Effects of Long-Term Blue Light Exposure on Body Fat Synthesis and Body Weight Gain in Mice and the Inhibitory Effect of Tranexamic Acid
Abstract
1. Introduction
2. Results
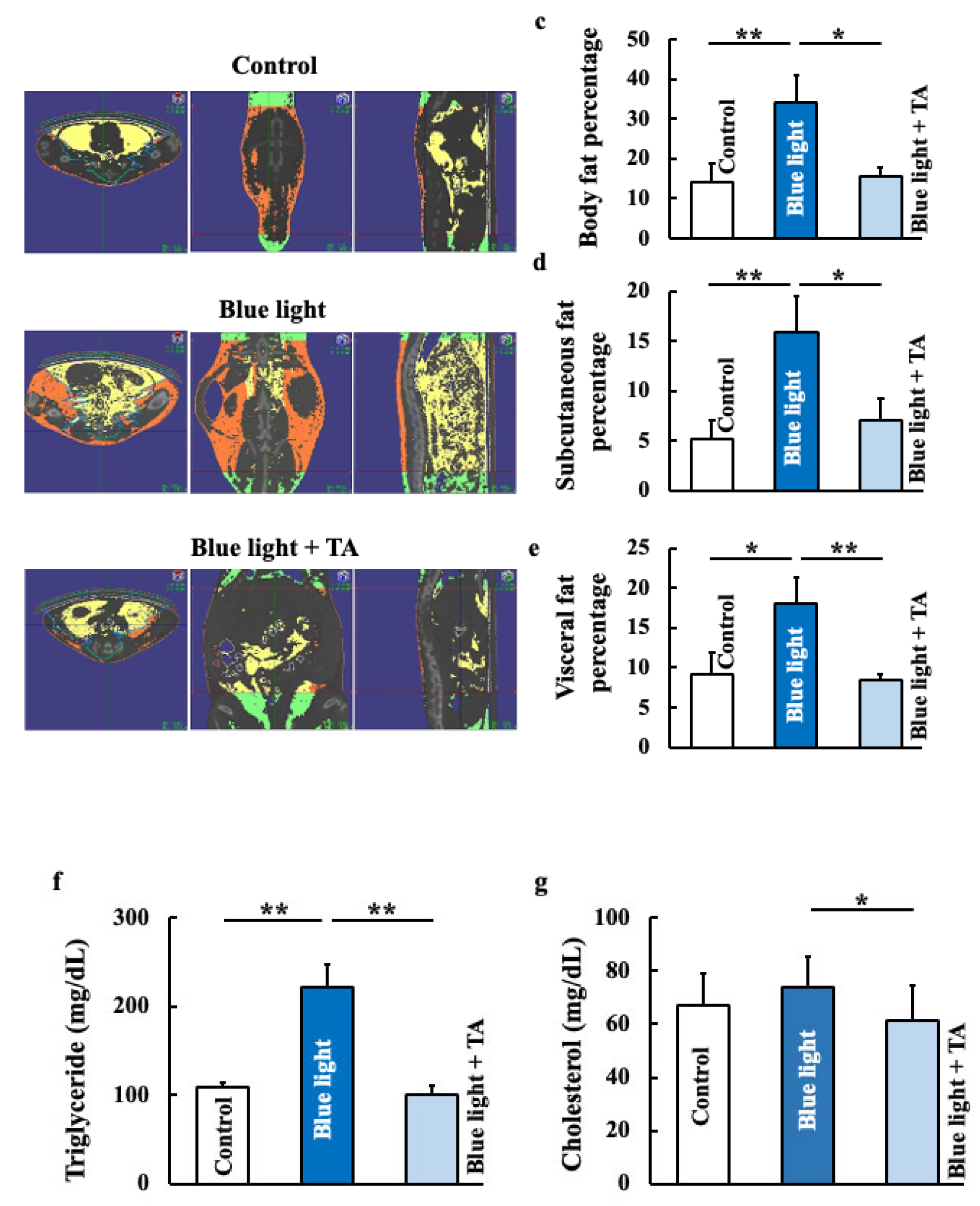
2.1. Effects of TA Treatment on Body Weight, Food Intake, Fat Accumulation, and Lipid Profiles in Blue Light-Exposed Mice
2.2. Effects of TA Treatment on p53, Clock Genes (Brain and Muscle Arnt-like 1 (Bmal1) and Clock), Sirt,1 and Sterol Regulatory Element-Binding Protein 1 (SREBP1) in Blue Light-Exposed Mice
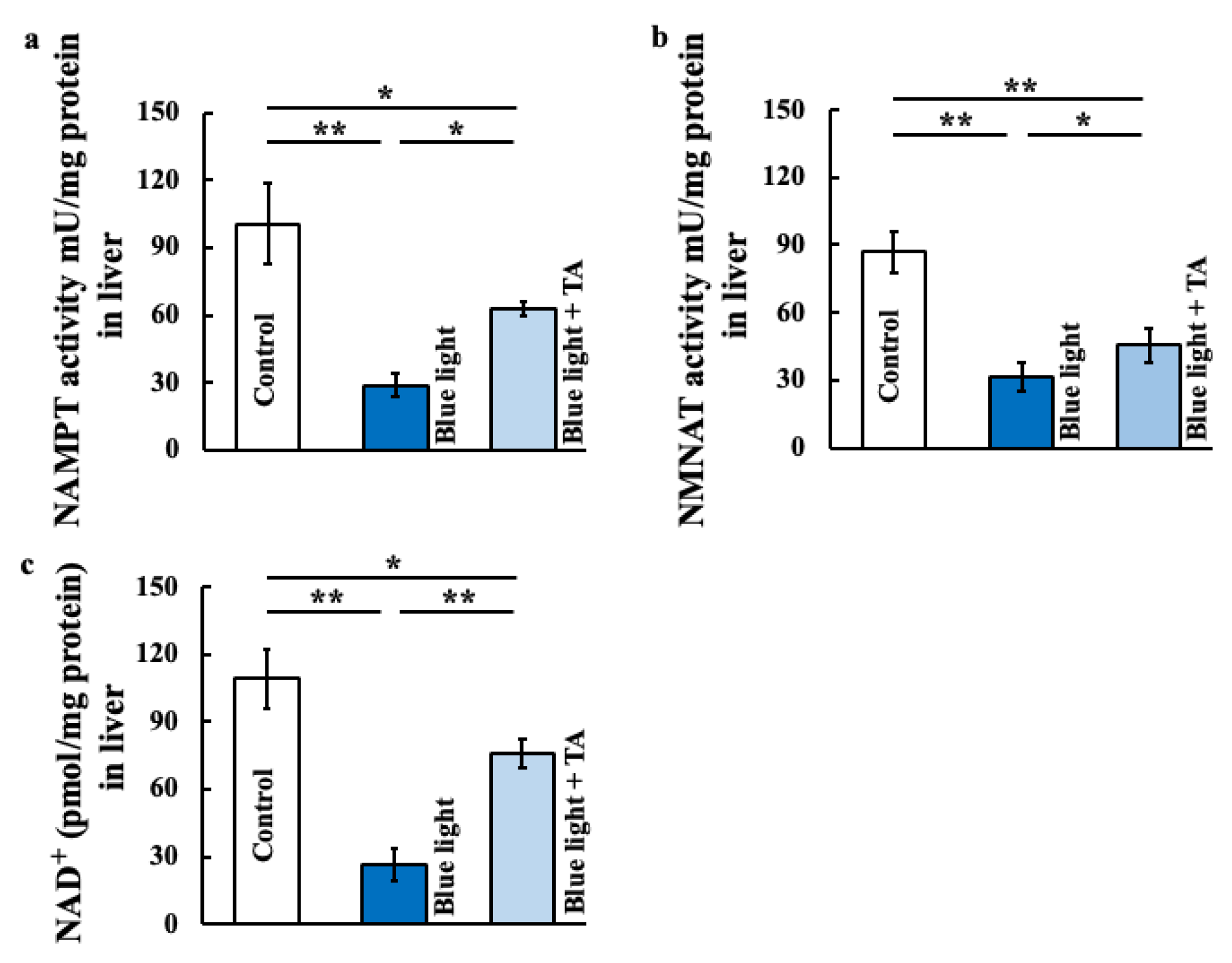
2.3. Effects of TA Treatment on Nicotinamide Mononucleotide Adnylyltransferase (NMNAT) Activity, Nicotimamide Phosphoribosyltransferase (NAMPT) Activity, and Nicotinamide Asenine Dinucleotide+ (NAD+) in Blue Light-Exposed Mice
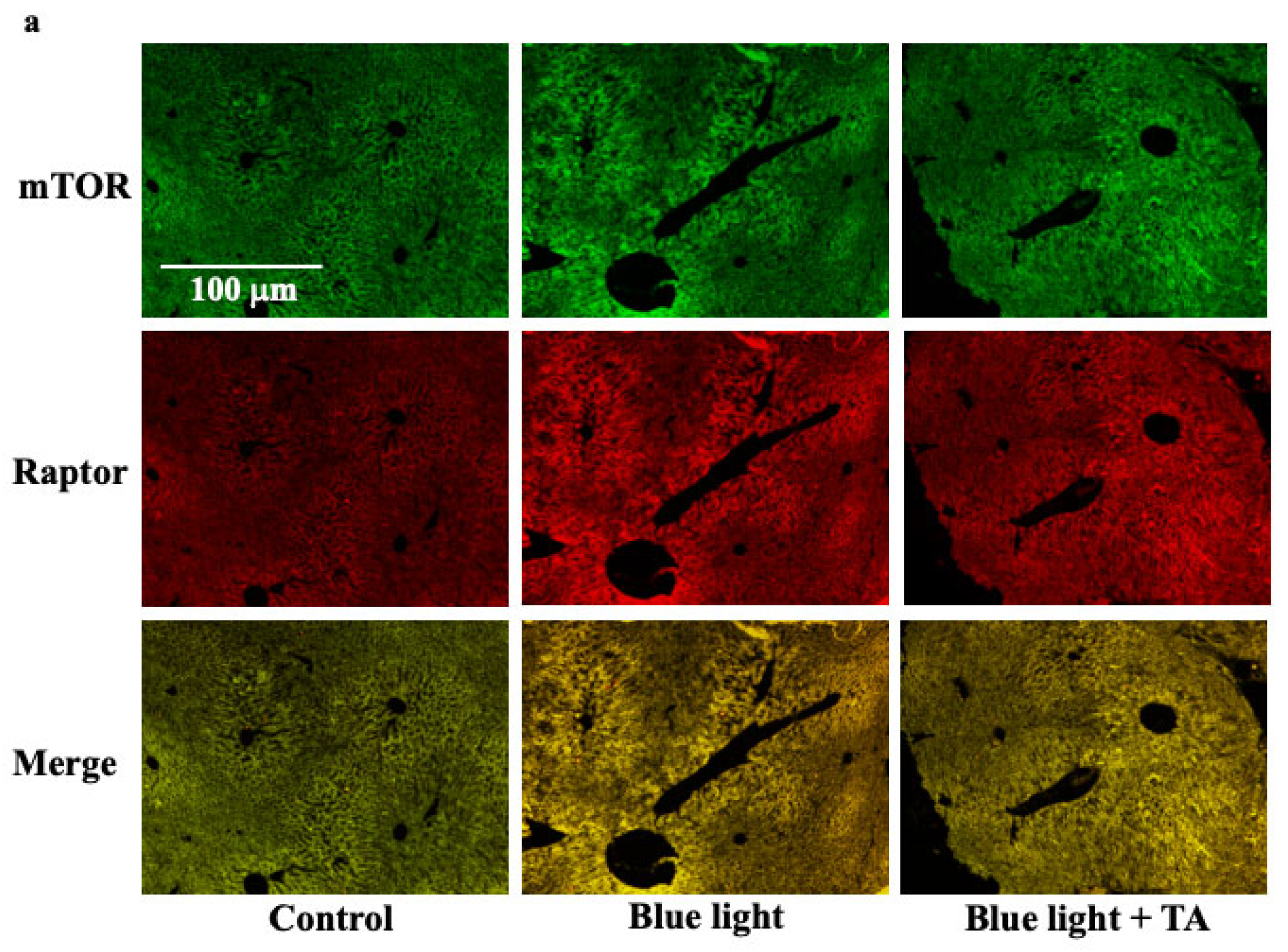
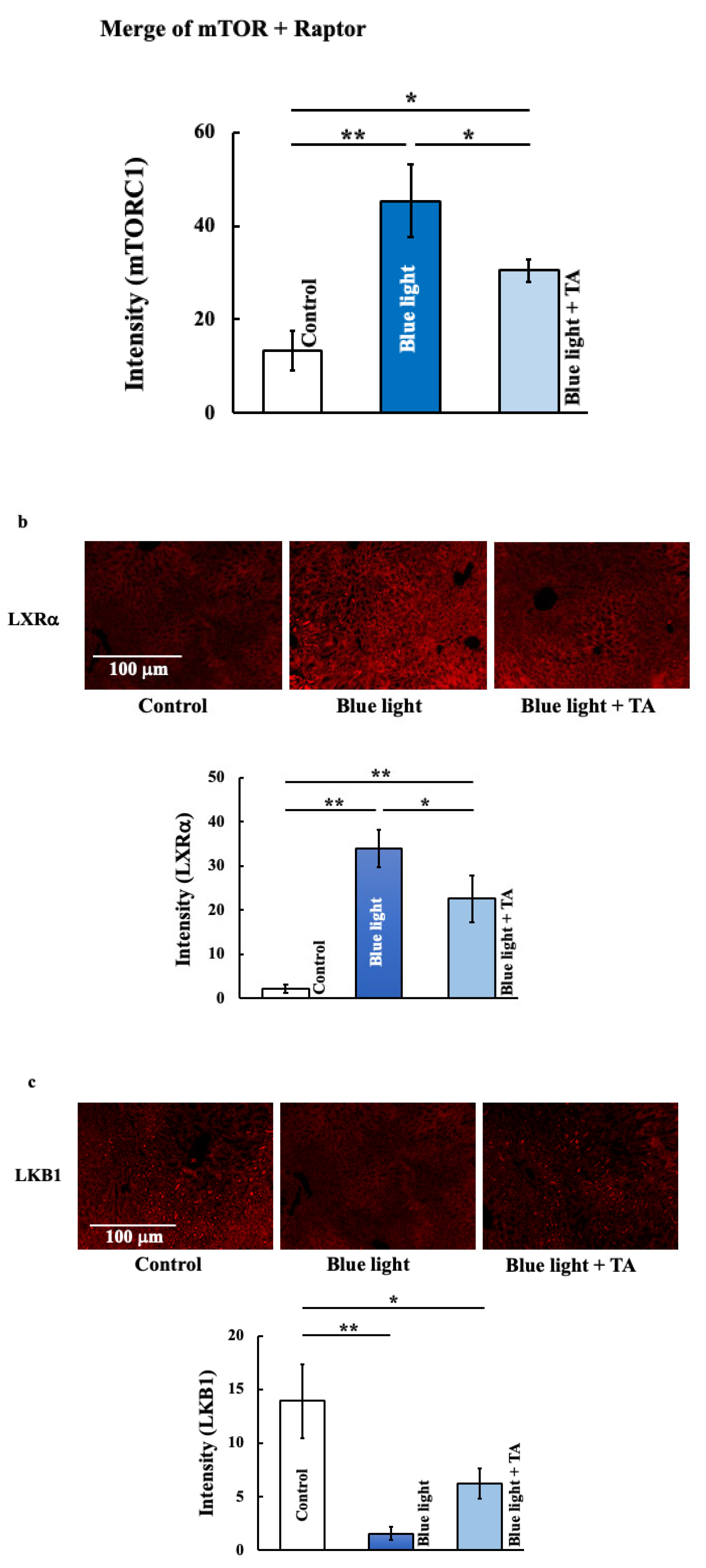
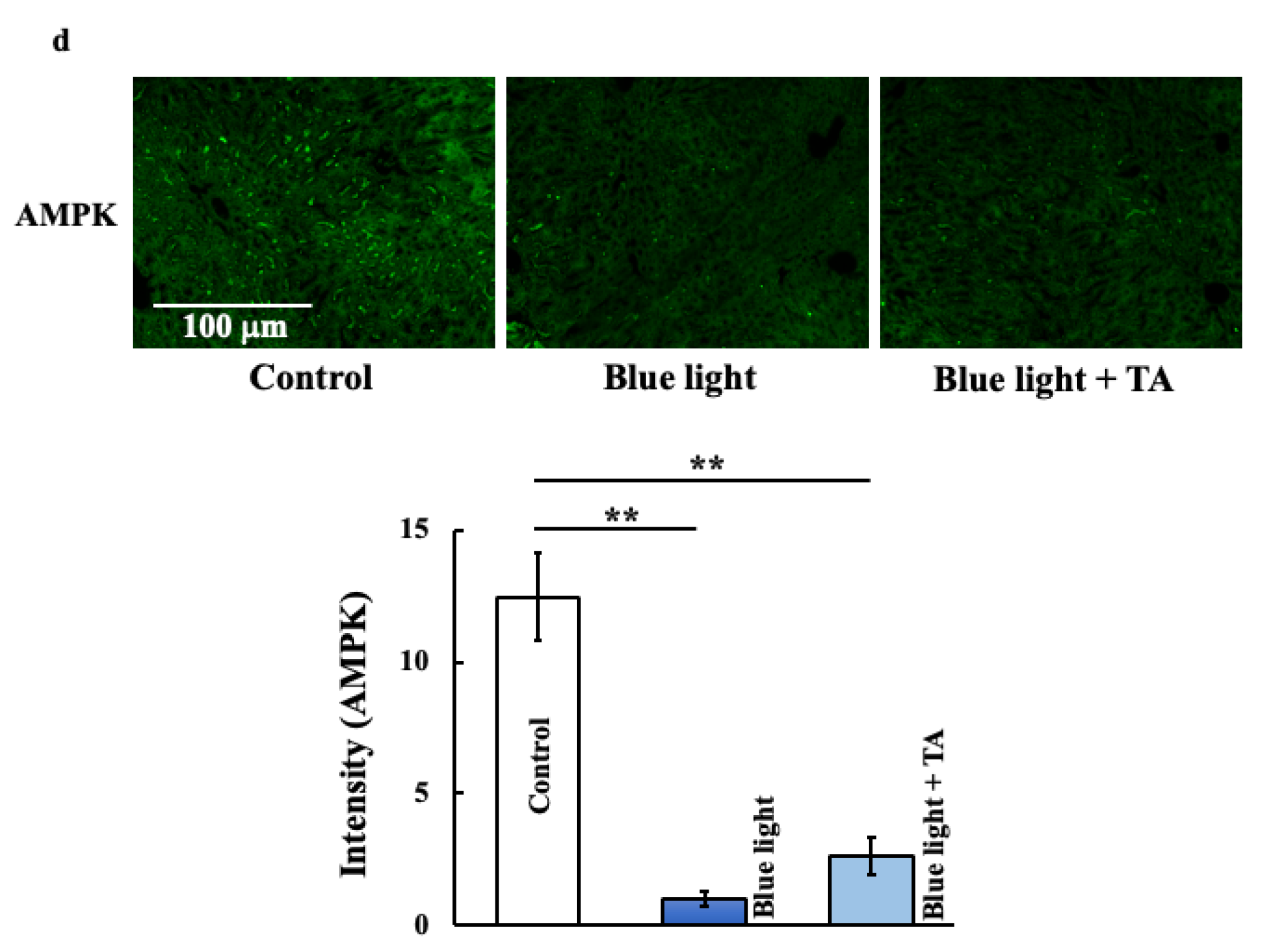
2.4. Effect of TA Treatment on Liver X Receptor α (LXRα), Liver Kinase B1 (LKB1), 5′AMP-Activated Protein Kinase a1 (AMPKa1), and Mammalian Target of Rapamycin Complex 1 (mTORC1) in Blue Light Irradiation Mice
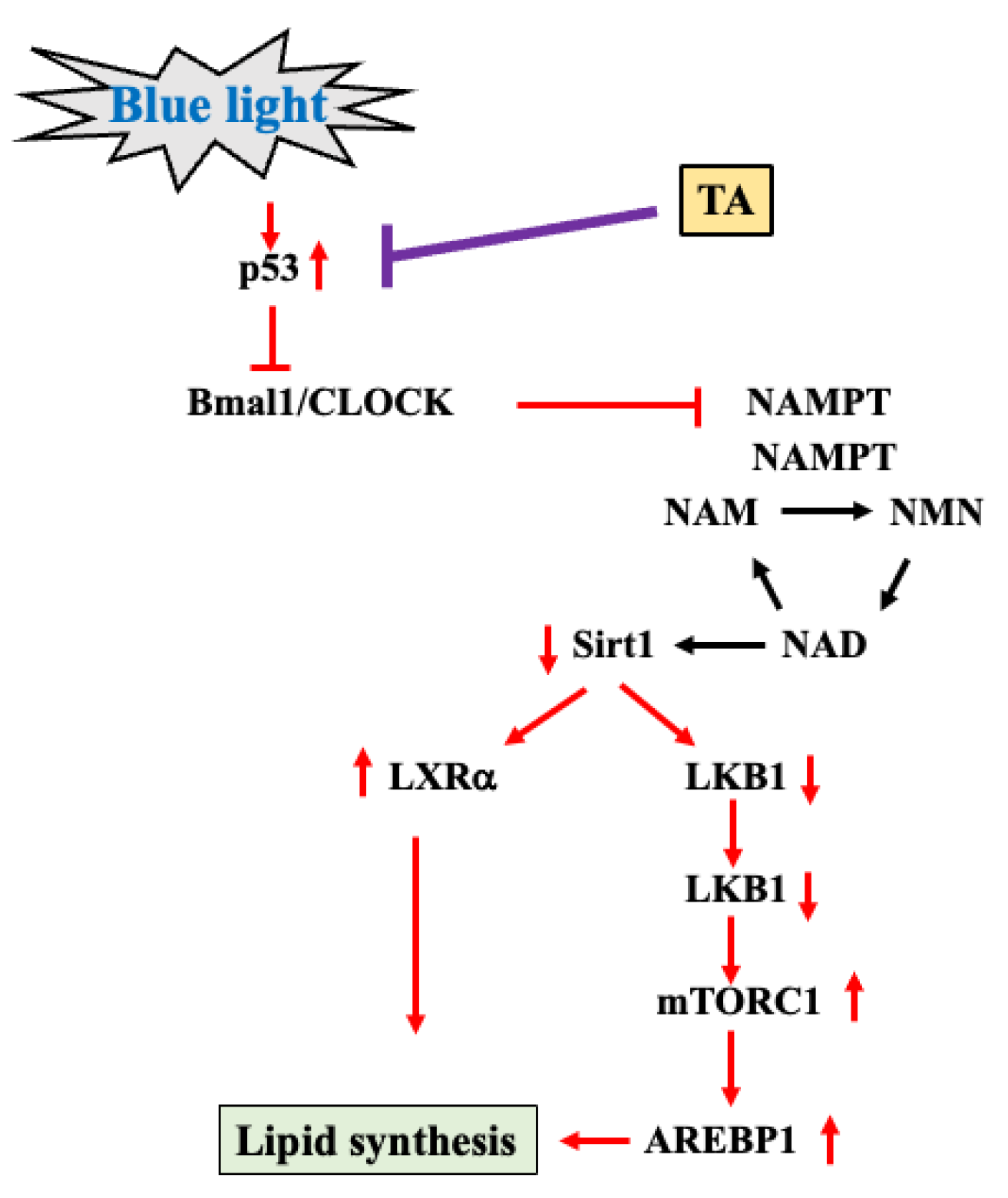
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Tranexamic Acid Treatment
