Single-Nucleotide Polymorphisms (SNPs) in Vitamin D Physiology Genes May Modulate Serum 25(OH)D Levels in Well-Trained CrossFit® Athletes, Which May Be Associated with Performance Outcomes
Abstract
1. Introduction
2. Results
2.1. CrossFit® Practitioners’ Characteristics
2.2. CrossFit® Practitioner’s Dietary Assessment
2.3. Circulating 25-Hydroxy-Vitamin D Plasma Levels by Ranges
2.4. Circulating of 25-Hydroxy Vitamin D, Vitamin D Binding Protein and Vitamin D Receptor Protein Plasma Levels
2.5. Genotyping and Phenotyping Frequencies
2.6. Correlations Between 25-Hydroxy Vitamin D and Single-Nucleotide Polymorphisms of the CYP2R1, GC and VDR Genes
2.7. Single-Nucleotide Polymorphisms of the CYP2R1, GC and VDR Genes Associated with 25-Hydroxy Vitamin D Plasma Level
2.8. CYP2R1, GC and VDR Gene Polymorphism; 25-Hydroxy Vitamin D Plasma Level; and Sports Performance Levels in the CrossFit® Total
3. Discussion
3.1. Limitations and Strengths
3.2. Future Directions
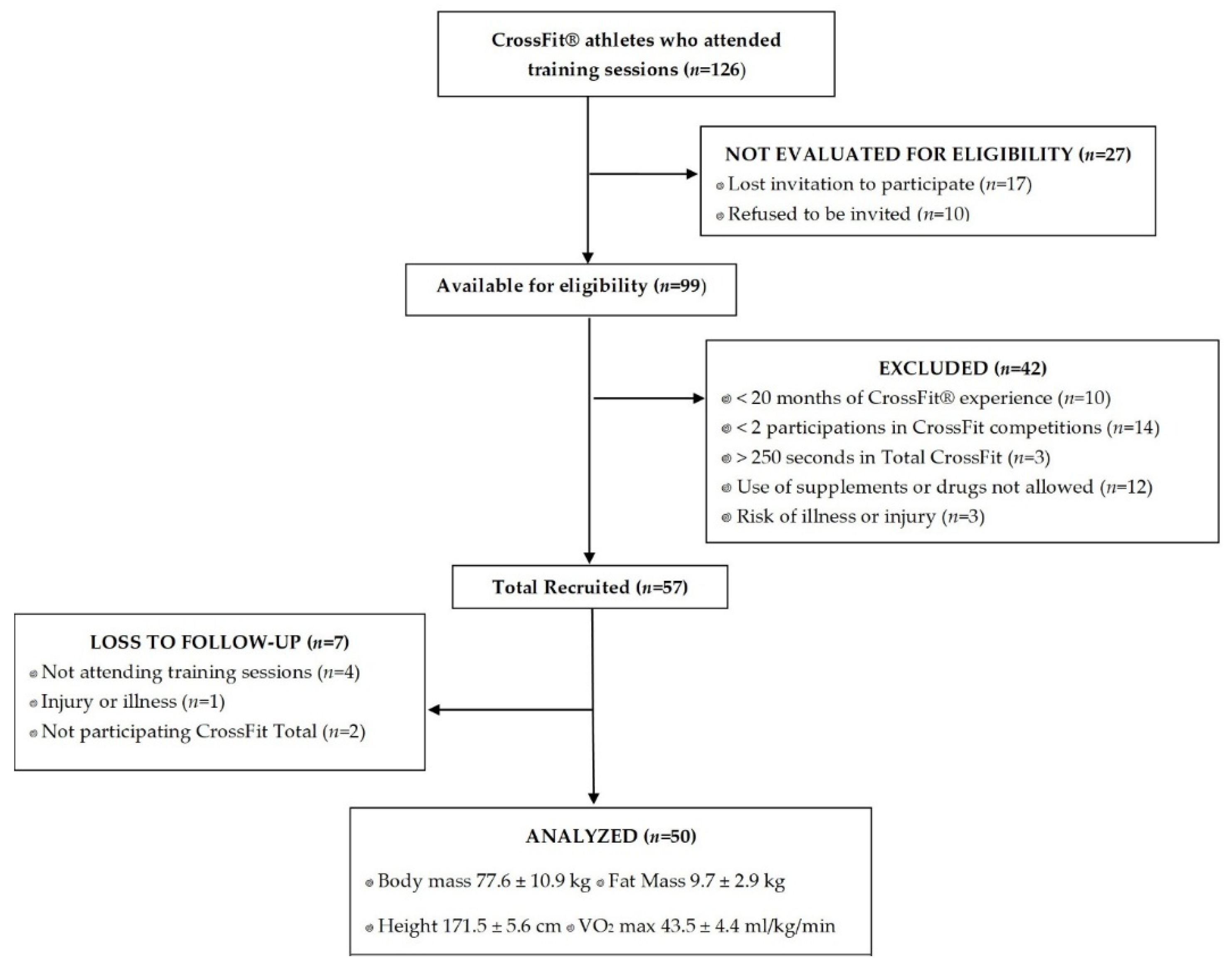
4. Material and Methods
4.1. Study Design
4.2. Inclusion Criteria
4.3. Data Collection
4.4. Blood Collection and 25(OH)D Determination in Peripheral Blood
4.5. Single-Nucleotide Polymorphism (SNPs) Selection
4.6. Single-Nucleotide Polymorphism (SNPs) Determination After DNA Isolation and Genotyping
4.7. Assessment of Genotyping and Phenotyping Frequencies
4.8. Determination of Serum Protein Levels for Vitamin D–Binding and Vitamin D Receptor
4.9. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (1,25(OH)2D) | 1,25-dihydroxy vitamin D |
| (24,25(OH)2D) | 24,25-dihydroxy vitamin D |
| 25(OH)D | 25-hydroxy-vitamin D |
| CI | confidence interval |
| CREC | Clinical Research Ethics Committee |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| eNOS | endothelial NOS |
| FFQ | Food Frequency Questionnaire |
| GDF-8 | myostatin |
| HIIT | high-intensity interval training |
| HWE | Hardy–Weinberg Equilibrium |
| IGF-1 | insulin-like growth factor-1 |
| MAF | macrophage activating factor |
| MAFr | mayor allele frequency |
| mRNA | messenger RNA |
| NO | Nitric oxide |
| NOS | endothelial NO synthase |
| RED-S | Relative Energy Deficiency in Sport |
| RM | repetition maximum |
| SC | satellite cell |
| SD | standard deviations |
| SNPs | single-nucleotide polymorphisms |
| STROBE | Reporting of Observational Studies in Epidemiology |
| VDBP | vitamin D-binding protein |
| VDR | vitamin D-receptor |
| VDRE | vitamin D responsive elements |
| WADA | World Anti-Doping Agency |
| WODs | Workouts of the Day |
Appendix A. Statistical Details
Appendix A.1. Assumptions and Data Validation
- Tests for normality (e.g., Shapiro–Wilk) were conducted for continuous variables to determine the suitability of parametric approaches.
