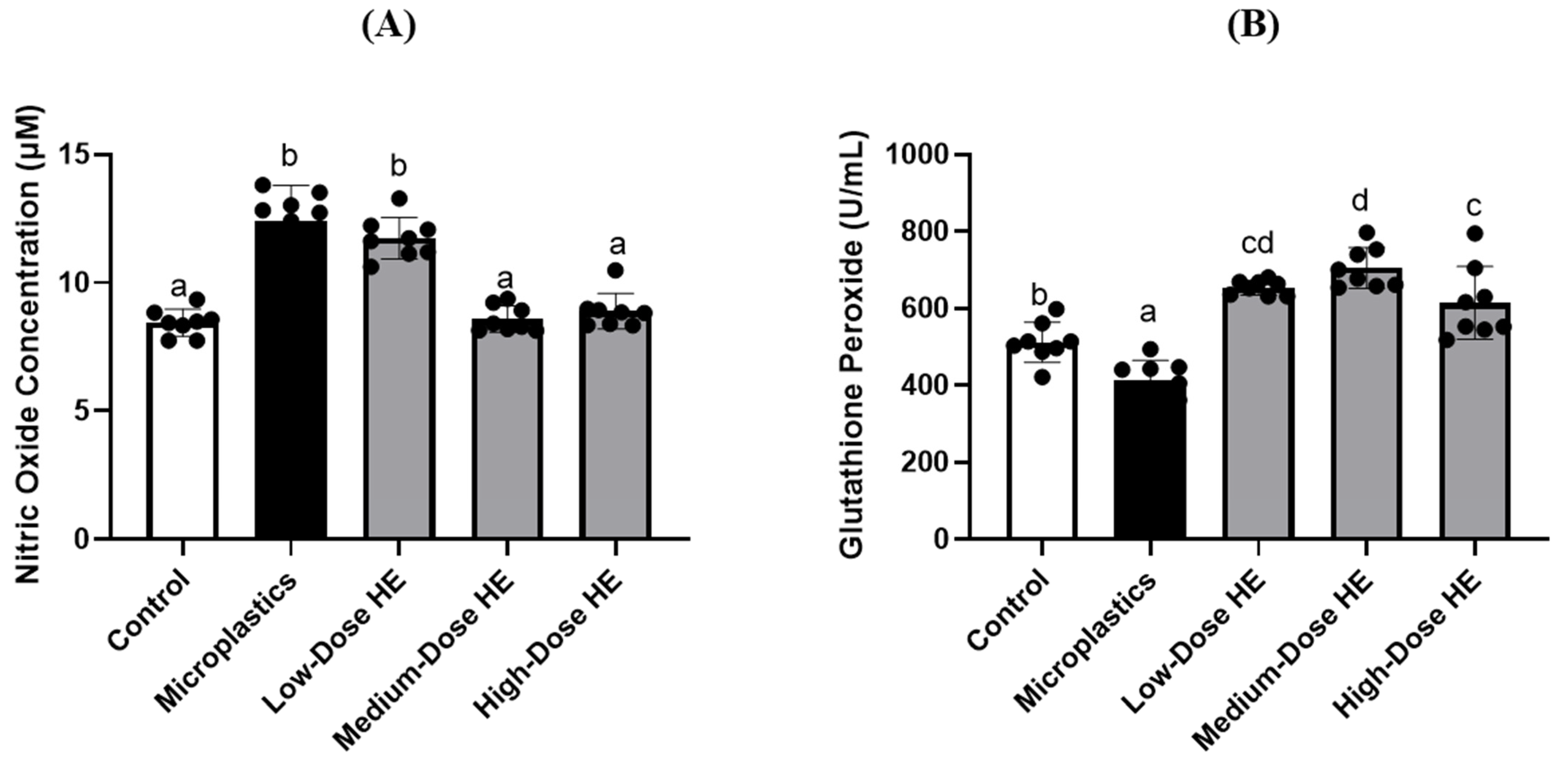
2.2. HE Supplementation Regulates Plasma Nitric Oxide Concentration and Glutathione Peroxidase Activity
2.3. HE Reduces the Levels of Interleukin-6 and Interleukin-1β in Rat Plasma
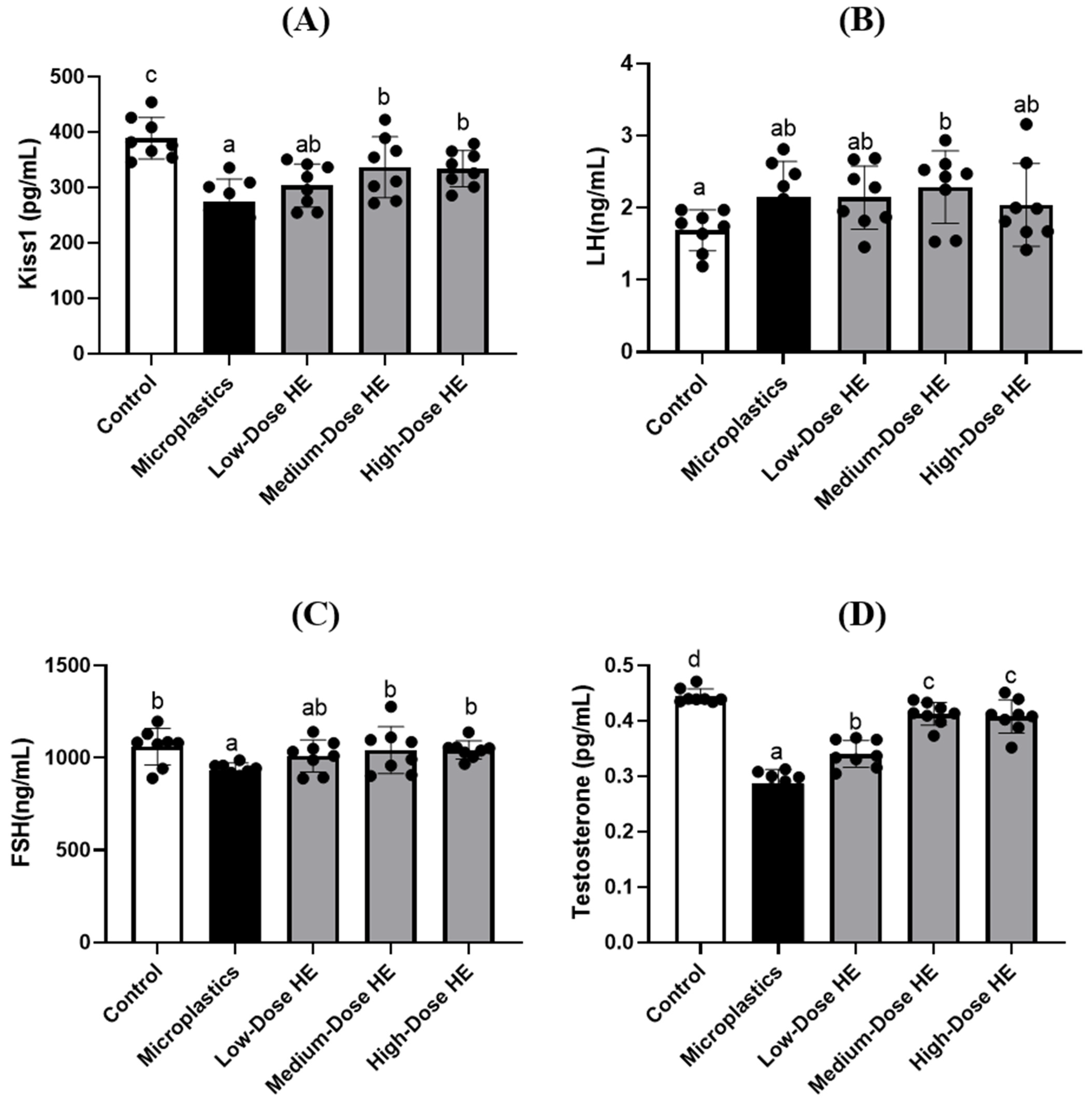
2.4. HE Regulates Kiss1 and Reproductive Hormone Concentrations
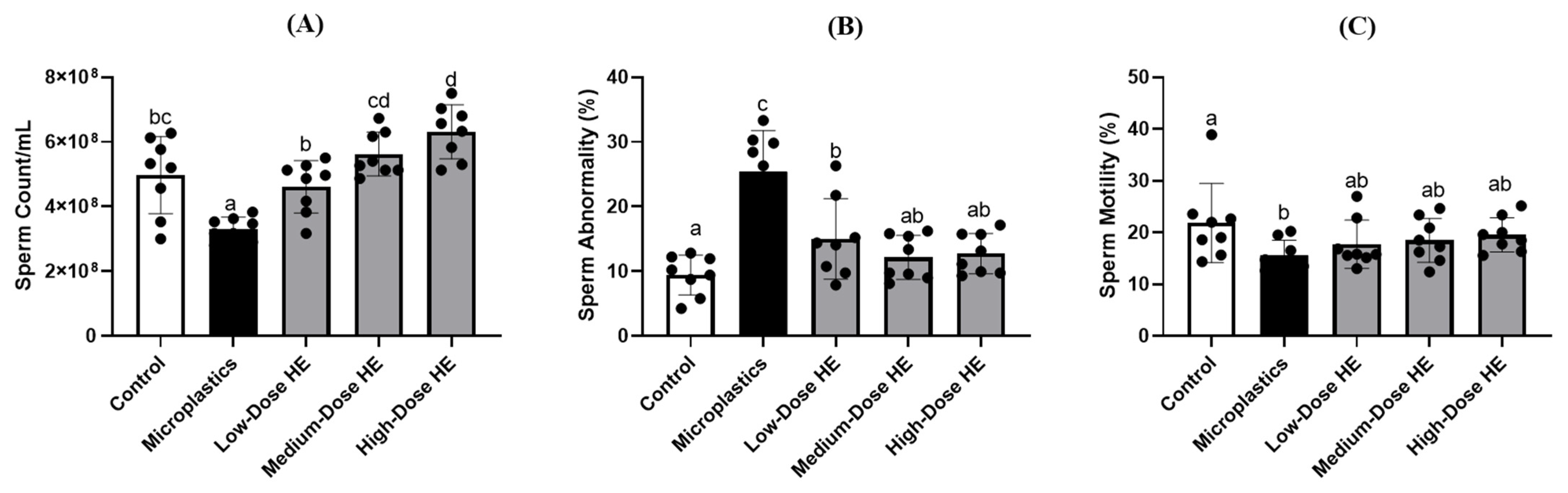
2.5. Effects of HE on the Sperm Count, Abnormality, and Motility
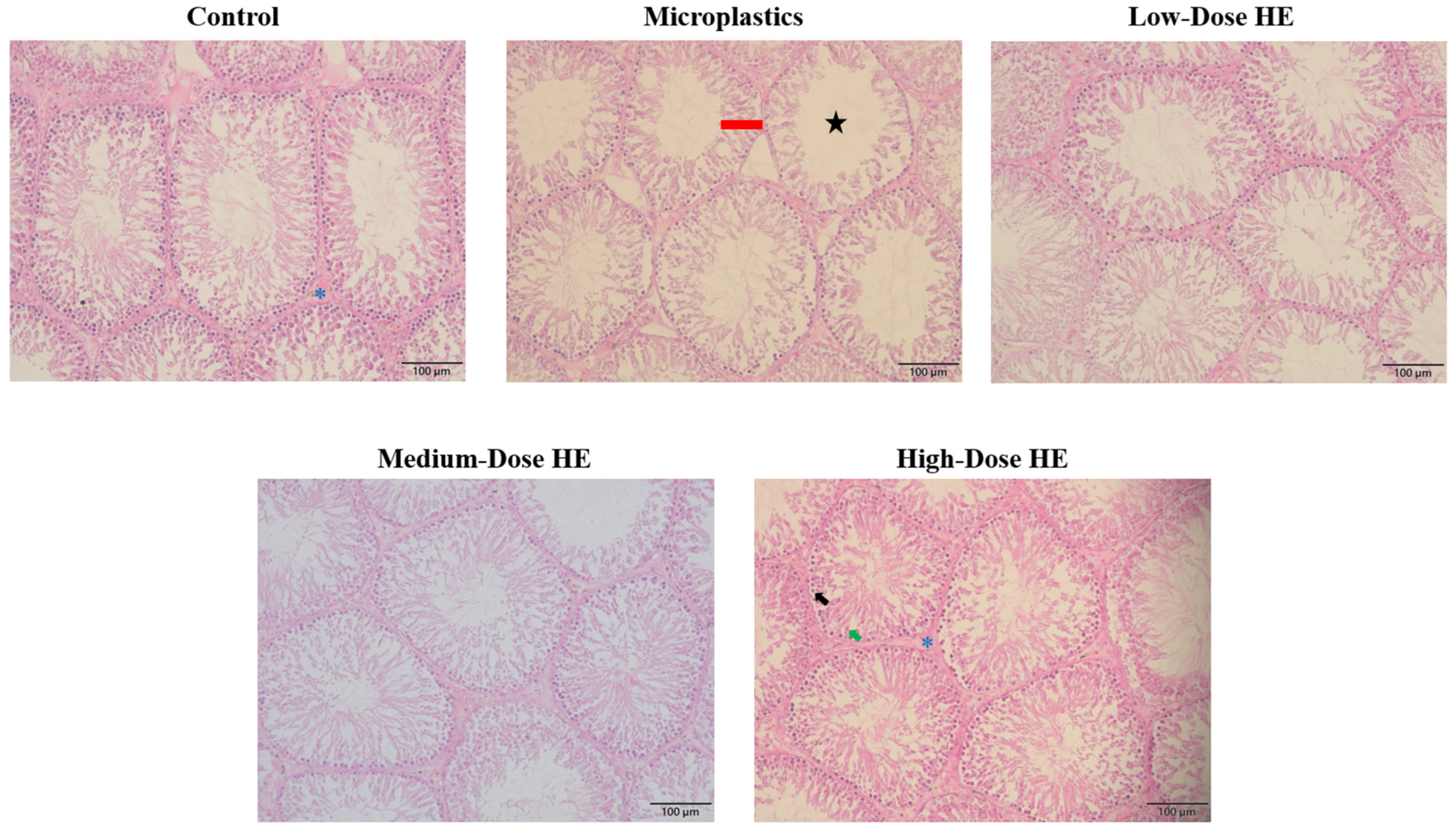
2.6. Effects of the HE on the Seminiferous Tubule’s Lumen Area and Thickness of the Epithelium
3. Discussion
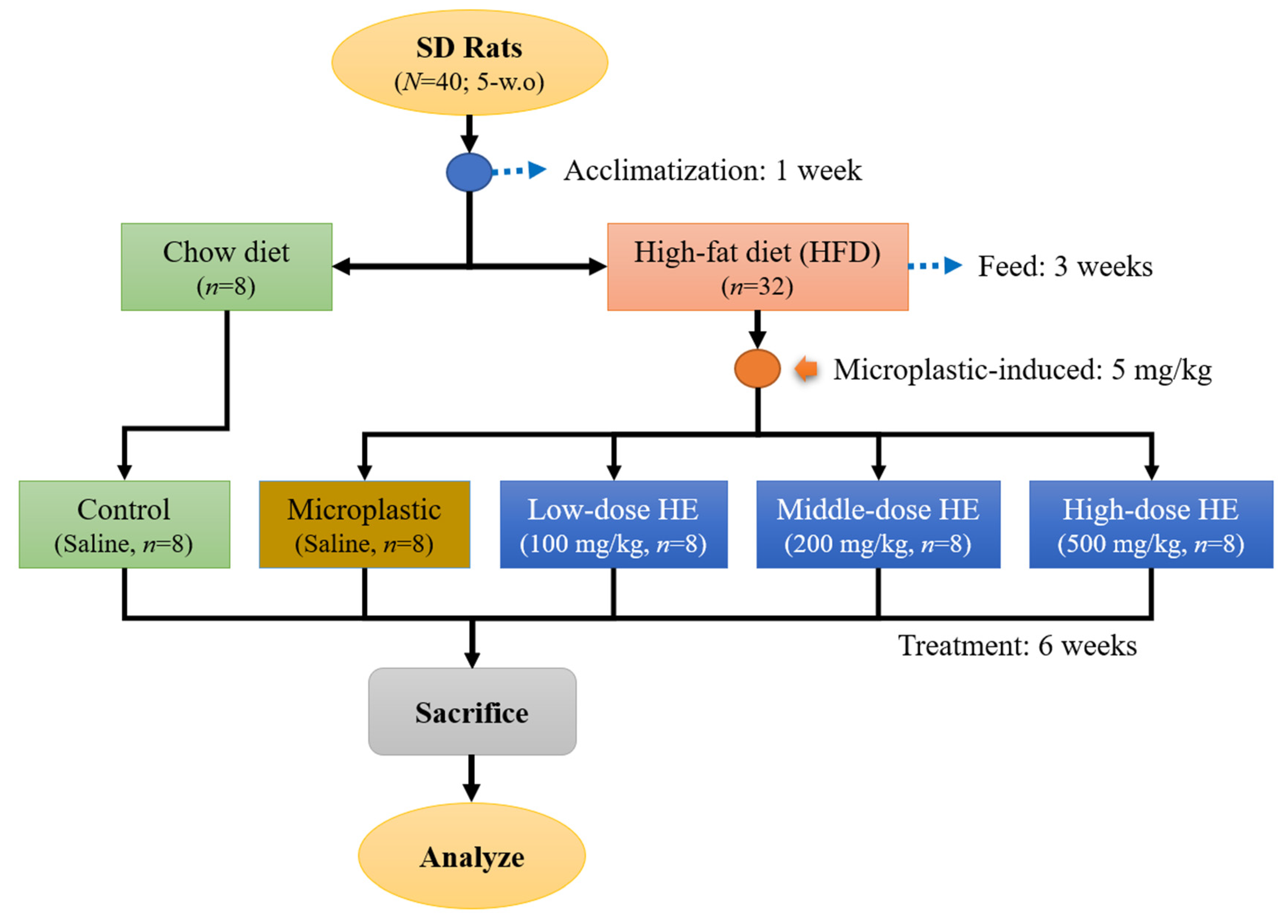
4. Materials and Methods
4.1. Materials
4.2. Polystyrene Microplastics Solution Preparation
4.3. Animal Study
4.3.1. Animal Treatment
4.3.2. Blood Sample Collection
4.3.3. Nitric Oxide and Glutathione Peroxidase Analysis
4.3.4. Pro-Inflammatory Cytokines, Kiss1, and Reproductive Hormones Analysis
4.3.5. Sperm Count, Abnormality, and Motility Analysis
4.3.6. Testis Histopathology Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assays |
| FSH | Follicle-stimulating hormone |
| GPx | Glutathione peroxidase |
| HE | Erinacine A-enriched H. erinaceus mycelium |
| IL | Interleukin |
| LH | Luteinizing hormone |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| PS-MPs | Polystyrene microplastics |
| ROS | Reactive oxygen species |
| RPMI | Roswell Park Memorial Institute |
| TNF | Tumor necrosis factor |
References
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an emerging threat to the global environment and human health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Wang, W.; Guan, J.; Feng, Y.; Nie, L.; Xu, Y.; Xu, H.; Fu, F. Polystyrene microplastics induced nephrotoxicity associated with oxidative stress, inflammation, and endoplasmic reticulum stress in juvenile rats. Front. Nutr. 2023, 9, 1059660. [Google Scholar] [CrossRef]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, G.; Xiong, Y.; Zhang, Y.; Zhang, M. The hidden threat: Unraveling the impact of microplastics on reproductive health. Sci. Total Environ. 2024, 935, 173177. [Google Scholar] [CrossRef]
