Nanoliposomes as Effective Vehicles of Antioxidant Compounds in Food and Health
Abstract
1. Introduction
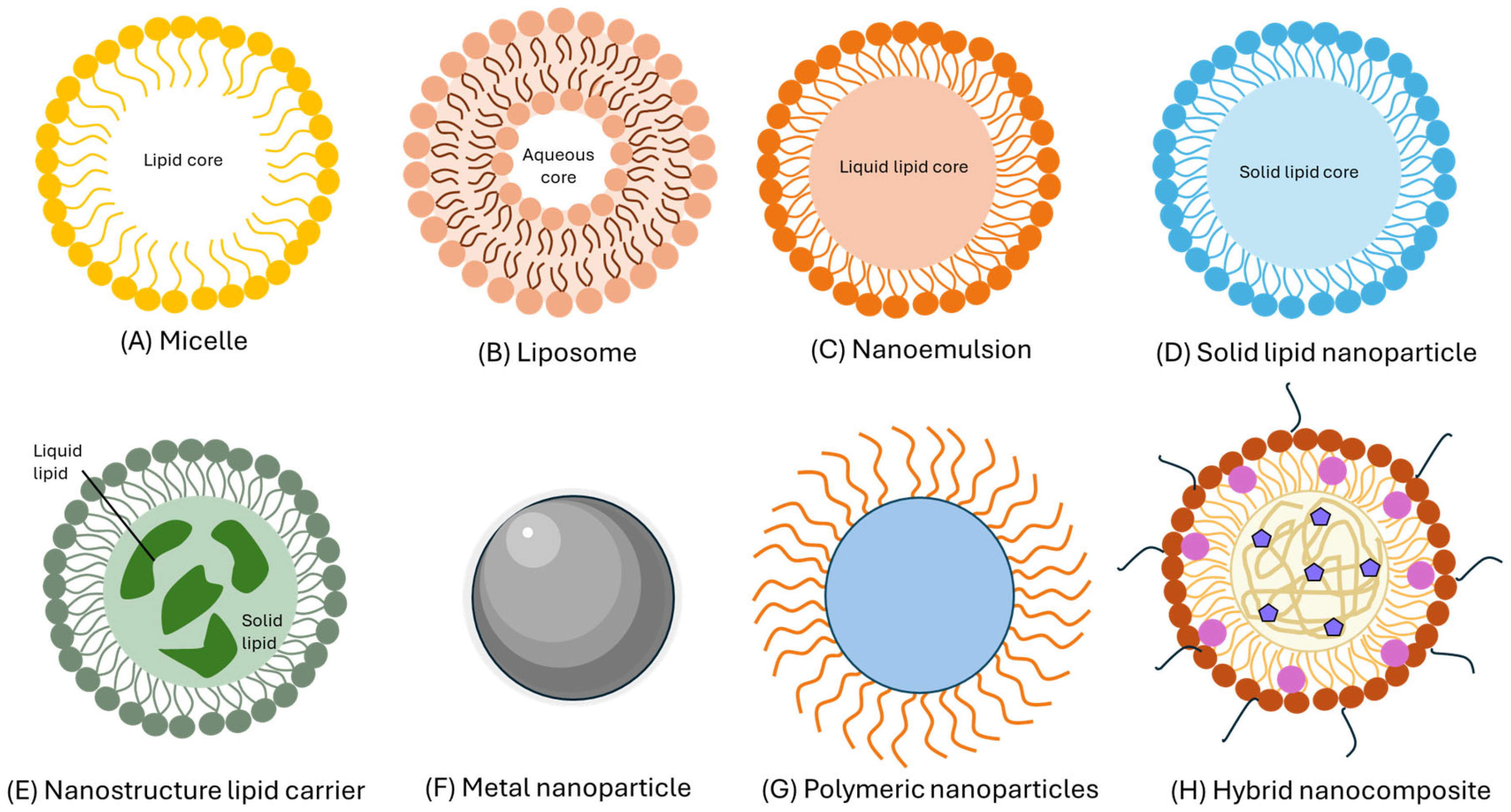
2. Nanoliposomes
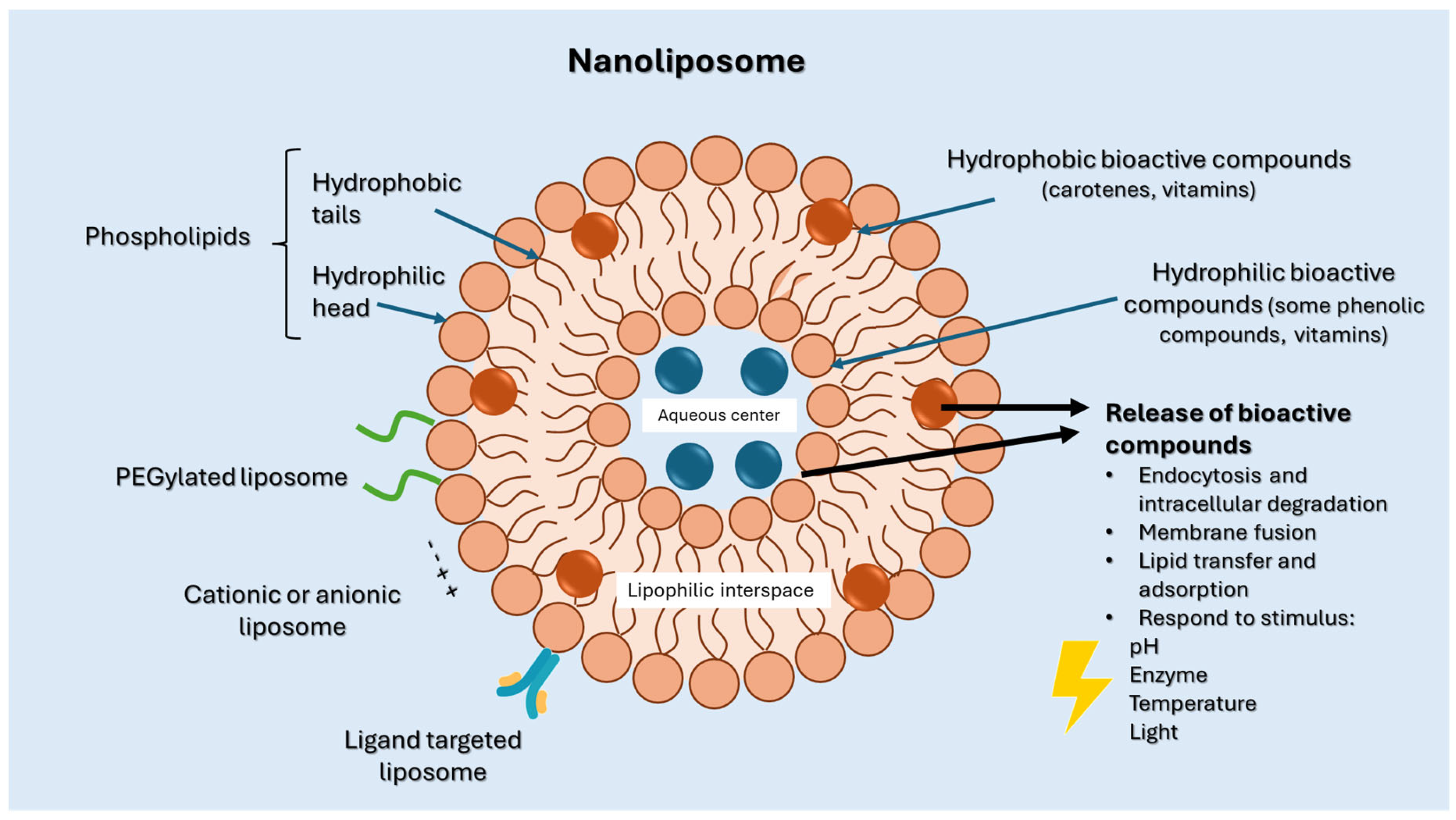
2.1. Structure and Properties of Nanoliposomes
| Natural | Synthetic | Function | Reference |
|---|---|---|---|
Phosphatidylcholine (PC)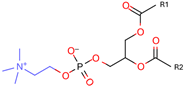 | 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)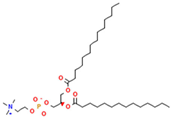 | Increase membrane fluidity and eicosanoid production | [14] |
Phosphatidylethanolamine (PE)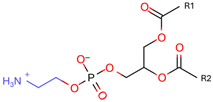 | 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)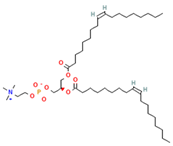 | PC precursor promotes membrane fusion, oxidative phosphorylation, and mitochondrial biogenesis | [15] |
Phosphatidylserine (PS)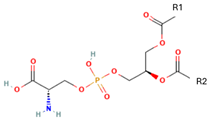 | 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC)  | PE decarboxylation, autophagosomes formation, morphology regulation and dynamics and functions of mitochondria | [16] |
Phosphatidylglycerol (PG) | 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG)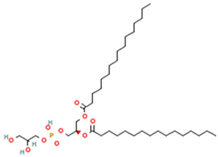 | Important role in apoptosis and blood clotting, besides serving as a conduit for the transfer of lipids between organelles | [17] |
Phosphatidylinositol (PI)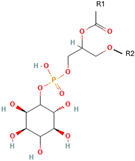 | 1,2-Distearoyl-sn-glycero-3-phosphoglycerol (DSPG)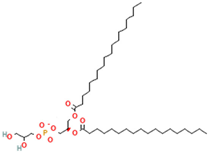 | Regulates traffic to and from Golgi apparatus and helps protect against hepatic viruses | [18] |
Phosphatidic acid (PA)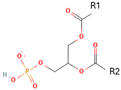 | Serve as a fusogenic lipid, altering membrane structure and promoting membrane fusion, especially in neurons | [19] |
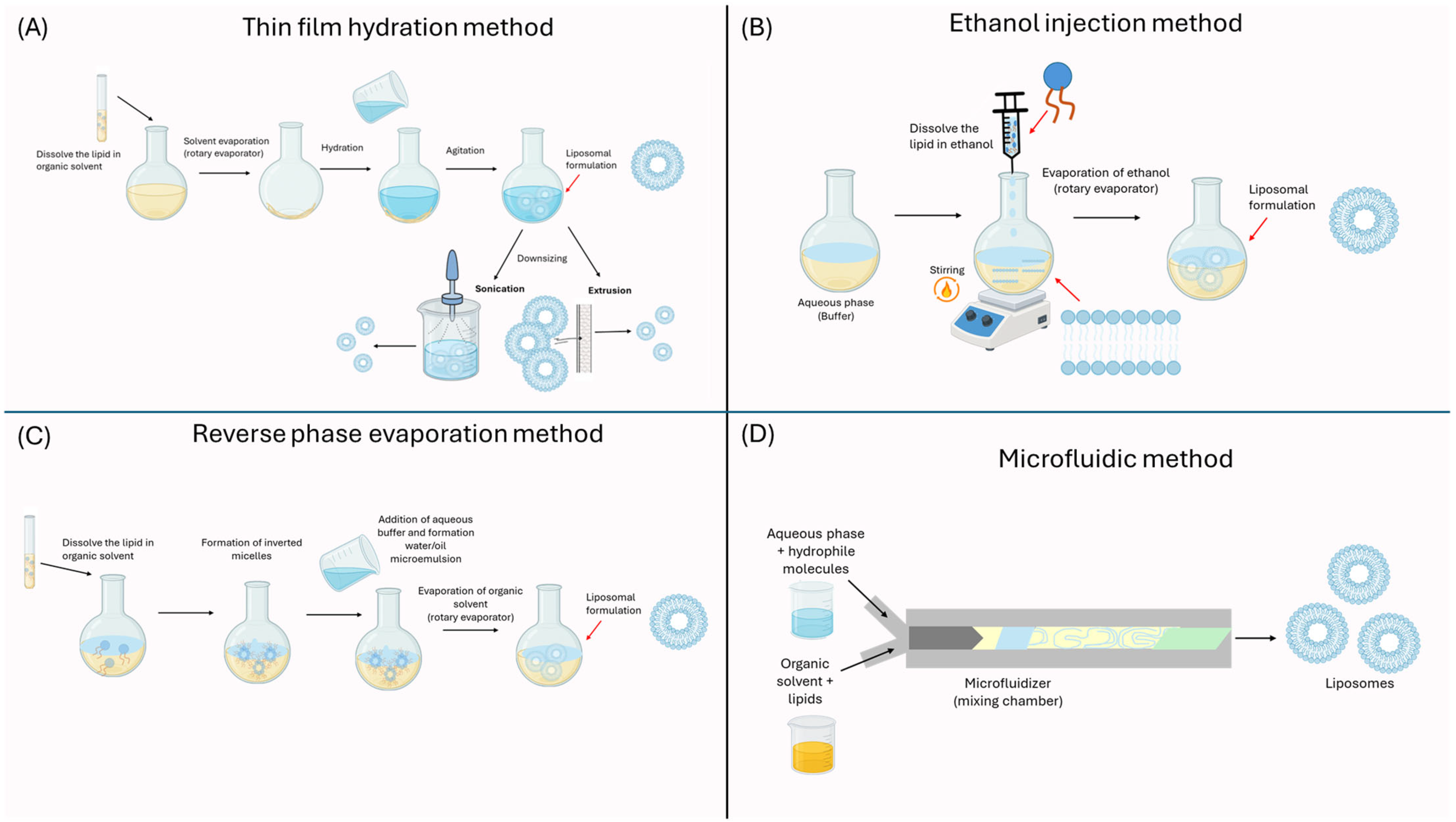
2.2. Preparation of Nanoliposomes
2.3. Stability of Nanoliposomes
2.4. Nanoliposome as Bioactive Compound Delivery
3. Biological Activity of the Encapsulated Compounds
3.1. Bioactive Compounds Encapsulated in Nanoliposomes
| Retinoids | ||||
|---|---|---|---|---|
| Name | Chemical Structure | Type of Nanoliposome | General Bioactivity | Reference |
| Retinol | 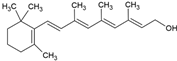 | Lecithin-cholesterol structure, small unilamellar (20–200 nm), retinol is contained in the lipid intermembrane section | Not evaluated in the study Antioxidant, regulation of cell differentiation, epithelial and vision maintenance | [21,66] |
| Retinoic acid | 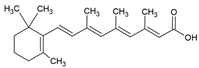 | Lecithin-cholesterol structure, small unilamellar (20–200 nm), retinoic acid contained in the lipid intermembrane section | Not evaluated in the study Gene expression modulator, cell differentiation, antitumor activity, tissue regeneration | [66,67] |
| Retinyl ester | 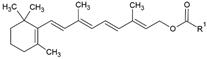 | Lecithin-cholesterol structure, small unilamellar (20–200 nm), retinyl ester contained in the lipid intermembrane section | Not evaluated in the study Storehouse of vitamin A, precursor of active retinol | [66,67] |
| Carotenoids | ||||
| Name | Chemical structure | Type of nanoliposome | General bioactivity | Reference |
| α-carotene | 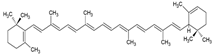 | Lecithin, cholesterol, and polysorbate 80 structure, giant unilamellar size (>1 µm), α-carotene contained in the lipid intermembrane section | Not evaluated in the study Antioxidant, precursor of vitamin A, protector against oxidative stress | [21,68] |
| β-carotene | 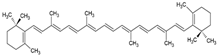 | Lecithin, cholesterol, and polysorbate 80 structure, giant unilamellar size (>1 µm), β-carotene contained in the lipid intermembrane section | Not evaluated in the study Powerful antioxidant, precursor of vitamin A, protection against free radicals | [21,68] |