4.3. Measurement of Body Fat Percentage, Subcutaneous Fat Percentage, and Visceral Fat Percentage
4.4. Preparation and Staining of the Liver
4.5. Western Blotting Analysis of the Liver
4.6. Measurement of Triglyceride, Cholesterol, NAMPT Activity, NMNAT Activity, NAD+, and p53 Levels in Mouse Liver
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoar, S.; Naderan, M.; Mahmoodzadeh, H.; Shoar, N.; Lotfi, D. Night eating syndrome: A psychiatric disease, a sleep disorder, a delayed circadian eating rhythm, and/or a metabolic condition? Expert Rev. Endocrinol. Metab. 2019, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Redd, S.C. Understanding and preventing computer vision syndrome. Malays. Fam. Physician 2008, 3, 128–130. [Google Scholar] [PubMed]
- Jaiswal, S.; Asper, L.; Long, J.; Lee, A.; Harrison, K.; Golebiowski, B. Ocular and visual discomfort associated with smartphones, tablets and computers: What we do and do not know. Clin. Exp. Optom. 2019, 102, 463–477. [Google Scholar] [CrossRef]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated chitosan-coated flexible liposomes with resveratrol for topical drug delivery to reduce blue-light-induced retinal damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef]
- Münch, M.; Kobialka, S.; Steiner, R.; Oelhafen, P.; Wirz-Justice, A.; Cajochen, C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1421–R1428. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Iizuka, Y.; Yamaguchi, T. Mechanism of the blue light-induced asthenopia and the ameliorating effect of tranexamic acid. BPB Rep. 2023, 6, 166–171. [Google Scholar] [CrossRef]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid. Med. Cell. Longev. 2015, 2015, 579675. [Google Scholar] [CrossRef]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.-P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Iwamoto, M. Plasminogen-Plasmin system. VII. Potentiation of antifibrinolytic action of a synthetic inhibitor, tranexamic acid, by alpha 2-macroglobulin antiplasmin. Biochim. Biophys. Acta 1970, 214, 411–418. [Google Scholar] [CrossRef]
- Chang, W.C.; Shi, G.Y.; Chow, Y.H.; Chang, L.C.; Hau, J.S.; Lin, M.T.; Jen, C.J.; Wing, L.Y.; Wu, H.L. Human plasma induces a receptor-mediated arachidonate release coupled with G protein in endothelial cells. Am. J. Physiol. 1993, 264, C271–C281. [Google Scholar] [CrossRef]