- Dubey, I.; Khan, S.; Kushwaha, S. Developmental and reproductive toxic effects of exposure to microplastics: A review of associated signaling pathways. Front. Toxicol. 2022, 4, 901798. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Najam, S.; Hamza, A.; Azmat, R.; Ashraf, A.; Unuofin, J.O.; Lebelo, S.L.; Simal-Gandara, J. Pinostrobin alleviates testicular and spermatological damage induced by polystyrene microplastics in adult albino rats. Biomed. Pharmacother. 2023, 162, 114686. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Hwang, Y.-Y.; Sudirman, S.; Wei, E.-Y.; Kong, Z.-L.; Hwang, D.-F. Fucoidan from cladosiphon okamuranus enhances antioxidant activity and prevents reproductive dysfunction in polystyrene microplastic-induced male rats. Biomed. Pharmacother. 2024, 170, 115912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Ma, S.; Sun, Z.; Wang, Z. Microplastics may be a significant cause of male infertility. Am. J. Men’s Health 2022, 16, 15579883221096549. [Google Scholar] [CrossRef]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the lh-mediated lhr/camp/pka/star pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, Y.; Yao, C.; Yu, S.; Qu, J.; Chen, G.; Wei, H. Combined effects of polystyrene nanoplastics and lipopolysaccharide on testosterone biosynthesis and inflammation in mouse testis. Ecotoxicol. Environ. Saf. 2024, 273, 116180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, B.; Liu, T.; Wu, Y.; Wang, Z. Probiotics improve polystyrene microplastics-induced male reproductive toxicity in mice by alleviating inflammatory response. Ecotoxicol. Environ. Saf. 2023, 263, 115248. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 mapk signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-Z. Hericium erinaceus: The enchanting medicinal-culinary mushroom of east asian tradition. Integr. Med. Discov. 2024, 8, e24013. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Phatthanamas, W.; Thepsilvisut, O.; Chantarachot, T.; Thongtip, A.; Chutimanukul, P. Commercial scale production of yamabushitake mushroom (hericium erinaceus (bull.) pers. 1797) using rubber and bamboo sawdust substrates in tropical regions. Sci. Rep. 2023, 13, 13316. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’Onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J. Gastroenterol. 2023, 29, 3048–3065. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and neuroprotective effects of hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wu, J.; Kang, S.; Gao, J.; Hirokazu, K.; Liu, H.; Liu, C. The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus hericium: A review. Chin. J. Nat. Med. 2024, 22, 676–698. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Sun, H.-L.; Chang, J.-C.; Lin, W.-Y.; Chen, P.-Y.; Chen, C.-C.; Lee, L.-Y.; Li, C.-C.; Hsieh, M.; Chen, H.-W.; et al. Erinacine a-enriched hericium erinaceus mycelium ethanol extract lessens cellular damage in cell and drosophila models of spinocerebellar ataxia type 3 by improvement of nrf2 activation. Antioxidants 2024, 13, 1495. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Huang, C.-S.; Chen, Y.-H.; Chen, C.-C.; Chen, C.-C.; Chuang, C.-H. Anti-inflammatory effect of erinacine c on no production through down-regulation of nf-κb and activation of nrf2-mediated ho-1 in bv2 microglial cells treated with lps. Molecules 2019, 24, 3317. [Google Scholar] [CrossRef]