| γ-carotene | 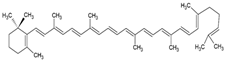 | Soy, egg, or marine lecithin and cholesterol structure, giant unilamellar size (>1 µm), γ-carotene contained in the lipid intermembrane section | Not evaluated in the study Antioxidant, less active than β-carotene; contributes to cellular homeostasis | [21,69] |
| Astaxanthin |  | Agarose oligosaccharides, phosphatidylcholine, phosphatidyl galactose and/or phosphatidyl neoagarobiose structure, small unilamellar (20–200 nm), astaxanthin contained in the lipid intermembrane section | Potent antioxidant Not evaluated in the study: anti-inflammatory, photoprotective, cardiovascular, and ocular protector | [45,70] |
| Lutein | 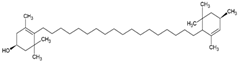 | Supercritical carbon-dioxide method, small unilamellar size (20–200 nm), lutein contained in the aqueous center | Not evaluated in the study Antioxidant, protects the retina, eye photoprotector | [71] |
| Zeaxanthin |  | Lecithin, cholesterol, and polysorbate 80 structure, giant unilamellar size (>1 µm), zeaxanthin contained in the lipid intermembrane section | Not evaluated in the study Eye protection against blue light, antioxidant in retina | [45,68] |
| β-criptoxanthin | 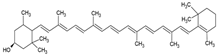 | Cholesterol and phosphatidylcholine structure, small unilamellar (20–200 nm), β-cryptoxanthin contained in the aqueous center | Antioxidant | [58] |
| Phenols | ||||
| Name | Chemical structure | Type of nanoliposome | General bioactivity | Reference |
| Gallic acid | 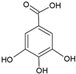 | Soy lecithin and cholesterol structure, small unilamellar size (20–200 nm), gallic acid contained in the aqueous center | Antioxidant, antimicrobial | [72] |
| Protocatechuic acid | 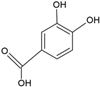 | Egg yolk phosphatidylcholine and cholesterol structure, small unilamellar size (20–200 nm), and protocatechuic acid contained in the aqueous center | Antioxidant, cytoprotective effect against H2O2-induced cytotoxicity on mouse fibroblast cells | [73,74] |
| Caffeic acid | 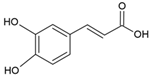 | Egg yolk phosphatidylcholine and cholesterol structure, small unilamellar size (20–200 nm), and protocatechuic acid contained in the aqueous center | Antioxidant, cytoprotective effect against H2O2-induced cytotoxicity on mouse fibroblast cells | [73,74] |
| p-cumaric acid | 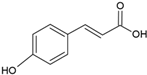 | Soy lecithin and cholesterol structure, small unilamellar size (20–200 nm), p-coumaric acid contained in the aqueous center | Antimicrobial, antioxidant | [68] |
| Salicylic acid | 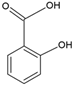 | Soy lecithin and cholesterol structure, small unilamellar size (20–200 nm), p-coumaric acid contained in the lipid intermembrane section | Not evaluated in the study Anti-inflammatory, moderate antioxidant properties | [75] |
| Vitamins | ||||
| Name | Chemical structure | Type of nanoliposome | General bioactivity | Reference |
| Vitamin A | 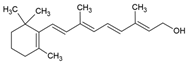 | Lecithin and cholesterol structure, small unilamellar size (20–200 nm), and vitamin A contained in the lipid intermembrane section | Not evaluated in the study Antioxidant, immunomodulator, regulator of cell differentiation. | [66,67] |
| Vitamin B2 | 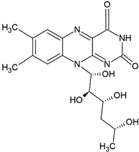 | Vegetable oil (chia, sunflower, and virgin olive) structure, giant unilamellar size (>1 µm), vitamin B2 contained in the lipid intermembrane section | Not evaluated in the study Enzyme cofactor, antioxidant, participation in energy metabolism | [67,76] |