- Weide, I.; Tippler, B.; Syrovets, T.; Simmet, T. Plasma is a specific stimulus of the 5-lipoxygenase pathway of human peripheral monocytes. Thromb. Heamost. 1996, 76, 561–568. [Google Scholar]
- Liao, L.L.; Pang, G.Y. Effect observation of acidum tranexamicum on treating chloasma. Chin. J. Dermatovenereol. 2006, 20, 675–676. [Google Scholar]
- Li, D.; Shi, Y.; Li, M.; Liu, J.; Feng, X. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur. J. Dermatol. 2010, 20, 289–292. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Abnormal circadian rhythms and cell death associated with neutrophil extracellular trap a role in skin cancer caused by long-term blue light irradiation. Arch. Dermatol. Res. 2024, 316, 177. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Sex differences and Bmal 1/acetylcholine- or Bmal 1/noradrenaline-mediated effects of blue light irradiation on dextran-sodium-sulfate-induced ulcerative colitis model mice. Gastrointest. Disord. 2024, 6, 720–732. [Google Scholar] [CrossRef]
- Chen, A.; Du, L.; Xu, Y.; Chen, L.; Wu, Y. The effect of blue light exposure on the expression of circadian genes: Bmal1 and cryptochrome 1 in peripheral blood mononuclear cells of jaundiced neonates. Pediatr. Res. 2005, 58, 1180–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiramoto, K.; Yamate, Y.; Sato, E.F. p53 