- Multicollinearity between predictor variables was assessed using variance inflation factors (VIF), ensuring independent contributions to the logistic regression model.
- Outliers were detected and evaluated using Cook’s distance to minimise undue influence on regression coefficients.
Appendix A.2. Hardy–Weinberg Equilibrium (HWE) Testing
- The HWE principle was tested using chi-square goodness-of-fit tests, ensuring that allele frequencies matched expected distributions under random mating conditions.
- SNPs deviating from HWE (p < 0.05) were examined for potential genotyping errors or population structure effects.
Appendix A.3. Regression Model Specifications
- The logistic regression models were fitted using both crude estimates and adjusted models controlling for BMI, VO2max, and age.
- Sensitivity analyses were performed by excluding participants with extreme 25(OH)D values (>100 ng/mL) to assess robustness of findings.
- Interaction terms between SNPs and VO2max were explored but did not yield statistically significant results (p > 0.05).
Appendix A.4. Statistical Software and Code Availability
- All statistical analyses were performed using STATA version 15.
- Custom scripts for HWE testing and correlation analyses can be provided upon request for reproducibility purposes.
References
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The effects of vitamin D3 supplementation on serum total 25[OH]D concentration and physical performance: A randomised dose-response study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and exercise performance in professional soccer players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef] [PubMed]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef]
- Todd, J.J.; Pourshahidi, L.K.; McSorley, E.M.; Madigan, S.M.; Magee, P.J. Vitamin D: Recent advances and implications for athletes. Sports Med. 2015, 45, 213–229. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef]
- de La Puente Yagüe, M.; Yurrita, L.C.; Cabañas, M.J.C.; Cenzual, M.A.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports health benefits of vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef]
- Casado, E.; Quesada, J.M.; Naves, M.; Peris, P.; Jódar, E.; Giner, M.; Neyro, J.L.; Del Pino, J.; Sosa, M.; De Paz, H.D.; et al. Recomendaciones de la SEIOMM en la prevención y tratamiento del déficit de vitamina D. Rev. Osteoporos. Metab. Miner. 2021, 13, 84–97. [Google Scholar] [CrossRef]
- Ip, T.S.T.; Fu, S.C.; Ong, M.T.Y.; Yung, P.S.H. Vitamin D deficiency in athletes: Laboratory, clinical and field integration. Asia-Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2022, 29, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Glassman, G. What Is CrossFit? Available online: http://journal.crossfit.com/2004/03/what-is-crossfit-mar-04-cfj.tpl (accessed on 10 January 2024).
