Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends
Abstract
1. Introduction
2. Characteristics of Biomaterials
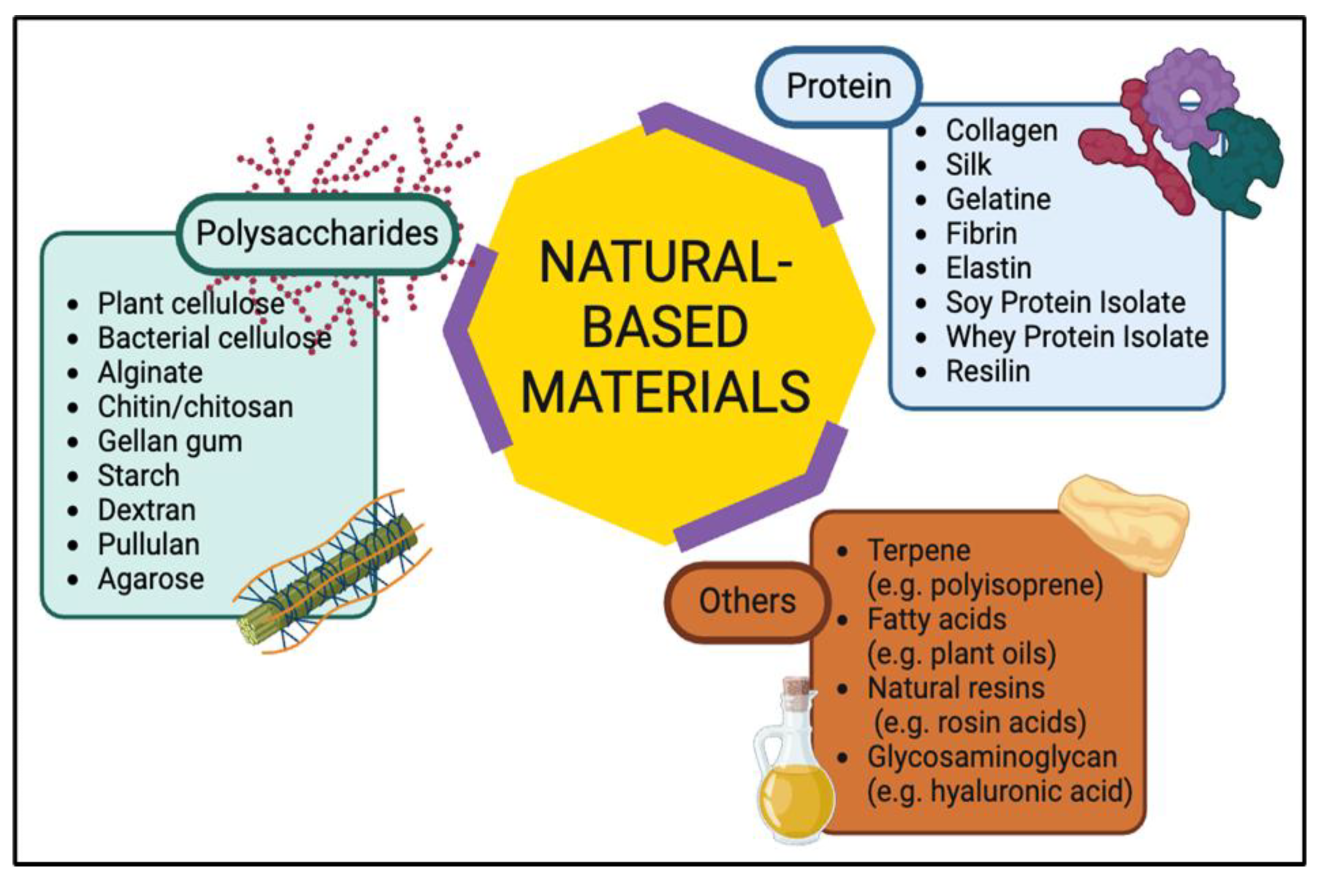
2.1. Polysaccharide-Based Materials
