Bisphenol AF Induced Neurodevelopmental Toxicity of Human Neural Progenitor Cells via Nrf2/HO-1 Pathway
Abstract
1. Introduction
2. Results
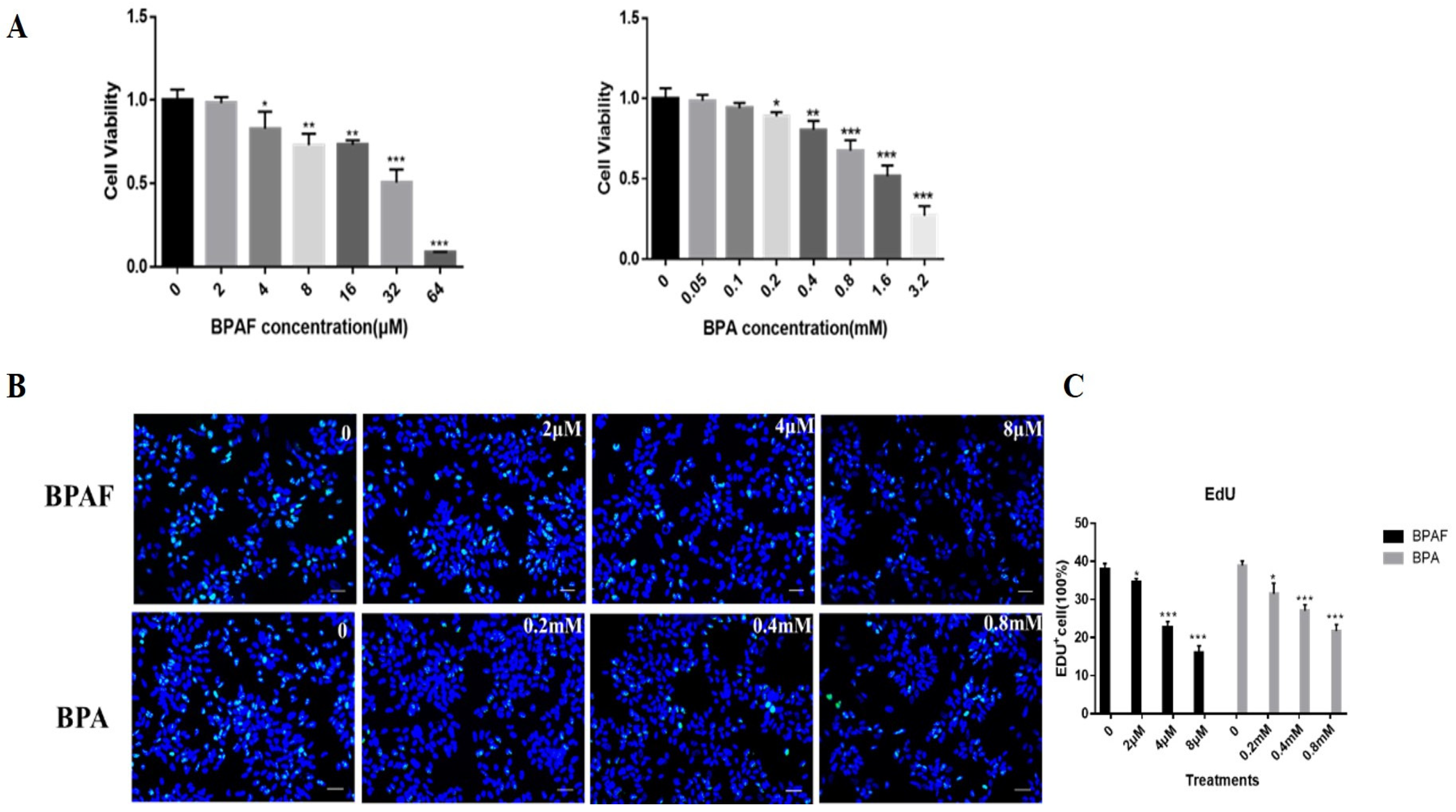
2.1. BPAF Reduced the Cell Activity and Proliferation of ReNcell CX Cells to a Greater Extent than BPA
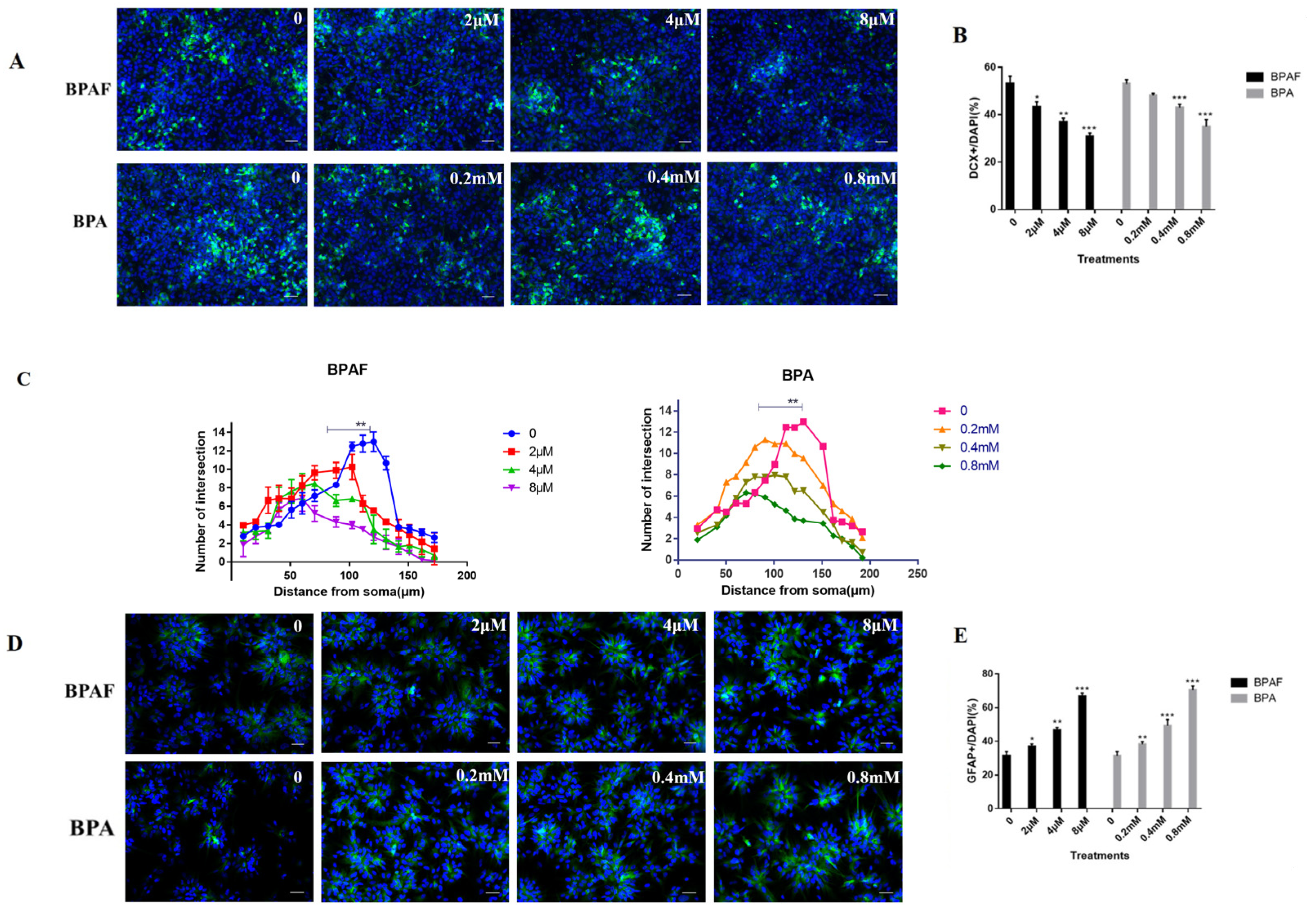
2.2. BPAF Impaired the Differentiation Direction of ReNcell CX Cells and Synaptic Growth
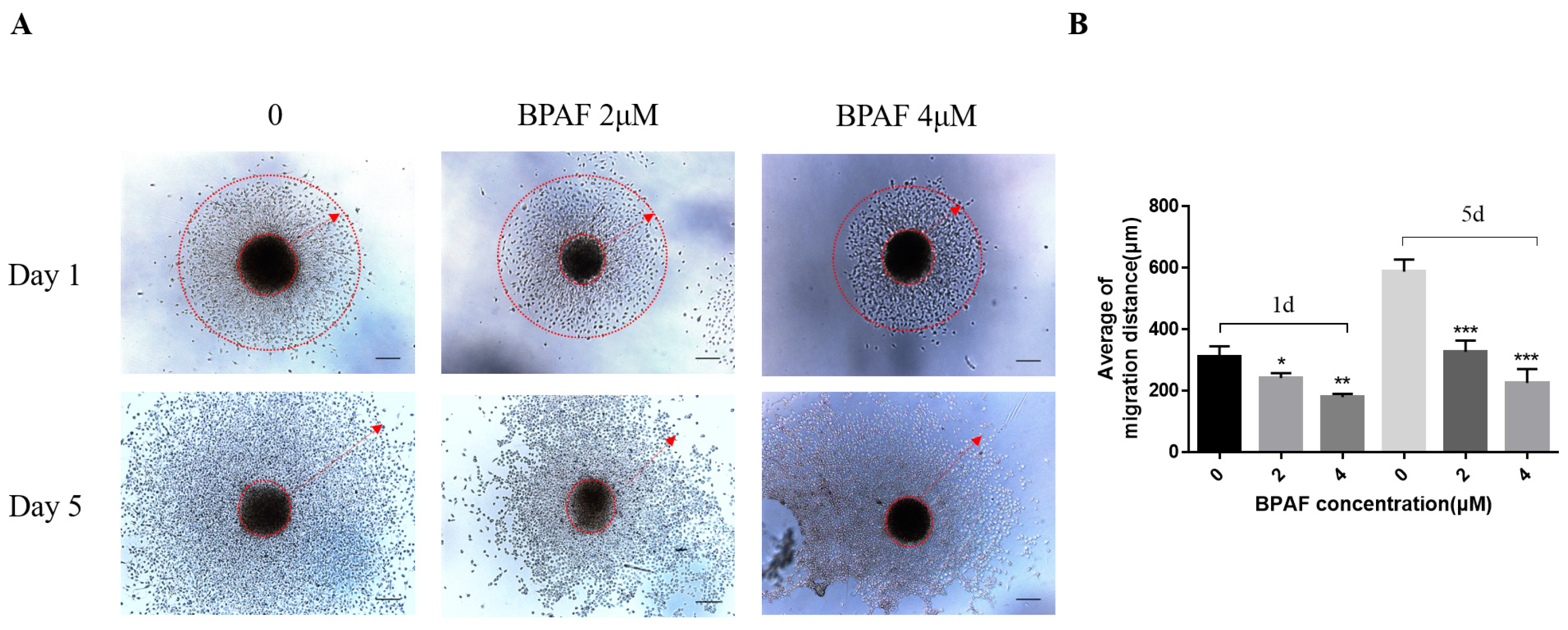
2.3. A Total of 2 μM of BPAF Compromised the Migration Ability of ReNcell CX Cells During Differentiation
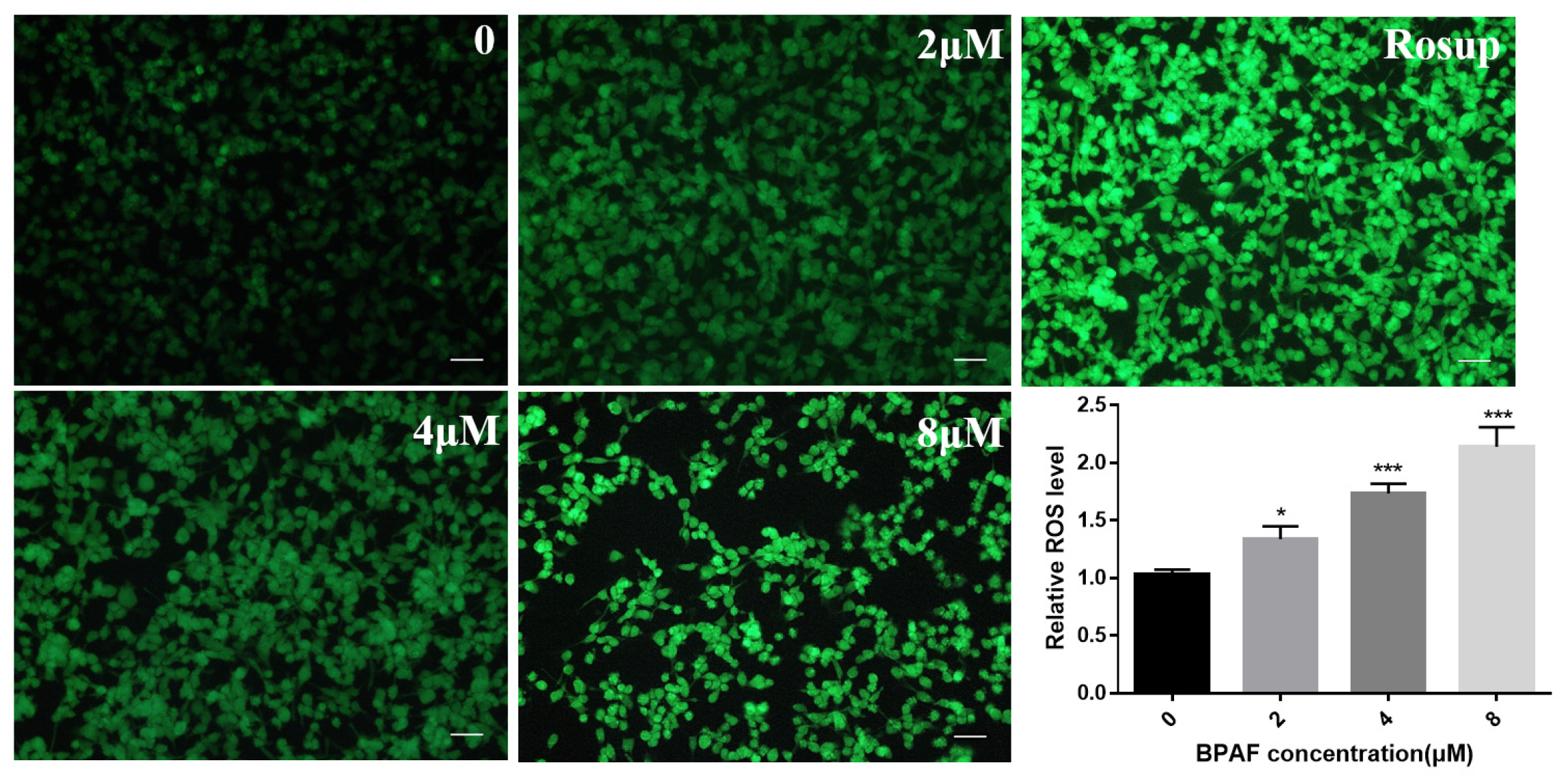
2.4. BPAF Increased ROS Levels in ReNcell CX Cells
2.5. Relationship Between AKT/Nrf2/HO-1 Pathway and Neurodevelopmental Toxicity of BPAF
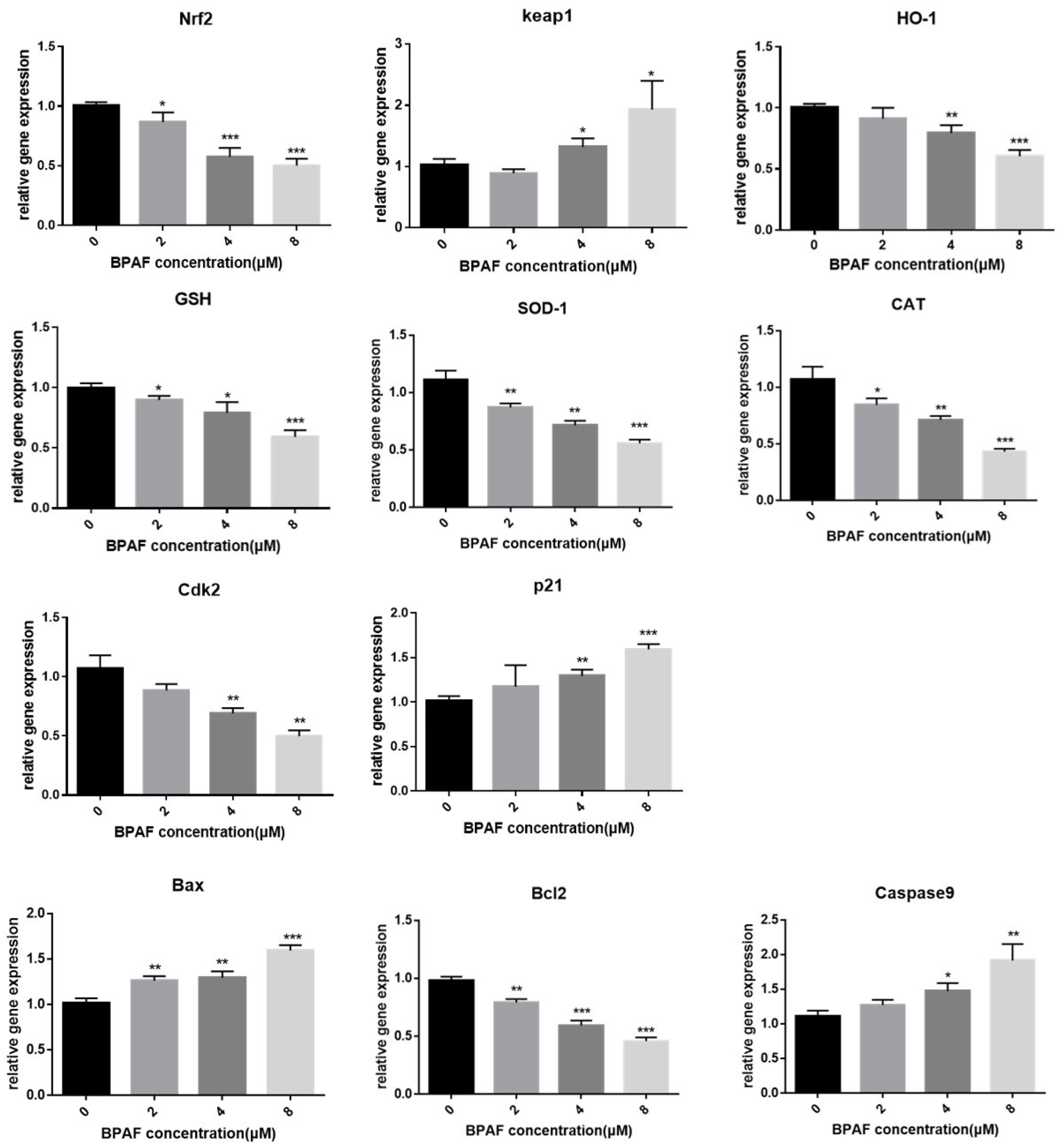
2.6. BPAF Altered Gene Expression Associated with the Nrf2/HO-1 Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Cell Activity and Proliferation Measurement
4.3. Immunocytochemistry
4.4. Intracellular ROS Levels
4.5. Proteome Profiling Array
4.6. Western Blot
4.7. Quantitative Real-Time PCR Analysis
4.8. Statistical Analysis
4.9. Ethics and Provenance Statement