- Glassman, G. Understanding CrossFit. Available online: http://journal.crossfit.com/2007/04/understanding-crossfit-by-greg.tpl (accessed on 10 January 2024).
- Claudino, J.G.; Gabbett, T.J.; Bourgeois, F.; de Sá Souza, H.; Miranda, R.C.; Mezêncio, B.; Soncin, R.; Cardoso Filho, C.A.; Bottaro, M.; Hernandez, A.J.; et al. CrossFit Overview: Systematic Review and Meta-analysis. Sports Med.-Open 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, J.; Sabido-Solana, R.; Moya, D.; Marin, S.J.M.; Ramón, M.M.; Fernandez-Fernandez, J. Acute physiological responses during crossfit® workouts. Eur. J. Hum. Mov. 2015, 35, 114–124. [Google Scholar]
- Fernández-Lázaro, D.; Seco-Calvo, J.; Pascual-Fernández, J.; Domínguez-Ortega, C.; Del Valle Soto, M.; Mielgo-Ayuso, J. 6-Week Supplementation with Tribulus terrestris L. to Trained Male CrossFit® Athletes on Muscle, Inflammation, and Antioxidant Biomarkers: A Randomized, Single-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16158. [Google Scholar] [CrossRef]
- Gogojewicz, A.; Śliwicka, E.; Durkalec-Michalski, K. Assessment of Dietary Intake and Nutritional Status in CrossFit-Trained Individuals: A Descriptive Study. Int. J. Environ. Res. Public Health 2020, 17, 4772. [Google Scholar] [CrossRef]
- De Souza, R.A.S.; Da Silva, A.G.; De Souza, M.F.; Ferreira Souza, L.K.; Roschel, H.; Da Silva, S.F.; Saunders, B. A Systematic Review of CrossFit® Workouts and Dietary and Supplementation Interventions to Guide Nutritional Strategies and Future Research in CrossFit®. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 187–205. [Google Scholar] [CrossRef]
- Cabre, H.E.; Moore, S.R.; Smith-Ryan, A.E.; Hackney, A.C. Relative Energy Deficiency in Sport (RED-S): Scientific, Clinical, and Practical Implications for the Female Athlete. Dtsch. Z. Sportmed. 2022, 73, 225–234. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- dos Santos Quaresma, M.V.L.; Guazzelli Marques, C.; Nakamoto, F.P. Effects of diet interventions, dietary supplements, and performance-enhancing substances on the performance of CrossFit-trained individuals: A systematic review of clinical studies. Nutrition 2021, 82, 110994. [Google Scholar] [CrossRef]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The Effects of Whey vs. Pea Protein on Physical Adaptations Following 8-Weeks of High-Intensity Functional Training (HIFT): A Pilot Study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Rountree, J.A.; Krings, B.M.; Peterson, T.J.; Thigpen, A.G.; McAllister, M.J.; Holmes, M.E.; Smith, J.W. Efficacy of Carbohydrate Ingestion on CrossFit Exercise Performance. Sports 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.A.; Ramirez, M.; Heinrich, K.M. Acute Caffeine Supplementation Does Not Improve Performance in Trained CrossFit® Athletes. Sports 2020, 8, 54. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Zawieja, E.E.; Podgórski, T.; Loniewski, I.; Zawieja, B.E.; Warzybok, M.; Jeszka, J. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: A double-blind, randomized cross-over trial. PLoS ONE 2018, 13, e0197480. [Google Scholar] [CrossRef]
- Kramer, S.J.; Baur, D.A.; Spicer, M.T.; Vukovich, M.D.; Ormsbee, M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sports Nutr. 2016, 13, 39. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Jeszka, J. The Effect of β-Hydroxy-β-Methylbutyrate on Aerobic Capacity and Body Composition in Trained Athletes. J. Strength Cond. Res. 2016, 30, 2617–2626. [Google Scholar] [CrossRef]
- Sadowska-Krȩpa, E.; Domaszewski, P.; Pokora, I.; Zebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 13. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Soto, M.D.V.; Adams, D.P.; González-Bernal, J.J.; Seco-Calvo, J. The Effects of 6 Weeks of Tribulus terrestris L. Supplementation on Body Composition, Hormonal Response, Perceived Exertion, and CrossFit® Performance: A Randomized, Single-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 3969. [Google Scholar] [CrossRef]