and Clock genes play an important role in memory and learning ability depression due to long-term ultraviolet A eye irradiation. Photochem. Photobiol. Sci. 2021, 20, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Matsumoto, T.; Zhao, Z.; Lee, C.C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 2013, 4, 2444. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Imai, S.; Yoshino, J. The importance of NAMPT/NAD/SIRT1 in the systhemic regulation of metabolism and ageing. Diabetes Obes. Metab. 2013, 15, 26–33. [Google Scholar] [CrossRef]
- Yoshino, J.; Imai, S.I. Accute measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Methods Mol. Biol. 2013, 1077, 203–215. [Google Scholar]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein sirt2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; McBurney, M.; Robbins, P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE 2010, 5, e9199. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; SenGupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yujiri, T.; Tanaka, M.; Tanaka, Y.; Nakamura, Y.; Tanizawa, Y. Altered expression of circadian clock genes during peripheral blood stem cell mobilization induced by granulocyte colony-stimulating factor. Chronobiol. Int. 2015, 32, 934–941. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.-E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef]
- Suzuki, K.; Hayano, Y.; Nakai, A.; Furuta, F.; Noda, M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J. Exp. Med. 2016, 213, 2567–2574. [Google Scholar] [CrossRef]
- Cooper, K.D.; Duraiswamy, N.; Hammerberg, C.; Allen, E.; Kimbrough-Green, C.; Dillon, W.; Thomas, D. Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. J. Investig. Dermatol. 1993, 101, 155–163. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Honda, A.; Zhao, Q.-L.; Makino, T.; Abe, R.; Matsui, K.; Shimizu, H.; Miyamoto, Y.; Kondo, T.; Shimizu, T. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 2010, 19, 1000–1006. [Google Scholar] [CrossRef]
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp. Dermatol. 2012, 21 (Suppl. S1), 11–17. [Google Scholar]
- Ostrakhovitch, E.A.; Cherian, M.G. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis 2005, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Neubert, E.; Bach, K.M.; Busse, J.; Bogeski, I.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Blue and long-wave ultraviolet light induce in vitro neutrophil extracellular trap (NET) formation. Front. Immunol. 