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, H.; Zhu, J.; Chen, Z.; Yanjun, H. Occurrence and Partitioning of Bisphenol Analogues in Adults’ Blood from China. Environ. Sci. Technol. 2018, 52, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Da, C.; Kurunthachalam, K.; Hongli, T.; Zhengui, Z.; Yong-Lai, F.; Yan, W.; Margaret, W. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef]
- Faust, T.E.; Gunner, G.; Schafer, D.P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat. Rev. Neurosci. 2021, 22, 657–673. [Google Scholar] [CrossRef]
- Gyimah, E.; Zhu, X.; Zhang, Z.; Guo, M.; Xu, H.; Mensah, J.K.; Dong, X.; Zhang, Z.; Gyimah, G.N.W. Oxidative Stress and Apoptosis in Bisphenol AF-Induced Neurotoxicity in Zebrafish Embryos. Environ. Toxicol. Chem. 2022, 41, 2273–2284. [Google Scholar] [CrossRef]
- Morales-Grahl, E.; Hilz, E.N.; Gore, A.C. Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements. Int. J. Mol. Sci. 2024, 25, 6887. [Google Scholar] [CrossRef]
- Harnett, K.G.; Chin, A.; Schuh, S.M. Cytotoxic and apoptotic data of BPA and BPA alternatives TMBPF, BPAF, and BPS in female adult rat and human stem cells. Data Brief. 2021, 37, 107183. [Google Scholar] [CrossRef]