| Vitamin C | 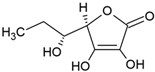 | Phosphatidylcholine, stearic acid, and stearic calcium structure, giant unilamellar (>1 µm), vitamin C contained in the aqueous center | Not evaluated in the study Neutralization of free radicals in aqueous media, collagen synthesis, absorption of non-heme iron, stimulation of the immune system | [3,21] |
| Vitamin D3 | 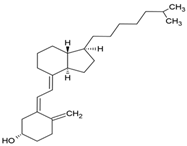 | Soy phosphatidylcholine and cholesterol structure, giant unilamellar size (>1 µm), vitamin D3 contained in the lipid intermembrane section | Not evaluated in the study Regulates calcium and phosphorus homeostasis, immunomodulator, essential for bone and immune health | [67,77] |
| Vitamin E |  | Phosphatidylcholine, stearic acid, and stearic calcium structure, giant unilamellar (>1 µm), vitamin E contained in the aqueous center | Not evaluated in the study Protection of cell membranes against oxidative stress, prevention of lipid peroxidation | [67] |
| Vitamin K |  | Phosphatidylcholine and cholesterol structure, giant unilamellar size (>1 µm), vitamin K contained in the lipid intermembrane section | Not evaluated in the study Activation of proteins involved in blood coagulation, bone metabolism, and osteoporosis prevention | [67,78] |
3.2. Carotenoids Encapsulated in Nanocarriers
4. Nanoliposomes Enhance Foods and Human Health
5. Conclusions
6. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Astaxanthin |
| CLA | Conjugated linoleic acid |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DPPG | 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol |
| DSPC | 1,2-Distearoyl-sn-glycero-3-phosphocholine |
| DSPG | 1,2-Distearoyl-sn-glycero-3-phosphoglycerol |
| EE | Encapsulation efficiency |
| NA | Data not available |
| NCs | Nanocapsules |
| NEs | Nanoemulsions |
| NFs | Nanofibers |
| NLCs | Nanostructured lipid carriers |
| NLs | Nanoliposomes |
| NPs | Nanoparticles |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PG | Phosphatidyl-glycerol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SLNPs | Solid lipid nanoparticles |
References
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ali, A.A.E.; Trivedi, L.R. An Updated Review on: Liposomes as Drug Delivery System. Pharmatutor 2018, 6, 50–62. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2019, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 79–108. [Google Scholar]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. In Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers; Human Press: Totowa, NJ, USA, 2009; pp. 29–50. [Google Scholar]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef]
- Al-Ekaid, N.M.; Al-Samydai, A.; Al-deeb, I.; Nsairat, H.; Khleifat, K.; Alshaer, W. Preparation, characterization, and anticancer activity of pegylated nano liposomal loaded with rutin against human carcinoma cells (HT-29). Chem. Biodivers. 2023, 20, e202301167. [Google Scholar] [CrossRef]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.-J. Understanding How Sterols Regulate Membrane Remodeling in Supported Lipid Bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef]
- Ortega-Galindo, A.S.; Díaz-Peralta, L.; Galván-Hernández, A.; Ortega-Blake, I.; Pérez-Riascos, A.; Rojas-Aguirre, Y. Los liposomas en nanomedicina: Del concepto a sus aplicaciones clínicas y tendencias actuales en investigación. Mundo Nano. Rev. Interdiscip. Nanociencias Nanotecnología 2023, 16, 1e–26e. [Google Scholar] [CrossRef]
- Kanno, K.; Wu, M.K.; Scapa, E.F.; Roderick, S.L.; Cohen, D.E. Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2007, 1771, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 29–88. [Google Scholar]
- Vance, J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.G.; Fairn, G.D. Distribution, dynamics and functional roles of phosphatidylserine within the cell. Cell Commun. Signal. 2019, 17, 126. [Google Scholar] [CrossRef]