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J. Sci. Med. Sport 2014, 17, 139–143. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández, J.L.G.; Lumbreras, E.; Mielgo-Ayuso, J.; Seco-Calvo, J. 25-Hydroxyvitamin D Serum Levels Linked to Single Nucleotide Polymorphisms (SNPs) (rs2228570, rs2282679, rs10741657) in Skeletal Muscle Aging in Institutionalized Elderly Men Not Supplemented with Vitamin D. Int. J. Mol. Sci. 2022, 23, 11846. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; Horne, J.; Vanderhout, S.M.; El-Sohemy, A. Sport Nutrigenomics: Personalized Nutrition for Athletic Performance. Front. Nutr. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Adamski, M.M.; Twohig, C.; Murgia, C. Opportunities for training for nutritional professionals in nutritional genomics: What is out there? Nutr. Diet. 2018, 75, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Levine, M.A.; Bell, N.H.; Mangelsdorf, D.J.; Russell, D.W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA 2004, 101, 7711–7715. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; Van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Sist, M.; Zou, L.; Galloway, S.D.R.; Rodriguez-Sanchez, N. Effects of vitamin D supplementation on maximal strength and power in athletes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1163313. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Yongjoo Park, C.; Jo, G.; Yoen Kim, O.; Shin, M.J. Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: Results from the Korea National Health and Nutrition Examination Survey 2011–2012. Endocr. J. 2018, 65, 881–891. [Google Scholar] [CrossRef]
- Slater, N.A.; Rager, M.L.; Havrda, D.E.; Harralson, A.F. Genetic Variation in CYP2R1 and GC Genes Associated With Vitamin D Deficiency Status. J. Pharm. Pract. 2017, 30, 31–36. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Bello, H.J.; Caballero-García, A.; Pérez-Valdecantos, D.; Roche, E.; Noriega, D.C.; Córdova-Martínez, A. Effects of Vitamin D in Post-Exercise Muscle Recovery. A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4013. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, A.; Pérez-Valdecantos, D.; Guallar, P.; Caballero-Castillo, A.; Roche, E.; Noriega, D.C.; Córdova, A. Effect of Vitamin D Supplementation on Muscle Status in Old Patients Recovering from COVID-19 Infection. Medicina 2021, 57, 1079. [Google Scholar] [CrossRef] [PubMed]
- Kamyshna, I.I.; Pavlovych, L.B.; Malyk, I.V.; Kamyshnyi, A.M. 25-OH Vitamin D blood serum linkage with VDR gene polymorphism (rs2228570) in thyroid pathology patients in the West-Ukrainian population. J. Med. Life 2021, 14, 549–556. [Google Scholar] [CrossRef]
- Redenšek, S.; Kristanc, T.; Blagus, T.; Trošt, M.; Dolžan, V. Genetic Variability of the Vitamin D Receptor Affects Susceptibility to Parkinson’s Disease and Dopaminergic Treatment Adverse Events. Front. Aging Neurosci. 2022, 14, 853277. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.J.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- Lurie, G.; Wilkens, L.R.; Thompson, P.J.; Carney, M.E.; Palmieri, R.T.; Pharoah, P.D.P.; Song, H.; Hogdall, E.; Kjaer, S.K.; Dicioccio, R.A.; et al. Vitamin D receptor rs2228570 polymorphism and invasive ovarian carcinoma risk: Pooled analysis in five studies within the Ovarian Cancer Association Consortium. Int. J. Cancer 2011, 128, 936–943. [Google Scholar] [CrossRef]
- Caballero-García, A.; Córdova-Martínez, A.; Vicente-Salar, N.; Roche, E.; Pérez-Valdecantos, D. Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise. Nutrients 2021, 13, 2336. [Google Scholar] [CrossRef]
- Von Hurst, P.R.; Beck, K.L. Vitamin D and skeletal muscle function in athletes. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 539–545. [Google Scholar] [CrossRef]
- Gussago, C.; Arosio, B.; Guerini, F.R.; Ferri, E.; Costa, A.S.; Casati, M.; Bollini, E.M.; Ronchetti, F.; Colombo, E.; Bernardelli, G.; et al. Impact of vitamin D receptor polymorphisms in centenarians. Endocrine 2016, 53, 558–564. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Skin colour and vitamin D: An update. Exp. Dermatol. 2020, 29, 864–875. [Google Scholar] [CrossRef]
- Malsagova, K.A.; Kopylov, A.T.; Sinitsyna, A.; Stepanov, A.A.; Izotov, A.A.; Butkova, T.V.; Chingin, K.; Klyuchnikov, M.S.; Kaysheva, A.L. Sports Nutrition: Diets, Selection Factors, Recommendations. Nutrients 2021, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- CrossFit Games Competition Rulebook CrossFit 2024. 2024. Available online: https://games.crossfit.com/rules (accessed on 23 May 2024).