- Liang, X.; Yin, N.; Liang, S.; Yang, R.; Liu, S.; Lu, Y.; Jiang, L.; Zhou, Q.; Jiang, G.; Faiola, F. Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length. Food Chem. Toxicol. 2020, 135, 111015. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxid. Med. Cell. Longel. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. Vitr. 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Gao, C.; Qiu, W.; Zheng, Y. Oxidative Stress, Endocrine Disturbance, and Immune Interference in Humans Showed Relationships to Serum Bisphenol Concentrations in a Dense Industrial Area. Environ. Sci. Technol. 2021, 56, 4688–4690. [Google Scholar] [CrossRef]
- Murakami, S.; Kusano, Y.; Okazaki, K.; Akaike, T.; Motohashi, H. NRF2 signalling in cytoprotection and metabolism. Br. J. Pharmacol. 2023, 1. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef]
- Vahidinia, Z.; Azami Tameh, A.; Barati, S.; Izadpanah, M.; Seyed Hosseini, E. Nrf2 activation: A key mechanism in stem cell exosomes-mediated therapies. Cell. Mol. Biol. Lett. 2024, 29, 30. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Sha, J.Y.; Zhou, Y.D.; Yang, J.Y.; Leng, J.; Li, J.H.; Hu, J.N.; Liu, W.; Jiang, S.; Wang, Y.P.; Chen, C.; et al. Maltol (3-Hydroxy-2-methyl-4-pyrone) Slows d-Galactose-Induced Brain Aging Process by Damping the Nrf2/HO-1-Mediated Oxidative Stress in Mice. J. Agric. Food Chem. 2019, 67, 10342–10351. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhao, C.; Yu, S.; Duan, D.; Xu, Q. Long-term expansion of human neural progenitor cells by epigenetic stimulation in vitro. Neurosci. Res. 2005, 51, 157–165. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Guan, T.; Yu, H.; Li, T. In vitro and in silico assessment of the structure-dependent binding of bisphenol analogues to glucocorticoid receptor. Anal. Bioanal. Chem. 2017, 409, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 2017, 47, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Karrer, C.; Roiss, T.; von Goetz, N.; Gramec Skledar, D.; Peterlin Mašič, L.; Hungerbühler, K. Physiologically Based Pharmacokinetic (PBPK) Modeling of the Bisphenols BPA, BPS, BPF, and BPAF with New Experimental Metabolic Parameters: Comparing the Pharmacokinetic Behavior of BPA with Its Substitutes. Environ. Health Perspect. 2018, 126, 077002. [Google Scholar] [CrossRef] [PubMed]
- Ovsepian, S.V.; O’Leary, V.B.; Ayvazyan, N.M.; Al-Sabi, A.; Ntziachristos, V.; Dolly, J.O. Neurobiology and therapeutic applications of neurotoxins targeting transmitter release. Pharmacol. Ther. 2017, 193, 135–155. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, W.; Liu, T.; Ni, Y.; Qin, Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins 2022, 15, 18. [Google Scholar] [CrossRef]
- Gu, J.; Guo, M.; Yin, X.; Huang, C.; Qian, L.; Zhou, L.; Wang, Z.; Wang, L.; Shi, L.; Ji, G. A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci. Total Environ. 2022, 805, 150210. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Li, J.; Wuliji, O.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Ding, J.; Liu, J. Neurodevelopmental toxicity of bisphenol AF in zebrafish larvae and the protective effects of curcumin. J. Appl. Toxicol. 2023, 43, 1806–1818. [Google Scholar] [CrossRef]
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 255–277. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Bio. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, S.; Xia, R.; Sun, M.; Sun, Y.; Ren, H.; Zhang, Y.; Xia, Z.; Yao, S.; Wang, T. Kinsenoside mitigates myocardial ischemia/reperfusion-induced ferroptosis via activation of the Akt/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2023, 956, 175985. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Ren, Y.; Yue, Q.; Ding, Z.; Wang, K.; Zheng, T.; Chen, G.; Chen, X.; Li, M.; Fan, L. α-Lipoic Acid Targeting PDK1/NRF2 Axis Contributes to the Apoptosis Effect of Lung Cancer Cells. Oxid. Med. Cell. Longev. 2021, 2021, 6633419. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xie, J.; Mao, L.; Zhao, M.; Bai, X.; Wen, J.; Shen, T.; Wu, P. Bisphenol analogue concentrations in human breast milk and their associations with postnatal infant growth. Environ. Pollut. 2020, 259, 113779. [Google Scholar] [CrossRef]
- Diermeier-Daucher, S.; Clarke, S.T.; Hill, D.; Vollmann-Zwerenz, A.; Bradford, J.A.; Brockhoff, G. Cell type specific applicability of 5-ethynyl-2′-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry. Cytometry A 2009, 75, 535–546. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Liu, C.; Deng, P.; Zhu, G.; Zhang, L.; He, M.; Lu, Y.; Duan, W.; Pei, L.; et al. Exposure to 1800 MHz radiofrequency radiation impairs neurite outgrowth of embryonic neural stem cells. Sci. Rep. 2014, 4, 5103. [Google Scholar] [CrossRef]
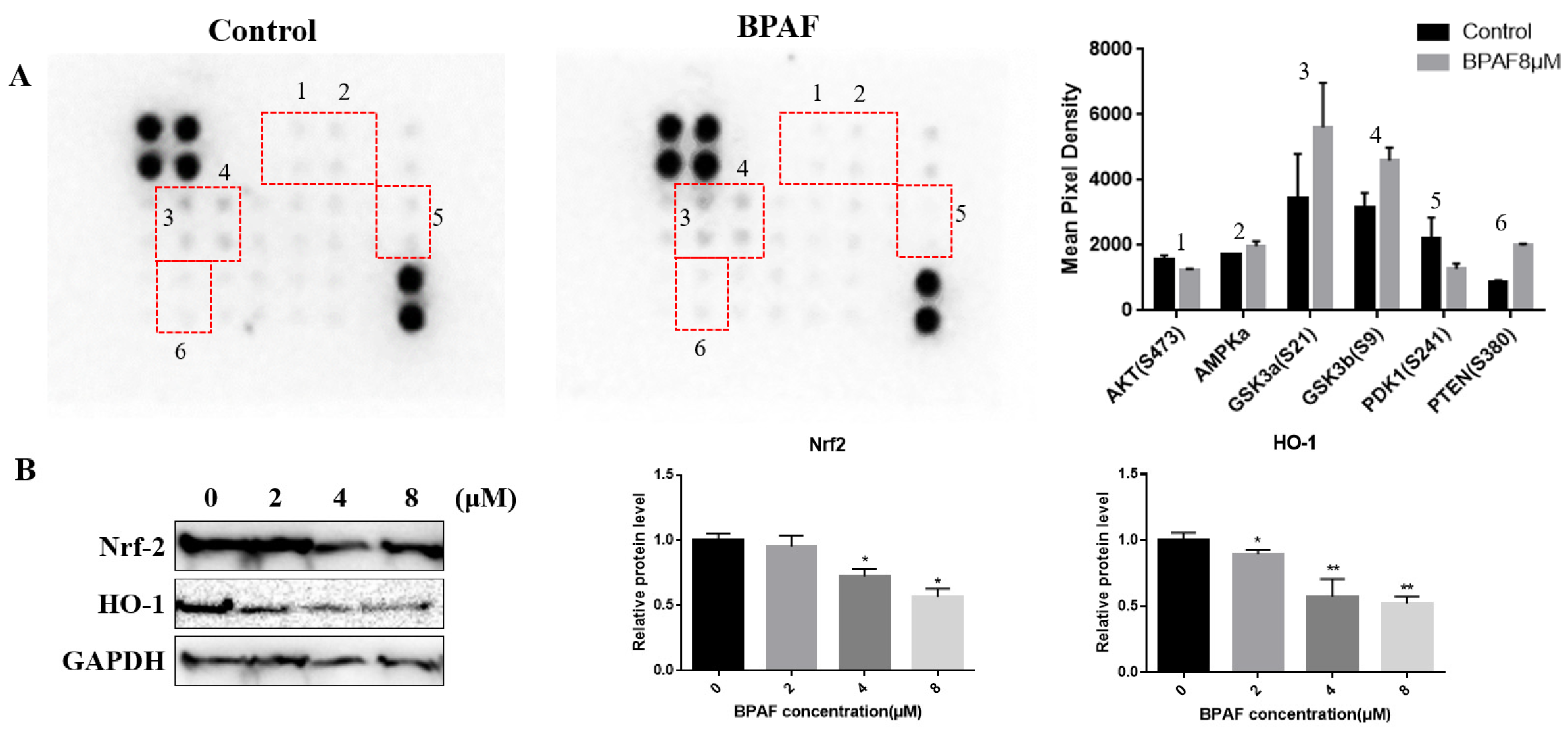
| A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|