- Boura, E.; Nencka, R. Corrigendum to: ‘‘Phosphatidylinositol 4-kinases: Function, structure, and inhibition’’ [Exp. Cell Res. 337/2 (2015) 136–145]. Exp. Cell Res. 2016, 341, 110. [Google Scholar] [CrossRef]
- Raben, D.M.; Barber, C.N. Phosphatidic acid and neurotransmission. Adv. Biol. Regul. 2017, 63, 15–21. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Farshi, P.; Ahmadi, P.; Ahmadi, A.; Yousefi, M.; Ghorbani, M.; Hosseini, S.M. Encapsulation of vitamins using nanoliposome: Recent advances and perspectives. Adv. Pharm. Bull. 2021, 13, 48. [Google Scholar] [CrossRef]
- Du, G.; Sun, X. Ethanol injection method for liposome preparation. In Liposomes: Methods and Protocols; Human Press: Totowa, NJ, USA, 2023; pp. 65–70. [Google Scholar]
- Shi, N.-Q.; Qi, X.-R. Preparation of drug liposomes by reverse-phase evaporation. Liposome-Based Drug Deliv. Syst. 2021, 37–46. [Google Scholar]
- Zhong, Q.; Zhang, H. Preparation of small unilamellar vesicle liposomes using detergent dialysis method. In Liposomes: Methods and Protocols; Human Press: Totowa, NJ, USA, 2023; pp. 49–56. [Google Scholar]
- Alavi, M.; Mozafari, M.; Hamblin, M.R.; Hamidi, M.; Hajimolaali, M.; Katouzian, I. Industrial-scale methods for the manufacture of liposomes and nanoliposomes: Pharmaceutical, cosmetic, and nutraceutical aspects. Micro Nano Bio Asp. 2022, 1, 26–35. [Google Scholar]
- Li, X.; Zhao, X.; Wang, J.; Xu, B.; Feng, J.; Huang, W. High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes. Biology 2025, 14, 568. [Google Scholar] [CrossRef]
- Delma, K.L.; Lechanteur, A.; Evrard, B.; Semdé, R.; Piel, G. Sterilization methods of liposomes: Drawbacks of conventional methods and perspectives. Int. J. Pharm. 2021, 597, 120271. [Google Scholar] [CrossRef] [PubMed]
- Sakar, F.; Özer, A.; Erdogan, S.; Ekizoglu, M.; Kart, D.; Özalp, M.; Colak, S.; Zencir, Y. Nano drug delivery systems and gamma radiation sterilization. Pharm. Dev. Technol. 2017, 22, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Matsuzaki, T.; Nakamura, A.; Nakatani, D.; Sanada, S.; Fu, H.Y.; Okuda, K.; Yamato, M.; Tsuchida, S.; Sakata, Y. Development of a novel one-step production system for injectable liposomes under GMP. Pharm. Dev. Technol. 2018, 23, 602–607. [Google Scholar] [CrossRef]
- Delma, K.L.; Penoy, N.; Sakira, A.K.; Egrek, S.; Sacheli, R.; Grignard, B.; Hayette, M.-P.; Somé, T.I.; Evrard, B.; Semdé, R. Use of supercritical CO2 for the sterilization of liposomes: Study of the influence of sterilization conditions on the chemical and physical stability of phospholipids and liposomes. Eur. J. Pharm. Biopharm. 2023, 183, 112–118. [Google Scholar] [CrossRef]
- Bondu, C.; Yen, F.T. Nanoliposomes, from food industry to nutraceuticals: Interests and uses. Innov. Food Sci. Emerg. Technol. 2022, 81, 103140. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Lee, M.-K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Omri, A. Process variables and design of experiments in liposome and nanoliposome research. Mini Rev. Med. Chem. 2018, 18, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.V.; Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Trends in drug delivery systems for natural bioactive molecules to treat health disorders: The importance of nano-liposomes. Pharmaceutics 2022, 14, 2808. [Google Scholar] [CrossRef] [PubMed]