2019, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, E.; Yoshimura, E.; Hatamoto, Y.; Tanaka, H.; Shimoda, S. Effect of sleep curtailment on dietary behavior and physical activity: A randomized crossover trial. Physiol. Behav. 2018, 184, 60–67. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E.V. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Covassin, N.; Singh, P.; McCrady-Spitzer, S.K.; St Louis, E.K.S.; Calvin, A.D.; Levine, J.A.; Somers, V.K. Effects of experimental sleep restriction on energy intake, energy expenditure, and visceral obesity. J. Am. Coll. Cardiol. 2022, 79, 1254–1265. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yamate, Y.; Sugiyama, D.; Matsuda, K.; Iizuka, Y.; Yamaguchi, T. Ameliorative effect of tranexamic acid on physiological skin aging and its sex difference in mice. Arch. Dermatol. Res. 2019, 311, 545–553. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramoto, K.; Koyama, M.; Ooi, K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp. Dermatol. 2014, 23, 659–663. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yamate, Y.; Sugiyama, D.; Matsuda, K.; Iizuka, Y.; Yamaguchi, T. Tranexamic acid ameliorates nonmelanoma skin cancer induced by long-term ultraviolet A irradiation. Photochem. Photobiol. 2019, 95, 612–617. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramoto, K.; Oikawa, H. Effects of Long-Term Blue Light Exposure on Body Fat Synthesis and Body Weight Gain in Mice and the Inhibitory Effect of Tranexamic Acid. Int. J. Mol. Sci. 2025, 26, 5554. https://doi.org/10.3390/ijms26125554
Hiramoto K, Oikawa H. Effects of Long-Term Blue Light Exposure on Body Fat Synthesis and Body Weight Gain in Mice and the Inhibitory Effect of Tranexamic Acid. International Journal of Molecular Sciences. 2025; 26(12):5554. https://doi.org/10.3390/ijms26125554
Chicago/Turabian StyleHiramoto, Keiichi, and Hirotaka Oikawa. 2025. "Effects of Long-Term Blue Light Exposure on Body Fat Synthesis and Body Weight Gain in Mice and the Inhibitory Effect of Tranexamic Acid" International Journal of Molecular Sciences 26, no. 12: 5554. https://doi.org/10.3390/ijms26125554
APA StyleHiramoto, K., & Oikawa, H. (2025). Effects of Long-Term Blue Light Exposure on Body Fat Synthesis and Body Weight Gain in Mice and the Inhibitory Effect of Tranexamic Acid. International Journal of Molecular Sciences, 26(12), 5554. https://doi.org/10.3390/ijms26125554