- Leong, A.; Rehman, W.; Dastani, Z.; Greenwood, C.; Timpson, N.; Langsetmo, L.; Berger, C.; Fu, L.; Wong, B.Y.L.; Malik, S.; et al. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: A Mendelian randomization study. PLoS Med. 2014, 11, e1001751. [Google Scholar] [CrossRef]
- Gorey, S.; Canavan, M.; Robinson, S.; O’Keeffe, S.T.; Mulkerrin, E. A review of vitamin D insufficiency and its management: A lack of evidence and consensus persists. QJM 2019, 112, 165–167. [Google Scholar] [CrossRef]
- Kotur, N.; Skakic, A.; Klaassen, K.; Gasic, V.; Zukic, B.; Skodric-Trifunovic, V.; Stjepanovic, M.; Zivkovic, Z.; Ostojic, O.; Stevanovic, G.; et al. Association of Vitamin D, Zinc and Selenium Related Genetic Variants with COVID-19 Disease Severity. Front. Nutr. 2021, 8, 689419. [Google Scholar] [CrossRef]
- Redenšek, S.; Bizjan, B.J.; Trošt, M.; Dolžan, V. Clinical-pharmacogenetic predictive models for time to occurrence of levodopa related motor complications in Parkinson’s disease. Front. Genet. 2019, 10, 461. [Google Scholar] [CrossRef]
- Fernández-Araque, A.; Giaquinta-Aranda, A.; Moreno-Sainz, C.; Martínez-Martínez, M.C.; Velasco-González, V.; Sainz-Gil, M.; Martín-Arias, L.H.; Carretero-Molinero, S.; García-Hidalgo, M.; Verde, Z. Haplotypes in the GC, CYP2R1 and CYP24A1 Genes and Biomarkers of Bone Mineral Metabolism in Older Adults. Nutrients 2022, 14, 259. [Google Scholar] [CrossRef]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Ksiażek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes-A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef]
- Bouillon, R.; Gielen, E.; Vanderschueren, D. Vitamin D receptor and vitamin D action in muscle. Endocrinology 2014, 155, 3210–3213. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W. Expression of the vitamin D receptor in skeletal muscle: Are we there yet? Endocrinology 2014, 155, 3214–3218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kew, R.R. The Vitamin D Binding Protein and Inflammatory Injury: A Mediator or Sentinel of Tissue Damage? Front. Endocrinol. 2019, 10, 470. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef]
- Hollis, B.W.; Horst, R.L. The assessment of circulating 25(OH)D and 1,25(OH)2D: Where we are and where we are going. J. Steroid Biochem. Mol. Biol. 2007, 103, 473–476. [Google Scholar] [CrossRef]
- Nagasawa, H.; Uto, Y.; Sasaki, H.; Okamura, N.; Murakami, A.; Kubo, S.; Kirk, K.L.; Hori, H. Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activity. Anticancer Res. 2005, 25, 3689–3695. [Google Scholar]
- Dueland, S.; Nenseter, M.S.; Drevon, C.A. Uptake and degradation of filamentous actin and vitamin D-binding protein in the rat. Biochem. J. 1991, 274 Pt 1, 237–241. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, O.; Lammert, F.; Gressner, A.M. Gc-globulin: Roles in response to injury. Clin. Chem. 2006, 52, 1247–1253. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, E. Vitamin D supplementation in athletes. Nestle Nutr. Inst. Workshop Ser. 2013, 75, 109–121. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Tuma, C.; Schick, A.; Pommerening, N.; Braun, H.; Thevis, M. Effects of an Individualized vs. Standardized Vitamin D Supplementation on the 25(OH)D Level in Athletes. Nutrients 2023, 15, 4747. [Google Scholar] [CrossRef]
- Pojednic, R.M.; Ceglia, L.; Olsson, K.; Gustafsson, T.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif. Tissue Int. 2015, 96, 256–263. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 735–756. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Centofanti, F.; Celi, M.; Gasbarra, E.; Novelli, G.; Botta, A.; Tarantino, U. Vitamin D Receptor in Muscle Atrophy of Elderly Patients: A Key Element of Osteoporosis-Sarcopenia Connection. Aging Dis. 2018, 9, 952–964. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 2014, 49, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Lee, S.M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef]
- Nunes, I.F.O.C.; Cavalcante, A.A.C.M.; Alencar, M.V.O.B.; Carvalho, M.D.F.; Sarmento, J.L.R.; Teixeira, N.S.C.C.A.; Paiva, A.A.; Carvalho, L.R.; Nascimento, L.F.M.; Cruz, M.S.P.; et al. Meta-Analysis of the Association Between the rs228570 Vitamin D Receptor Gene Polymorphism and Arterial Hypertension Risk. Adv. Nutr. 2020, 11, 1403. [Google Scholar] [CrossRef]
- Balta, B.; Gumus, H.; Bayramov, R.; Korkmaz Bayramov, K.; Erdogan, M.; Oztop, D.B.; Dogan, M.E.; Taheri, S.; Dundar, M. Increased vitamin D receptor gene expression and rs11568820 and rs4516035 promoter polymorphisms in autistic disorder. Mol. Biol. Rep. 2018, 45, 541–546. [Google Scholar] [CrossRef]
- Vidigal, V.M.; Silva, T.D.; de Oliveira, J.; Pimenta, C.A.M.; Felipe, A.V.; Forones, N.M. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int. J. Biol. Markers 2017, 32, e224–e230. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approachin pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 27–53. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 12 January 2024).