- Kozik, V.; Pentak, D.; Paździor, M.; Zięba, A.; Bąk, A. From design to study of liposome-driven drug release part 1: Impact of temperature and pH on environment. Int. J. Mol. Sci. 2023, 24, 11686. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, S.; Singh, S.; Wani, M.Y. Overcoming resistance: Chitosan-modified liposomes as targeted drug carriers in the fight against multidrug resistant bacteria-a review. Int. J. Biol. Macromol. 2024, 278, 135022. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Taléns-Visconti, R.; Díez-Sales, O.; de Julián-Ortiz, J.V.; Nácher, A. Nanoliposomes in cancer therapy: Marketed products and current clinical trials. Int. J. Mol. Sci. 2022, 23, 4249. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–36. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Sadeghi Mahoonak, A.; Allafchian, A.; Ghorbani, M. Fabrication of β-carotene loaded glucuronoxylan-based nanostructures through electrohydrodynamic processing. Int. J. Biol. Macromol. 2019, 139, 773–784. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Giada, M.d.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; IntechOpen: London, UK, 2013. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef]
- Cuomo, F.; Ceglie, S.; Miguel, M.; Lindman, B.; Lopez, F. Oral delivery of all-trans retinoic acid mediated by liposome carriers. Colloids Surf. B Biointerfaces 2021, 201, 111655. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.d.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Dutta, D. Designing a nanoliposome loaded with provitamin A xanthophyll β-cryptoxanthin from K. marina DAGII to target a population suffering from hypovitaminosis A. Process Biochem. 2024, 138, 97–110. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control Release 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Uemori, C.; Kon, T.; Honda, M.; Wahyudiono; Machmudah, S.; Kanda, H.; Goto, M. Preparation of liposomes encapsulating β–carotene using supercritical carbon dioxide with ultrasonication. J. Supercrit. Fluids 2020, 161, 104848. [Google Scholar] [CrossRef]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes loaded with phenolic extracts of Spirulina LEB-18: Physicochemical characterization and behavior under simulated gastrointestinal conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Yin, Y.; Xia, J. Preparation and evaluation of vitamin C and folic acid-coloaded antioxidant liposomes. Part. Sci. Technol. 2018, 37, 453–459. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; John Swing, C. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Manthey, J.A.; Hunter, W.B.; Bai, J. Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix. Foods 2020, 9, 1200. [Google Scholar] [CrossRef]
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Wahyudiono; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51. [Google Scholar] [CrossRef]
- Pezeshky, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Moghadam, M.; Babazadeh, A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Liu, P.; Shen, J.; Cao, J.; Jiang, W. p-Coumaric acid-loaded nanoliposomes: Optimization, characterization, antimicrobial properties and preservation effects on fresh pod pepper fruit. Food Chem. 2024, 435, 137672. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, T.B.R.; Mendonça, C.R.B.; Zambiazi, R.C. Methods of protection and application of carotenoids in foods—A bibliographic review. Food Biosci. 2022, 48, 101829. [Google Scholar]
- Wu, H.; Zhang, H.; Li, X.; Secundo, F.; Mao, X. Preparation and characterization of phosphatidyl-agar oligosaccharide liposomes for astaxanthin encapsulation. Food Chem. 2023, 404, 134601. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Res. Int. 2017, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chem. 2019, 293, 315–322. [Google Scholar] [CrossRef]
- Noubigh, A.; Aydi, A.; Abderrabba, M. Experimental Measurement and Correlation of Solubility Data and Thermodynamic Properties of Protocatechuic Acid in Four Organic Solvents. J. Chem. Eng. Data 2015, 60, 514–518. [Google Scholar] [CrossRef]