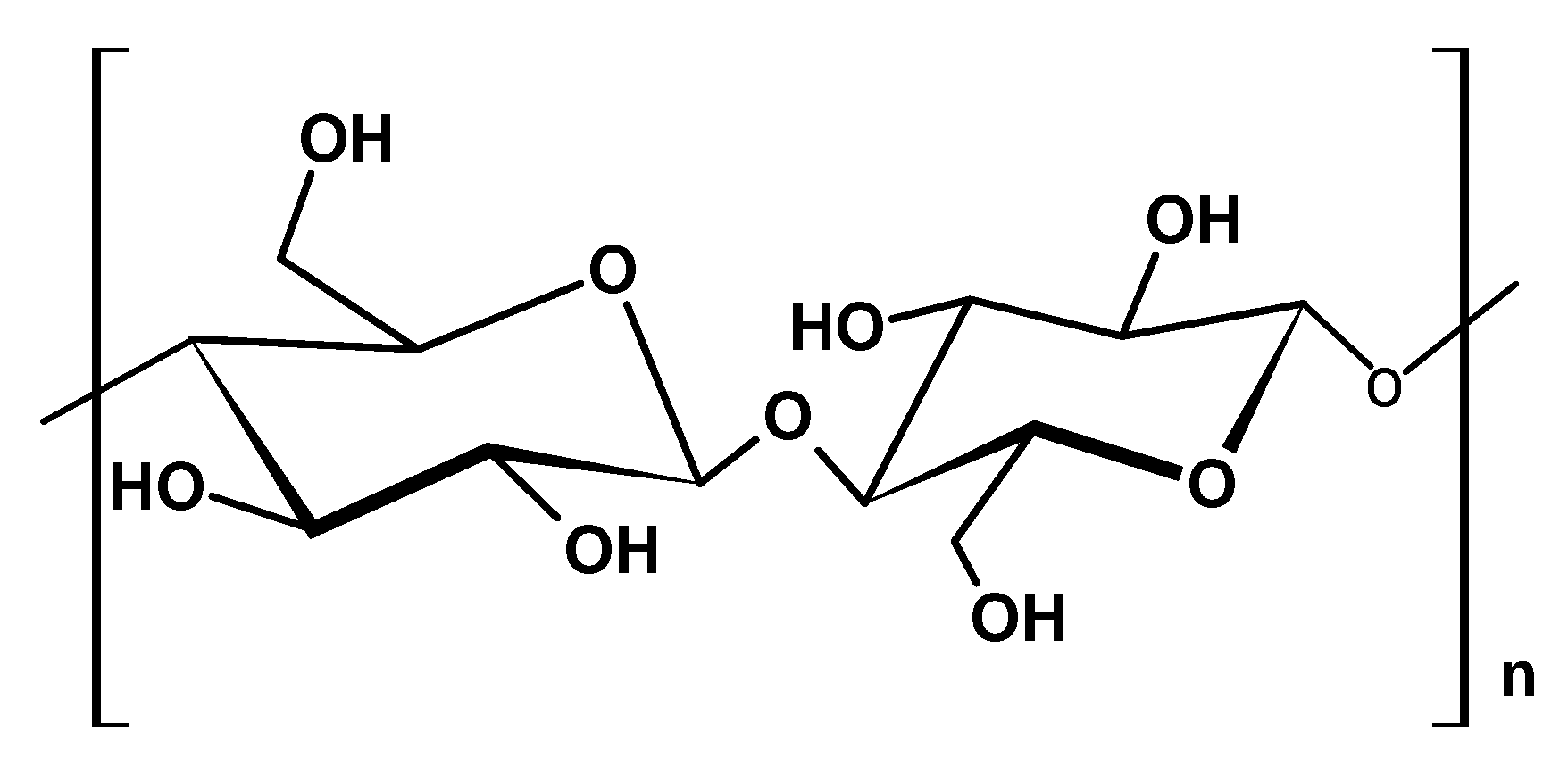
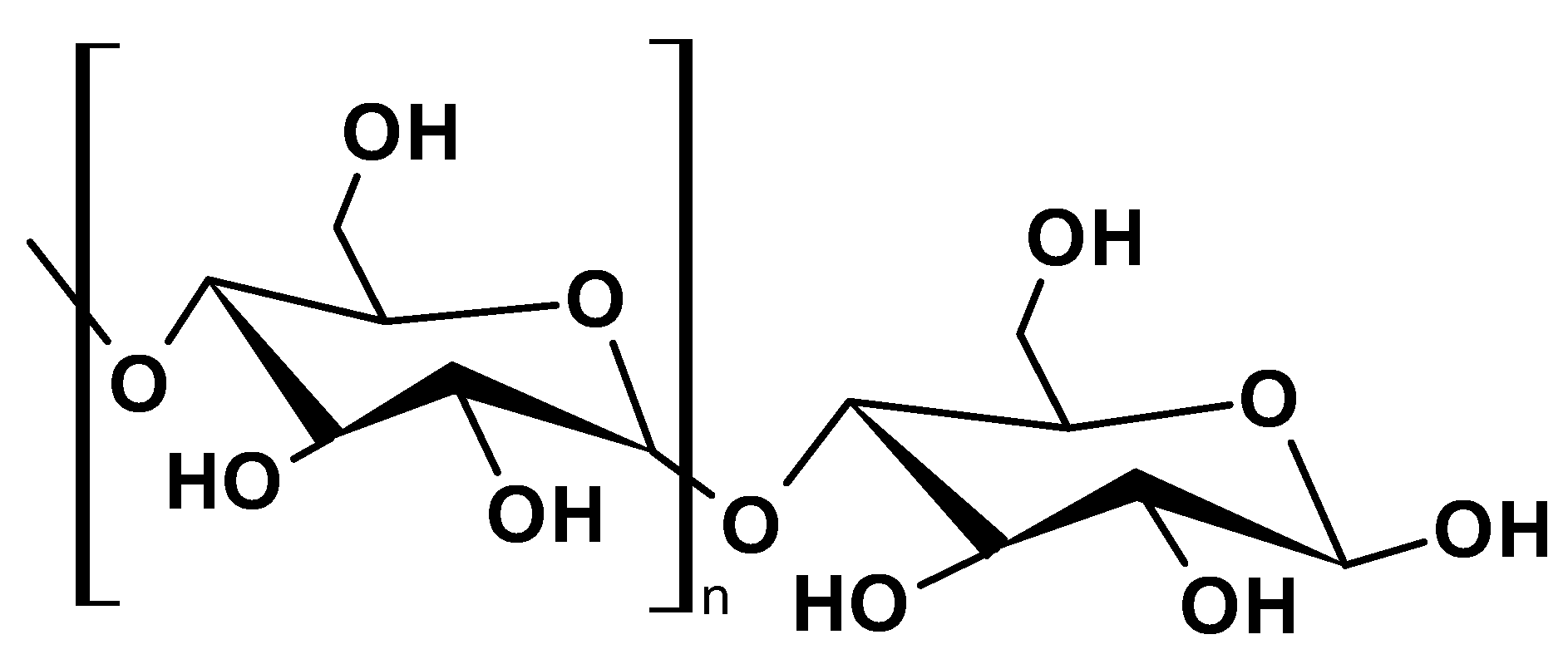
2.1.1. Cellulose
2.1.2. Alginate