- Verney, J.; Metz, L.; Chaplais, E.; Cardenoux, C.; Pereira, B.; Thivel, D. Bioelectrical impedance is an accurate method to assess body composition in obese but not severely obese adolescents. Nutr. Res. 2016, 36, 663–670. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Practitioners: Evaluation of the Effectiveness of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectorized Training of the Inspiratory Muscles. Ph.D. Thesis, Universidad de León, León, Spain, 2020. [Google Scholar]
- Gómez-Landero, L.A.; Frías-Menacho, J.M. Analysis of Morphofunctional Variables Associated with Performance in Crossfit® Competitors. J. Hum. Kinet. 2020, 73, 83–91. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef]
- Cashman, K.D.; van den Heuvel, E.G.H.M.; Schoemaker, R.J.W.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef]
- Cannell, J.J.; Hollis, B.W.; Zasloff, M.; Heaney, R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2008, 9, 107–118. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Al-Bluwi, G.S.M.; Yasin, J. Frequency of Vitamin D Receptor Gene Polymorphisms in a Population with a very High Prevalence of Vitamin D Deficiency, Obesity, Diabetes and Hypertension. Biomedicines 2023, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Maughan, P.J. SNP genotyping using KASPar assays. Methods Mol. Biol. 2015, 1245, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308. [Google Scholar] [CrossRef]
- Ryckman, K.; Williams, S.M. Calculation and use of the Hardy-Weinberg model in association studies. Curr. Protoc. Hum. Genet. 2008, 57, 1–8. [Google Scholar] [CrossRef]
- Mirković, K.; Doorenbos, C.R.C.; Dam, W.A.; Lambers Heerspink, H.J.; Slagman, M.C.J.; Nauta, F.L.; Kramer, A.B.; Gansevoort, R.T.; van den Born, J.; Navis, G.; et al. Urinary vitamin D binding protein: A potential novel marker of renal interstitial inflammation and fibrosis. PLoS ONE 2013, 8, e55887. [Google Scholar] [CrossRef]
- Al-Ghafari, A.B.; Balamash, K.S.; Al Doghaither, H.A. Serum vitamin D receptor (VDR) levels as a potential diagnostic marker for colorectal cancer. Saudi J. Biol. Sci. 2020, 27, 827–832. [Google Scholar] [CrossRef]

| Characteristics | CrossFit® Athletes | |
|---|---|---|
| Sample size (n) | 50 | |
| Age (years) | 35.7 ± 11.3 | |
| Gender n (%) | Male | 50 (100) |
| Female | 0 (0) | |
| Ethnicity n (%) | Caucasian | 50 (100) |
| Other | 0 (0) | |
| Body mass (Kg) | 77.6 ± 10.9 | |
| Fat Mass (Kg) | 9.7 ± 2.9 | |
| Fat Mass (%) | 12.5 ± 2.3 | |
| Fat Free Mass (Kg) | 67.9 ± 4.1 | |
| Fat Free Mass (%) | 65.2 ± 2.6 | |
| Height (cm) | 171.5 ± 5.6 | |
| VO2max (mL/kg/min) | 43.5 ± 4.4 | |
| Crossfit® experience (months) | 35.3 ± 11.7 | |
| Fran WODs * (seconds) | 231 ± 15 | |
| CrossFit® participants (n) | 50 |
| Energy (kcal/kg) | 40.3 ± 4.8 |
| Proteins (g) | 141.3 ± 37.9 |
| Fats (g) | 134.3 ± 43.2 |
| Carbohydrates (g) | 341.2 ± 97.6 |
| Ca (mg) | 1026.3 ± 224.1 |
| Mg (mg) | 544.3 ± 97.2 |
| P (mg) | 2120.6 ± 67.1 |
| Fe (mg) | 23.1 ± 3.6 |