- Lee, K.-F.; Chen, J.-H.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.-C.; Lu, C.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Protective effects of hericium erinaceus mycelium and its isolated erinacine a against ischemia-injury-induced neuronal cell death via the inhibition of inos/p38 mapk and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lin, C.; Wang, Z.-X.; Zhang, Y.-L.; Li, S.-X.; Hu, X.-S.; Hui, H.-P.; Wang, Z.; Zhao, Y.-Q.; Wang, X.-J.; et al. Metabolites of culinary-medicinal mushroom hericium erinaceus showed anti-neuroinflammatory activity in bv2 cells and anti-h2o2-induced oxidative stress activity in sh-sy5y cells. Nat. Prod. J. 2025, 15, e030624230635. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, G.; Liu, W.; Zhang, F.; Linhardt, R.J.; Wang, X.; Zhang, A. Bioactive compounds in hericium erinaceus and their biological properties: A review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Bao, L.; Yang, Y.; Ma, K.; Liu, H. Three new cyathane diterpenes with neurotrophic activity from the liquid cultures of hericium erinaceus. J. Antibiot. 2018, 71, 818–821. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lin, T.-W.; Li, I.C.; Tsung, L.; Hou, C.-H.; Yang, C.-Y.; Li, T.-J.; Chen, C.-C. A pilot pharmacokinetic and metabolite identification study of erinacine a in a single landrace pig model. Heliyon 2024, 10, e37850. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Panday, S.; Talreja, R.; Kavdia, M. The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Chou, W.; Chen, W.-P.; Wang, M.-F.; Chen, Y.-J.; Chen, C.-C.; Tung, K.-C. Erinacine a-enriched hericium erinaceus mycelium delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (samp8) mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine a-enriched hericium erinaceus mycelium produces antidepressant-like effects through modulating bdnf/pi3k/akt/gsk-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef]
- Lee, S.-L.; Hsu, J.-Y.; Chen, T.-C.; Huang, C.-C.; Wu, T.-Y.; Chin, T.-Y. Erinacine a prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef]