| POS | POS | NEG | NEG | AKT(S473) | AMPKa | BAD(S112) | 4E-BP1(T36) |
| POS | POS | NEG | NEG | AKT(S473) | AMPKa | BAD(S112) | 4E-BP1(T36) |
| IC | GSK3a(S21) | GSK3b(S9) | mTOR(S2448) | p27(T198) | IC | P70S6K(T421/S424) | PDK1(S241) |
| IC | GSK3a(S21) | GSK3b(S9) | mTOR(S2448) | p27(T198) | IC | P70S6K(T421/S425) | PDK1(S242) |
| PRAS(S40) | PTEN(S380) | RAF-1(S301) | RPS6(S235/236) | IC | IC | NEG | POS |
| PRAS(S41) | PTEN(S381) | RAF-1(S302) | RPS6(S235/237) | IC | IC | NEG | POS |
| Primer Name | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| Nrf2 | ACGGTATGCAACAGGACATTGAGC | TTGGCTTCTGGACTTGGAACCATG |
| Keap1 | ATTCAGCTGAGTGTTACTACCC | CAGCATAGATACAGTTGTGCAG |
| HO-1 | CCTCCCTGTACCACATCTATGT | GCTCTTCTGGGAAGTAGACAG |
| SOD | ATCCTCTATCCAGAAAACACGG | GCGTTTCCTGTCTTTGTACTTT |
| GSH-Px | GTTGCCTGGAACTTTGAGAAG | CTCGATGTCAATGGTCTGGAAG |
| CAT | GAGCACAGCATCCAATATTCTG | CTCATTCAGCACGTTCACATAG |
| Bax | AGGATGCGTCCACCAAGAAGCT | TCCGTGTCCACGTCAGCAATCA |
| Bcl2 | CCTGTGGATGACTGAGTACCTG | AGCCAGGAGAAATCAAACAGAGG |
| Caspase9 | CTGCTGCGTGGTGGTCATTCTC | CACAATCTTCTCGACCGACACAGG |
| Cdk2 | CGCACCTTTCAGACCGCTGTTT | CCATCTCCTCTATGACAGC |
| P21 | TCGCTGTCTTGCACTCTGGTGT | CCAATCTGCGCTTGGAGTGATAG |