| Zn (mg) | 13.4 ± 1.1 |
| Vitamin A (µg RE) | 1862.3 ± 1177.1 |
| Vitamin E (mg) | 16.0 ± 1.8 |
| Vitamin B1 (mg) | 2.9 ± 0.4 |
| Vitamin B2 (mg) | 2.6 ± 0.3 |
| Vitamin B3 (mg NE) | 40.9 ± 6.1 |
| Vitamin B6 (mg) | 4.3 ± 0.5 |
| Vitamin B9 (mg) | 637.2 ± 172.1 |
| Vitamin B12 (µg) | 9.6 ± 2.7 |
| Vitamin C (µg) | 351.1 ± 140.2 |
| CrossFit Competitive Categories (Years) | Sample (n) | 25-(OH)D (ng/mL) | Deficiency n (%) <20 ng/mL | Insufficiency n (%) <30–32 ng/mL | Sufficiency n (%) >30–32 ng/mL | Optimum n (%) 40–100 ng/mL | Toxic n (%) >150 ng/mL |
|---|---|---|---|---|---|---|---|
| Absolute/Elite 18–35 | 19 | 36.2 ± 4.3 | - | 2 (10.5) | 14 (73.7) | 3 (15.8) | - |
| Master >35 | 31 | 33.1 ± 6.8 | - | 6 (19.3) | 20 (64.5) | 5 (16.2) | - |
| Elite + Master | 50 | 34.7 ± 5.2 | - | 8 (16.0) | 34 (68.0) | 8 (16.0) | - |
| Gen | SNPs | Alleles | 25(OH)D (ng/mL) | p-Value | VDBP (µg/mL) | p-Value | VDR (ng/mL) | p-Value |
|---|---|---|---|---|---|---|---|---|
| CYP2R1 | rs10741657 | AA | 38.2 ± 11.2 | 0.076 | 323.6 ± 21.2 | 0.96 | 3.8 ± 0.5 | 0.67 |
| GA | 26.9 ± 7.5 | 318.1 ± 19.1 | 3.4 ± 0.6 | |||||
| GG | 21.5 ± 4.7 | 316. ± 23.5 | 3.2 ± 0.8 | |||||
| GC | rs2282679 | TT | 42.6 ± 3.2 | <0.05 | 344.1 ± 14.3 | 0.05 | 3.9 ± 0.9 | 0.19 |
| GT | 25.4 ± 5.7 * | 323.6 ± 16.2 | 3.3 ± 1.1 | |||||
| GG | 21.6 ± 5.1 * | 301.6 ± 10.2 # | 3.1 ± 0.7 | |||||
| VDR | rs2228570 | AA | 35.9 ± 8.3 | <0.05 | 319.6 ± 17.2 | 1.1 | 4.2 ± 0.5 | 0.05 |
| GA | 24.4 ± 5.6 | 314.6 ± 19.3 | 3.6 ± 0.8 | |||||
| GG | 18.9 ± 4.9 $ | 311.7 ± 26.7 | 3.0 ± 0.3 & |
| Gen (SNPs) | n (%) a | Genotypic Freq b | Allelic Freq c | Alleles | Allele Freq d | HWE e | |
|---|---|---|---|---|---|---|---|
| CYP2R1 (rs10741657) | A (p) 0.59 G (q) 0.41 | A > G | Ref A: 0.379 | Alt G: 0.614 | Yes 0.023 | ||
| AA | 17 (34) | 0.34 | |||||
| GA | 25 (50) | 0.50 | |||||
| GG | 8 (16) | 0.16 | |||||
| A | 59 (59) | ||||||
| G | 41 (41) | ||||||
| GC (rs2282679) | T (p) 0.60 G (q) 0.40 | T > G | Ref T: 0.716 | Alt G: 0.283 | Yes 0.34 | ||
| TT | 19 (38) | 0.38 | |||||
| GT | 22 (44) | 0.44 | |||||
| GG | 9 (18) | 0.18 | |||||
| T | 60 (60) | ||||||
| G | 40 (40) | ||||||
| VDR (rs2228570) | A (p) 0.60 G (q) 0.40 | A > G | Ref A: 0.387 | Alt G: 0.612 | Yes 0.34 | ||
| AA | 21 (42) | 0.42 | |||||
| GA | 18 (36) | 0.36 | |||||
| GG | 11 (22) | 0.22 | |||||
| A | 60 (60) | ||||||
| G | 40 (40) | ||||||
| Gen (SNPs) | (n = 50) | |
|---|---|---|
| r | p | |
| CYP2R1 (rs10741657) | ||
| AA | 0.17 * | 0.034 |
| GA | 0.089 | 0.424 |
| GG | −0.34 * | 0.016 |
| GC (rs2282679) | ||
| TT | 0.29 * | 0.041 |
| GT | 0.07 | 0.526 |
| GG | −0.33 * | 0.012 |
| VDR (rs2228570) | ||
| AA | 0.15 * | 0.030 |
| GA | 0.06 | 0.172 |
| GG | −0.43 * | <0.001 |
| Variable | Full Cohort (n = 50) | |
|---|---|---|
| OR (95% CI) Crude | OR (95% CI) Multivariate 1 | |
| Body mass index (BMI), (Kg/m2) | 1.00 (ref) | -- |
| VO2 max (mL/Kg/min) | 1.62 (0.81–3.27) | 1.77 (0.54–3.65) |
| Age (years) | 0.91 (0.71–1.18) | 0.92 (0.62–1.46) |