- Ogawa, S.; Parhar, I.S. Biological significance of kisspeptin–kiss 1 receptor signaling in the habenula of teleost species. Front. Endocrinol. 2018, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-gnrh pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Trussell, J.C.; Coward, R.M.; Santoro, N.; Stetter, C.; Kunselman, A.; Diamond, M.P.; Hansen, K.R.; Krawetz, S.A.; Legro, R.S.; Heisenleder, D.; et al. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil. Steril. 2019, 111, 1129–1134. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.; Mongioì, L.; Calogero, A. Fsh dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Grande, G.; Barrachina, F.; Soler-Ventura, A.; Jodar, M.; Mancini, F.; Marana, R.; Chiloiro, S.; Pontecorvi, A.; Oliva, R.; Milardi, D. The role of testosterone in spermatogenesis: Lessons from proteome profiling of human spermatozoa in testosterone deficiency. Front. Endocrinol. 2022, 13, 852661. [Google Scholar] [CrossRef]
- Guardo, F.D.; Vloeberghs, V.; Bardhi, E.; Blockeel, C.; Verheyen, G.; Tournaye, H.; Drakopoulos, P. Low testosterone and semen parameters in male partners of infertile couples undergoing ivf with a total sperm count greater than 5 million. J. Clin. Med. 2020, 9, 3824. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of inflammation on male reproductive tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The impact of oxidative stress on male reproductive function: Exploring the role of antioxidant supplementation. Cureus 2023, 17, e42583. [Google Scholar] [CrossRef]
- Walczak–Jedrzejowska, R.; Wolski, J.K.; Slowikowska–Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 65, 60–67. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of inflammatory associated impairment of sperm function, spermatogenesis and steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Owembabazi, E.; Nkomozepi, P.; Calvey, T.; Mbajiorgu, E.F. Co-administration of alcohol and combination antiretroviral therapy (cart) in male sprague dawley rats: A study on testicular morphology, oxidative and cytokines perturbations. Anat. Cell Biol. 2023, 56, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Kuchakulla, M.; Narasimman, M.; Khodamoradi, K.; Khosravizadeh, Z.; Ramasamy, R. How defective spermatogenesis affects sperm DNA integrity. Andrologia 2020, 53, e13615. [Google Scholar] [CrossRef]
- Ojo, O.A.; Nwafor-Ezeh, P.I.; Rotimi, D.E.; Iyobhebhe, M.; Ogunlakin, A.D.; Ojo, A.B. Apoptosis, inflammation, and oxidative stress in infertility: A mini review. Toxicol. Rep. 2023, 10, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Magata, F.; Tsukamura, H.; Matsuda, F. The impact of inflammatory stress on hypothalamic kisspeptin neurons: Mechanisms underlying inflammation-associated infertility in humans and domestic animals. Peptides 2023, 162, 170958. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. Nf-κb signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Wu, Y.-K.; Hu, J.-H.; Li, I.C.; Lin, T.-W.; Chen, C.-C.; Kuo, C.-F. Preclinical bioavailability, tissue distribution, and protein binding studies of erinacine a, a bioactive compound from hericium erinaceus mycelia using validated lc-ms/ms method. Molecules 2021, 26, 4510. [Google Scholar] [CrossRef]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.-J. Erinacine a-enriched hericium erinaceus mycelium ameliorates alzheimer’s disease-related pathologies in appswe/ps1de9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.-F.; Vazquez, C.I.; Chiang, C.-Y.; Chiu, M.-H.; Chen, C.-S.; Ni, C.-W.; Gong, G.-C.; Quigg, A.; Santschi, P.H.; Chin, W.-C. Nano- and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton. Sci. Total Environ. 2020, 748, 141469. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, C.S.; Yang, M.F.; Chen, S.; Soni, M.; Mahadevan, B. Subchronic toxicity and genotoxicity studies of hericium erinaceus β-glucan extract preparation. Curr. Res. Toxicol. 2022, 3, 100068. [Google Scholar] [CrossRef]