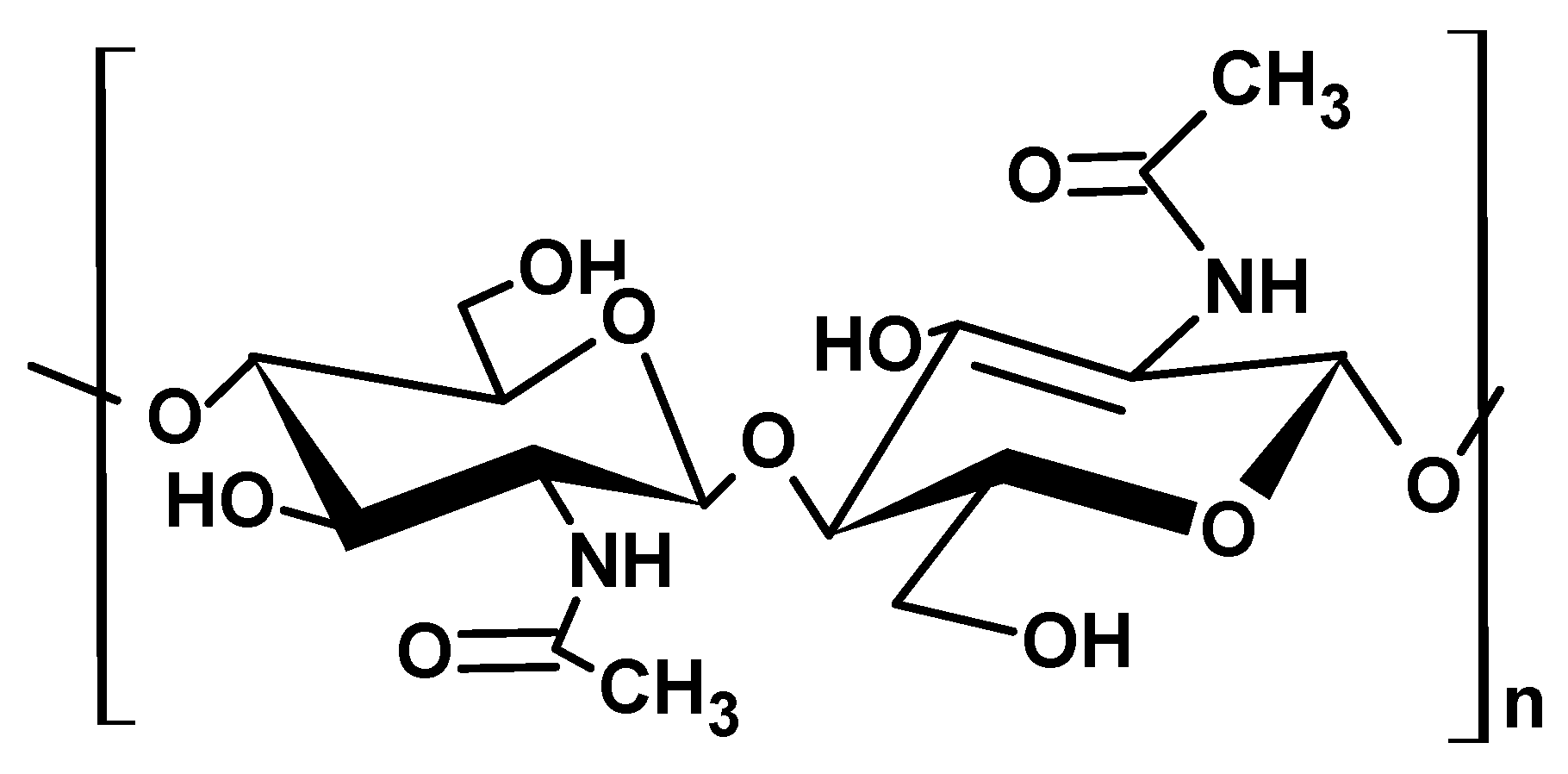
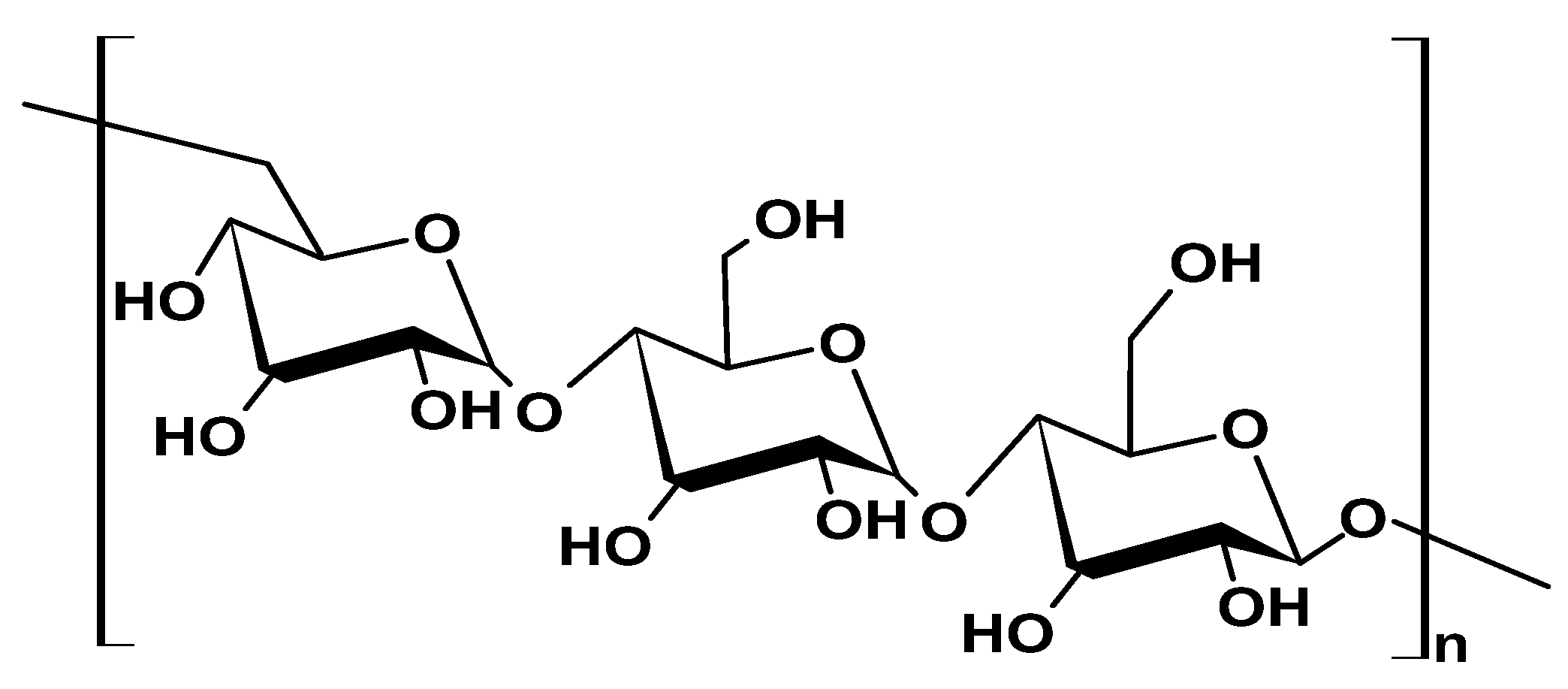
2.1.3. Chitin
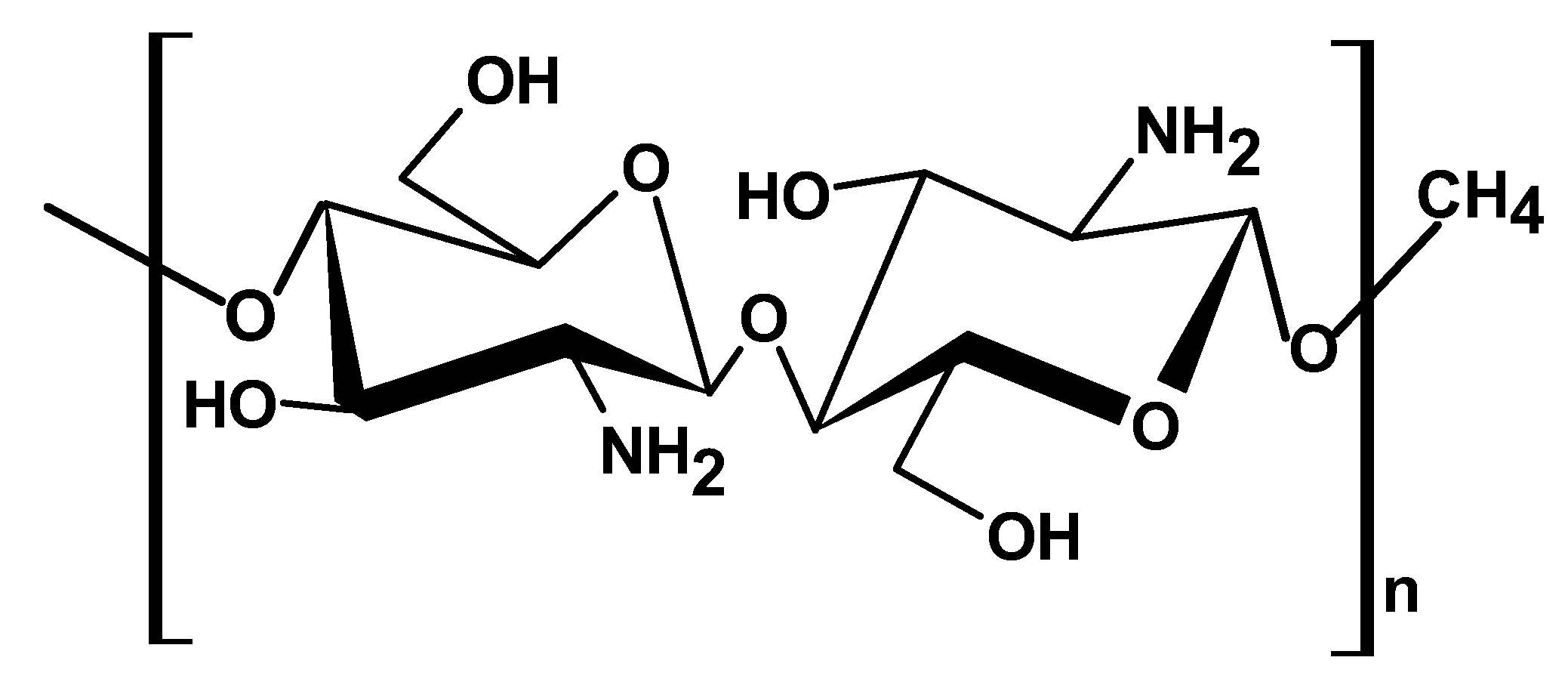