| CYP2R1 (rs10741657) | 1.00 (ref) | -- |
| AA | 1.44 (0.74–2.87) | 2.01 (0.77–5.48) * |
| GA | 0.93 (0.782–1.05) | 1.02 (0.83–1.27) |
| GG | 0.83 (0.74–0.93) | 0.76 (0.65–0.89) |
| GC (rs2282679) | 1.00 (ref) | -- |
| TT | 3.69 (2.28–5.99) | 3.67 (2.11–6.41) * |
| GT | 0.83 (0.68–1.02) | 0.76 (0.49–1.21) |
| GG | 0.67 (0.53–0.85) | 0.66 (0.51–0.89) * |
| VDR (rs2228570) | 1.00 (ref) | -- |
| AA | 2.93 (1.58–5.47) | 2.88 (1.43–5.92) * |
| GA | 1.01 (0.42–2.64) | 1.24 (0.29–6.11) |
| GG | 0.53 (0.23–1.42) | 0.31 (0.12–1.27) * |
| Gen | SNPs | Allele | n (%) | Levels of CrossFit® Total (n) 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Beginner (Level 0) <270 Kg | Intermediate (Level 1) 271–360 Kg | Advanced (Level 2) 361–450 Kg | Elite (Level 3) ≥451 Kg | Competitors 2 (Levels 2 + 3) ≥360 Kg | ||||
| CYP2R1 | rs10741657 | AA | 17 (34) | 0 | 6 | 8 | 3 | 11 |
| GA | 25 (50) | 2 | 18 | 4 | 1 | 5 | ||
| GG | 8 (16) | 4 | 3 | 1 | 0 | 1 | ||
| AA/GA/GG | 50 (100) | 6 | 27 | 13 | 4 | 17 | ||
| GC | rs2282679 | TT | 19 (38) | 1 | 8 | 7 | 3 | 10 |
| GT | 22 (44) | 3 | 15 | 4 | 0 | 4 | ||
| GG | 9 (18) | 2 | 4 | 2 | 1 | 3 | ||
| TT/GT/GG | 50 (100) | 6 | 27 | 13 | 4 | 17 | ||
| VDR | rs2228570 | AA | 21 (42) | 0 | 11 | 6 | 4 | 10 |
| GA | 18 (36) | 2 | 10 | 6 | 0 | 6 | ||
| GG | 11 (22) | 4 | 6 | 1 | 0 | 1 | ||
| AA/GA/GG | 50 (100) | 6 | 27 | 13 | 4 | 17 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco-Calvo, J.; Gutiérrez-Abejón, E.; Roche, E.; Garrosa, M. Single-Nucleotide Polymorphisms (SNPs) in Vitamin D Physiology Genes May Modulate Serum 25(OH)D Levels in Well-Trained CrossFit® Athletes, Which May Be Associated with Performance Outcomes. Int. J. Mol. Sci. 2025, 26, 5602. https://doi.org/10.3390/ijms26125602
Fernández-Lázaro D, Mielgo-Ayuso J, Seco-Calvo J, Gutiérrez-Abejón E, Roche E, Garrosa M. Single-Nucleotide Polymorphisms (SNPs) in Vitamin D Physiology Genes May Modulate Serum 25(OH)D Levels in Well-Trained CrossFit® Athletes, Which May Be Associated with Performance Outcomes. International Journal of Molecular Sciences. 2025; 26(12):5602. https://doi.org/10.3390/ijms26125602
Chicago/Turabian StyleFernández-Lázaro, Diego, Juan Mielgo-Ayuso, Jesús Seco-Calvo, Eduardo Gutiérrez-Abejón, Enrique Roche, and Manuel Garrosa. 2025. "Single-Nucleotide Polymorphisms (SNPs) in Vitamin D Physiology Genes May Modulate Serum 25(OH)D Levels in Well-Trained CrossFit® Athletes, Which May Be Associated with Performance Outcomes" International Journal of Molecular Sciences 26, no. 12: 5602. https://doi.org/10.3390/ijms26125602
APA StyleFernández-Lázaro, D., Mielgo-Ayuso, J., Seco-Calvo, J., Gutiérrez-Abejón, E., Roche, E., & Garrosa, M. (2025). Single-Nucleotide Polymorphisms (SNPs) in Vitamin D Physiology Genes May Modulate Serum 25(OH)D Levels in Well-Trained CrossFit® Athletes, Which May Be Associated with Performance Outcomes. International Journal of Molecular Sciences, 26(12), 5602. https://doi.org/10.3390/ijms26125602