- Păvăloiu, R.-D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery. Nanomaterials 2021, 11, 1938. [Google Scholar] [CrossRef]
- Bhalerao, S.S.; Harshal, A.R. Preparation, Optimization, Characterization, and Stability Studies of Salicylic Acid Liposomes. Drug Dev. Ind. Pharm. 2003, 29, 451–467. [Google Scholar] [CrossRef]
- Couto, R.; Alvarez, V.; Temelli, F. Encapsulation of Vitamin B2 in solid lipid nanoparticles using supercritical CO2. J. Supercrit. Fluids 2017, 120, 432–442. [Google Scholar] [CrossRef]
- Chaves, M.A.; Oseliero Filho, P.L.; Jange, C.G.; Sinigaglia-Coimbra, R.; Oliveira, C.L.P.; Pinho, S.C. Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 112–121. [Google Scholar] [CrossRef]
- Jansses, B. Encapsulation of Vitamin D3 and Vitamin K2 in Chitosan Coated Liposomes. Master’s Dissertation, Ghent University, Ghent, Belgium, Università degli Studi di Salerno (UNISA), Fisciano, Italy, 2017. [Google Scholar]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Jafari, S.M. Nanoencapsulation of bioactive food ingredients. In Handbook of Food Nanotechnology; Academic Press: Cambridge, MA, USA, 2020; pp. 279–344. [Google Scholar]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An Overview of Nanotechnology in Food Science: Preparative Methods, Practical Applications, and Safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Mansur, M.C.P.P.R.; Campos, C.; Vermelho, A.B.; Nobrega, J.; da Cunha Boldrini, L.; Balottin, L.; Lage, C.; Rosado, A.S.; Ricci-Júnior, E.; dos Santos, E.P. Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: Efficacy and safety study. Arab. J. Chem. 2020, 13, 6741–6752. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Arranz, E.; Odriozola-Serrano, I.; Martín-Belloso, O.; Giblin, L. Delivery of β-carotene to the in vitro intestinal barrier using nanoemulsions with lecithin or sodium caseinate as emulsifiers. LWT 2021, 135, 110059. [Google Scholar] [CrossRef]
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, B.; Jia, S.; Ma, M.; Hao, J. Novel supramolecular organogel based on β-cyclodextrin as a green drug carrier for enhancing anticancer effects. J. Mol. Liq. 2018, 250, 19–25. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT—Food Sci. Technol. 2015, 63, 100–107. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; de Matos, M.F.R.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control Release 2017, 248, 117–124. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef]
- dos Santos, P.P.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Costa, T.H.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Development of lycopene-loaded lipid-core nanocapsules: Physicochemical characterization and stability study. J. Nanoparticle Res. 2015, 17, 107. [Google Scholar] [CrossRef]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of Lutein-Loaded PVA/Sodium Alginate Nanofibers and Investigation of Its Release Behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Hafezi Ghahestani, Z.; Alebooye Langroodi, F.; Mokhtarzadeh, A.; Ramezani, M.; Hashemi, M. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif. Cells Nanomed. Biotechnol. 2016, 45, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; Baskaran, V. Biodegradable chitosan-glycolipid hybrid nanogels: A novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015, 43, 717–725. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Huang, W.-C.; Xue, C.; Mao, X. Comparison of β-carotene loaded marine and egg phospholipids nanoliposomes. J. Food Eng. 2020, 283, 110055. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA—J. Food 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and Physicochemical Characterization of Lycopene-Loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [Google Scholar] [CrossRef]