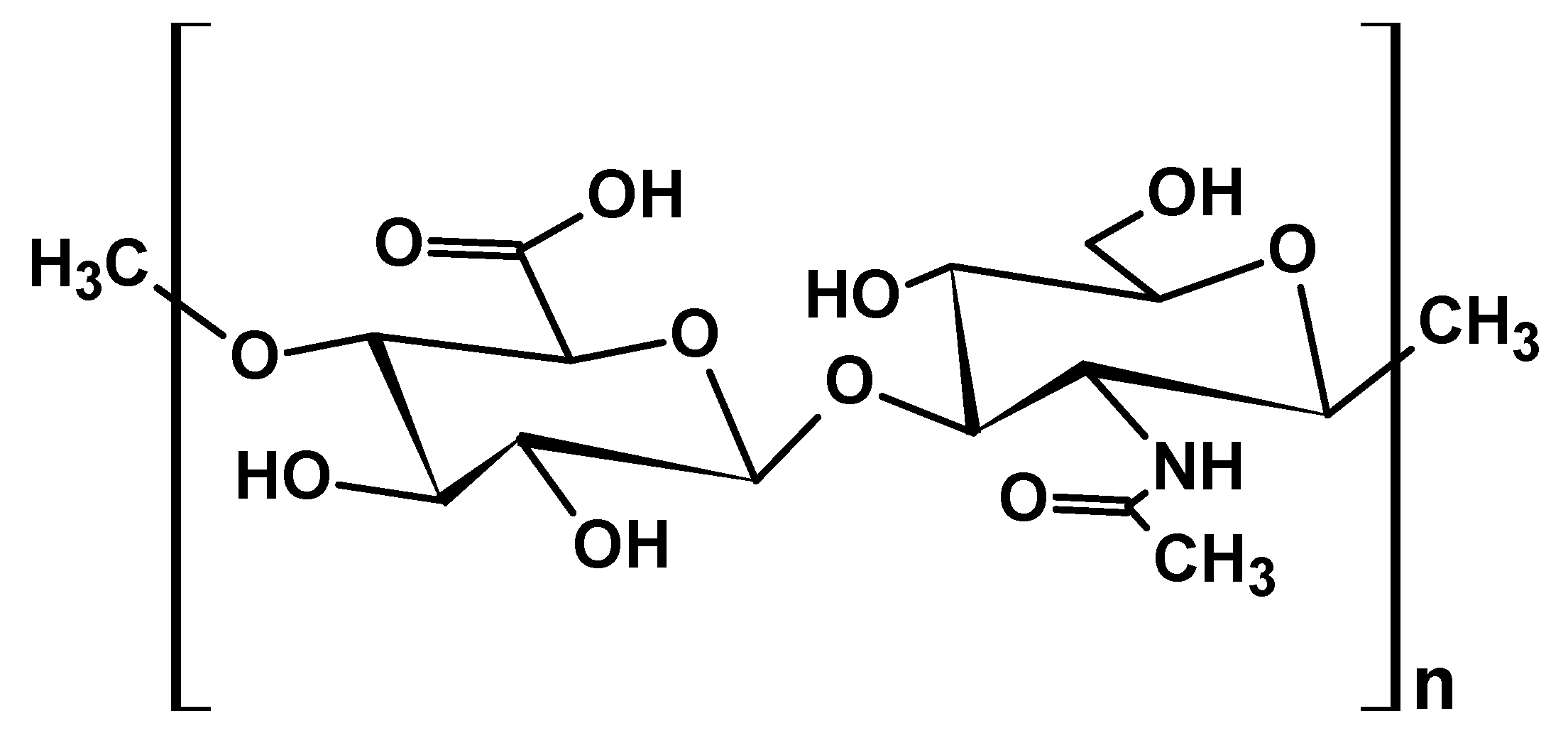
2.1.4. Chitosan
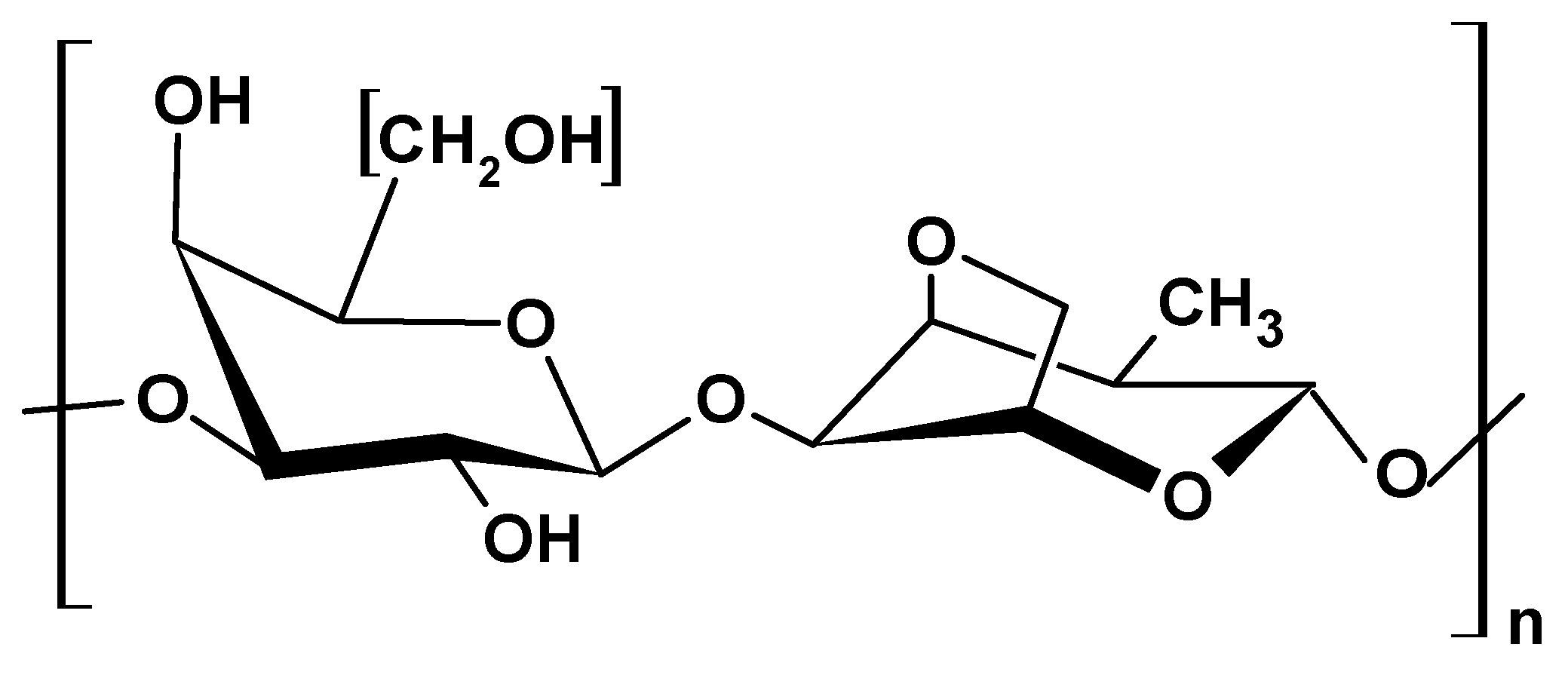