- Li, I.C.; Chen, Y.-L.; Lee, L.-Y.; Chen, W.-P.; Tsai, Y.-T.; Chen, C.-C.; Chen, C.-S. Evaluation of the toxicological safety of erinacine a-enriched hericium erinaceus in a 28-day oral feeding study in sprague–dawley rats. Food Chem. Toxicol. 2014, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sudirman, S.; Su, C.-Y.; Tsou, D.; Lee, M.-C.; Kong, Z.-L. Hippocampus kuda protein hydrolysate improves male reproductive dysfunction in diabetic rats. Biomed. Pharmacother. 2021, 140, 111760. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Schrottmaier, W.C.; Salzmann, M.; Brostjan, C.; Schmid, J.A.; Starlinger, P.; Assinger, A. Optimized plasma preparation is essential to monitor platelet-stored molecules in humans. PLoS ONE 2017, 12, e0188921. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Park, T.; Kang, M.-G.; Park, D. Anti-inflammatory activity and ros regulation effect of sinapaldehyde in lps-stimulated raw 264.7 macrophages. Molecules 2020, 25, 4089. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Meng, L.-J.; Xu, Y.-J.; Gong, T.; Yang, Y. Effects of 4% paraformaldehyde and modified davidson’s fluid on the morphology and immunohistochemistry of xiang pig testes. J. Toxicol. Pathol. 2020, 33, 97–104. [Google Scholar] [CrossRef]

| Group | Kidney | Spleen | Abdominal Fat | Testes | Epididymis Fat |
|---|---|---|---|---|---|
| Control | 4.23 ± 0.35 | 0.83 ± 0.18 | 8.83 ± 1.26 | 3.89 ± 0.89 | 8.02 ± 1.26 |
| Microplastics | 3.98 ± 0.37 | 0.74 ± 0.08 | 7.18 ± 1.98 | 3.74 ± 0.78 | 6.41 ± 1.75 |
| Low-Dose HE | 4.08 ± 0.48 | 0.70 ± 0.10 | 8.76 ± 2.45 | 4.43 ± 1.38 | 7.07 ± 2.24 |
| Medium-Dose HE | 4.26 ± 0.27 | 0.74 ± 0.10 | 7.93 ± 2.75 | 3.84 ± 0.93 | 7.40 ± 2.16 |