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin encapsulation in ethyl cellulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release profile and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136. [Google Scholar] [CrossRef]
- Baek, K.H.; Patra, J.K. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Huang, R.-F.S.; Wei, Y.-J.; Stephen Inbaraj, B. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef] [PubMed]
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green Synthesis of Gold Nanoparticles Using Sumac Aqueous Extract and Their Antioxidant Activity. Mater. Res. 2016, 20, 264–270. [Google Scholar] [CrossRef]
- Yi, J.; Zhong, F.; Zhang, Y.; Yokoyama, W.; Zhao, L. Effects of lipids on in vitro release and cellular uptake of β-carotene in nanoemulsion-based delivery systems. J. Agric. Food Chem. 2015, 63, 10831–10837. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical Fluid Technology as a Green Alternative During the Preparation of Drug Delivery Systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]
- Seabra, A.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Birch, C.S.; Bonwick, G.A. Ensuring the future of functional foods. Int. J. Food Sci. Technol. 2018, 54, 1467–1485. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E.; et al. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gaucheron, F. Iron fortification in dairy industry. Trends Food Sci. Technol. 2000, 11, 403–409. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]

| Flavonoid | Structure |
|---|---|
| Flavones | 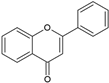 |
| Flavonols | 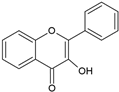 |
| Flavanones | 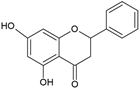 |
| Flavanols | 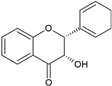 |
| Anthocyanidins |  |
| Isoflavones | 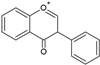 |
| Nanosystem | Carotenoids | Particle Size (nm) | EE (%) | Zeta Potential (mV) | Storage Stability (Days) | References |
|---|---|---|---|---|---|---|
| Nanoemulsions | β-carotene | 218 | NA | 40 | 21 at 37 °C | [86] |
| 143.7 | −38.2 | 30 at 25 °C | ||||
| Microbial carotenoids | 142.1 | NA | 30 at 25 °C | [86] | ||
| Carotenoids | 290 to 350 | −53.4 to −58.8 | 21 at 25 °C | [87] | ||
| β-carotene | 198.4 to 315.6 | −29.9 to −38.5 | 90 at 4, 25, and 37 °C | [88] | ||
| Carotenoids | <200 | −30 to −45 | 35 at 25 °C | [89] | ||
| Lycopene | 145.1 to 161.9 | −19.7 to −20.7 | 1 at 25 °C | [90] | ||
| 200.1 to 287.1 | 61 to 89.1 | 20 to 45 | 42 at 4, 25, and 37 °C | [91] | ||
| Polymeric/biopolymeric NPs | Carotenoids | 153 | 83.7 | NA | NA | [92] |
| 84.4 | >96 | −41.3 to −43.6 | 60 at 41 °C | [93] | ||
| β-carotene | 77.8 to 371.8 | 98.7 to 99.1 | −37.8 to −29.9 | NA | [94] | |
| β-carotene | 70.4 | 97.4 | NA | NA | [59] | |
| Lycopene | 152 | 89 | 58.3 | NA | [95] | |
| ~200 | >95 | −36 | 210 at 5 °C | [96] | ||
| 193 | NA | −11.5 | 14 at 25 °C | [97] | ||
| Lutein | <250 | 74.5 | −27.2 | NA | [98] | |
| Lutein | 240 to 340 | ~91.9 | NA | NA | [99] | |
| Crocetin | 288 to 584 | 59.6 to 97.2 | NA | NA | [100] | |
| Fucoxanthin | 200 to 500 | 47 to 90 | 30 to 50 | 6 at 37 °C | [101] | |
| Nanoliposomes/liposomes | Carotenoids | 70 to 100 | 75 | −5.3 | NA | [102] |
| β-carotene | 162.8 to 365.8 | ~98 | 64.5 to 42.6 | 70 at 4 °C | [103] | |
| Astaxanthin | 80.6 | 97.6 | 31.8 | 15 at 4 and 25 °C | [104] | |
| 60 to 80 | 97.4 | NA | NA | [105] | ||
| Lutein | 264.8 to 367.1 | 91.8 to 92.9 | −34.3 to −27.9 | NA | [62] | |
| SLNPs and NLCs | β-carotene SLNPs | 200 to 400 | 53.4 to 68.3 | −6.1 to −9.3 | 90 at 5, 25, and 40 °C | [106] |