| β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Ying, M.; Yang, Y.; Huo, Q.; Hong, X.; Tao, G.; Xiao, P. Bisphenol AF Induced Neurodevelopmental Toxicity of Human Neural Progenitor Cells via Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2025, 26, 5685. https://doi.org/10.3390/ijms26125685
Luo H, Ying M, Yang Y, Huo Q, Hong X, Tao G, Xiao P. Bisphenol AF Induced Neurodevelopmental Toxicity of Human Neural Progenitor Cells via Nrf2/HO-1 Pathway. International Journal of Molecular Sciences. 2025; 26(12):5685. https://doi.org/10.3390/ijms26125685
Chicago/Turabian StyleLuo, Huan, Mengchao Ying, Yun Yang, Qian Huo, Xinyu Hong, Gonghua Tao, and Ping Xiao. 2025. "Bisphenol AF Induced Neurodevelopmental Toxicity of Human Neural Progenitor Cells via Nrf2/HO-1 Pathway" International Journal of Molecular Sciences 26, no. 12: 5685. https://doi.org/10.3390/ijms26125685
APA StyleLuo, H., Ying, M., Yang, Y., Huo, Q., Hong, X., Tao, G., & Xiao, P. (2025). Bisphenol AF Induced Neurodevelopmental Toxicity of Human Neural Progenitor Cells via Nrf2/HO-1 Pathway. International Journal of Molecular Sciences, 26(12), 5685. https://doi.org/10.3390/ijms26125685