| High-Dose HE | 4.08 ± 0.55 | 0.76 ± 0.17 | 9.17 ± 2.34 | 3.79 ± 0.84 | 6.82 ± 2.29 |
| Groups | Seminiferous Tubule Area (%) | Thickness of Epithelium (µm) |
|---|---|---|
| Control | 17.55 ± 2.22 a | 110.23 ± 31.89 b |
| Microplastics | 41.74 ± 8.54 b | 62.03 ± 9.19 a |
| Low-Dose HE | 20.77 ± 8.03 a | 91.91 ± 13.72 b |
| Medium-Dose HE | 12.86 ± 0.61 a | 104.25 ± 13.93 b |
| High-Dose HE | 13.14 ± 1.57 a | 109.64 ± 14.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-Y.; Sudirman, S.; Wei, E.-Y.; Shiu, R.-F.; Kong, Z.-L.; Hwang, D.-F. Yamabushitake Mushroom (Hericium erinaceus (Bull.) Pers. 1797) Mycelium Improves Reproductive System Dysfunction in Male Rats Induced by Polystyrene Microplastics. Int. J. Mol. Sci. 2025, 26, 5735. https://doi.org/10.3390/ijms26125735
Hwang Y-Y, Sudirman S, Wei E-Y, Shiu R-F, Kong Z-L, Hwang D-F. Yamabushitake Mushroom (Hericium erinaceus (Bull.) Pers. 1797) Mycelium Improves Reproductive System Dysfunction in Male Rats Induced by Polystyrene Microplastics. International Journal of Molecular Sciences. 2025; 26(12):5735. https://doi.org/10.3390/ijms26125735
Chicago/Turabian StyleHwang, Yi-Yuh, Sabri Sudirman, En-Yu Wei, Ruei-Feng Shiu, Zwe-Ling Kong, and Deng-Fwu Hwang. 2025. "Yamabushitake Mushroom (Hericium erinaceus (Bull.) Pers. 1797) Mycelium Improves Reproductive System Dysfunction in Male Rats Induced by Polystyrene Microplastics" International Journal of Molecular Sciences 26, no. 12: 5735. https://doi.org/10.3390/ijms26125735
APA StyleHwang, Y.-Y., Sudirman, S., Wei, E.-Y., Shiu, R.-F., Kong, Z.-L., & Hwang, D.-F. (2025). Yamabushitake Mushroom (Hericium erinaceus (Bull.) Pers. 1797) Mycelium Improves Reproductive System Dysfunction in Male Rats Induced by Polystyrene Microplastics. International Journal of Molecular Sciences, 26(12), 5735. https://doi.org/10.3390/ijms26125735