| <220 | NA | 20 to 30 | 10 at 25 °C | [106] | ||
| 120 | NA | −30 | 56 at 25 °C | |||
| Lycopene SLNPs | 125 to 166 | 86.6 to 98.4 | NA | 60 at 4 °C | [107] | |
| Lycopene NLCs | 157 to 166 | > 99 | −74.2 to −74.6 | 120 at 4, 30, and 40 °C | [79] | |
| 121.9 | 84.50 | −29 | 90 at 25 °C | [7] | ||
| Supercritical fluid-based NPs | Astaxanthin | 150 to 175 | NA | NA | NA | [65] |
| 266 | 84 | NA | NA | [108] | ||
| Metal/metal oxide-based NPs and hybrid nanocomposites | Carotenoids | 20 to 140 | NA | NA | NA | [109] |
| Lycopene | 3 to 5 | −48.5 | 90 at 4 and 25 °C | [110] | ||
| 20.8 | −25.3 | NA | [111] |
| Nanosystem | Advantages | Disadvantages | Main Physiological Phenomena | References |
|---|---|---|---|---|
| Nanoemulsions |
|
| Internalized mainly by clathrin- or caveolin-mediated endocytosis. Intracellular trafficking: endosomal escape. | [43,57,112] |
| Polymeric/biopolymeric NPs |
|
| NPs entry into cell using different endocytotic pathways: macropinocytosis. phagocytosis, clathrin-mediated endocytosis, clathrin-caveolin independent endocytosis, and caveolae-mediated endocytosis. Intracellular trafficking: endosomal escape | [43,57,83] |
| Nanoliposomes/liposomes |
|
| Fusion with cell membranes or endocytosis; intracellular trafficking to lysosomes or cytosol | [57,80,83] |
| SLNPs |
|
| Internalized mainly by clathrin- or caveolin-mediated endocytosis. Intracellular trafficking: endosomal escape, lysosome | [43,57,80,85] |
| NLCs |
|
| Internalized mainly by clathrin- or caveolin-mediated endocytosis. Intracellular trafficking: endosomal escape, lysosome | [57,80,85] |
| Supercritical fluid-based NPs |
|
| Internalized mainly by clathrin- or caveolin-mediated endocytosis. Intracellular trafficking: endosomal escape, lysosome | [43,80,84,113] |
| Metal/metal oxide-based NPs and hybrid nanocomposites |
|
| Internalized mainly by clathrin- or caveolin-mediated endocytosis. Intracellular trafficking to lysosomes | [84,114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Morales, J.; Fimbres-Olivarría, D.; González-Vega, R.I.; Bernal-Mercado, A.T.; Aubourg-Martínez, S.P.; López-Gastélum, K.A.; Robles-García, M.Á.; de Jesús Ornelas-Paz, J.; Ruiz-Cruz, S.; Del-Toro-Sánchez, C.L. Nanoliposomes as Effective Vehicles of Antioxidant Compounds in Food and Health. Int. J. Mol. Sci. 2025, 26, 5523. https://doi.org/10.3390/ijms26125523
García-Morales J, Fimbres-Olivarría D, González-Vega RI, Bernal-Mercado AT, Aubourg-Martínez SP, López-Gastélum KA, Robles-García MÁ, de Jesús Ornelas-Paz J, Ruiz-Cruz S, Del-Toro-Sánchez CL. Nanoliposomes as Effective Vehicles of Antioxidant Compounds in Food and Health. International Journal of Molecular Sciences. 2025; 26(12):5523. https://doi.org/10.3390/ijms26125523
Chicago/Turabian StyleGarcía-Morales, Jonathan, Diana Fimbres-Olivarría, Ricardo Iván González-Vega, Ariadna Thalía Bernal-Mercado, Santiago Pedro Aubourg-Martínez, Karla Alejandra López-Gastélum, Miguel Ángel Robles-García, José de Jesús Ornelas-Paz, Saúl Ruiz-Cruz, and Carmen Lizette Del-Toro-Sánchez. 2025. "Nanoliposomes as Effective Vehicles of Antioxidant Compounds in Food and Health" International Journal of Molecular Sciences 26, no. 12: 5523. https://doi.org/10.3390/ijms26125523
APA StyleGarcía-Morales, J., Fimbres-Olivarría, D., González-Vega, R. I., Bernal-Mercado, A. T., Aubourg-Martínez, S. P., López-Gastélum, K. A., Robles-García, M. Á., de Jesús Ornelas-Paz, J., Ruiz-Cruz, S., & Del-Toro-Sánchez, C. L. (2025). Nanoliposomes as Effective Vehicles of Antioxidant Compounds in Food and Health. International Journal of Molecular Sciences, 26(12), 5523. https://doi.org/10.3390/ijms26125523