2.1.5. Gellan Gum
2.1.6. Starch
2.1.7. Dextran
2.1.8. Pullulan
2.1.9. Agarose
2.2. Protein-Based Materials
2.2.1. Collagen
2.2.2. Gelatin
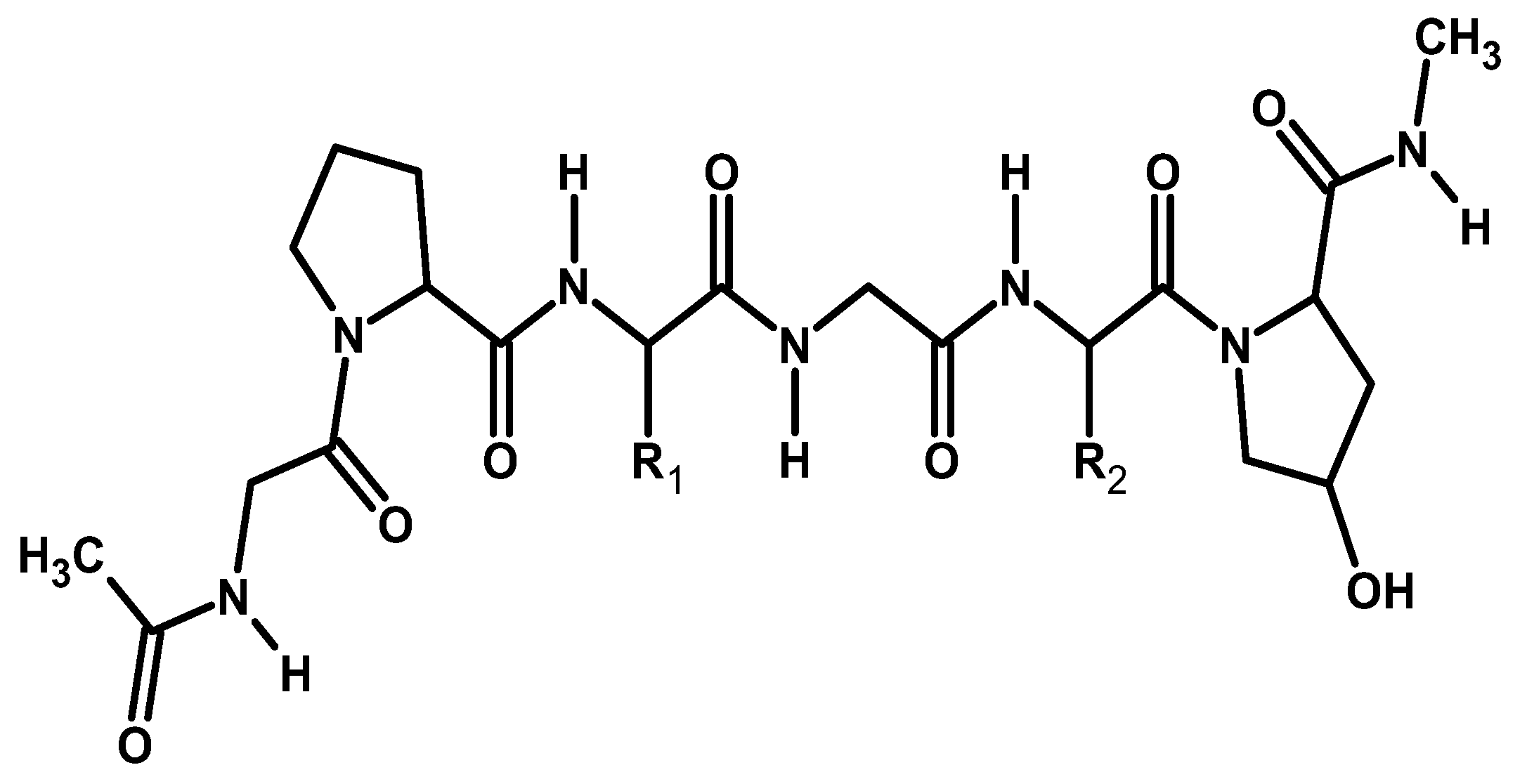
2.2.3. Silk
2.2.4. Fibrin
2.2.5. Elastin
2.2.6. Soy Protein
2.2.7. Whey Protein
2.2.8. Resilin
2.3. Other Natural-Based Materials
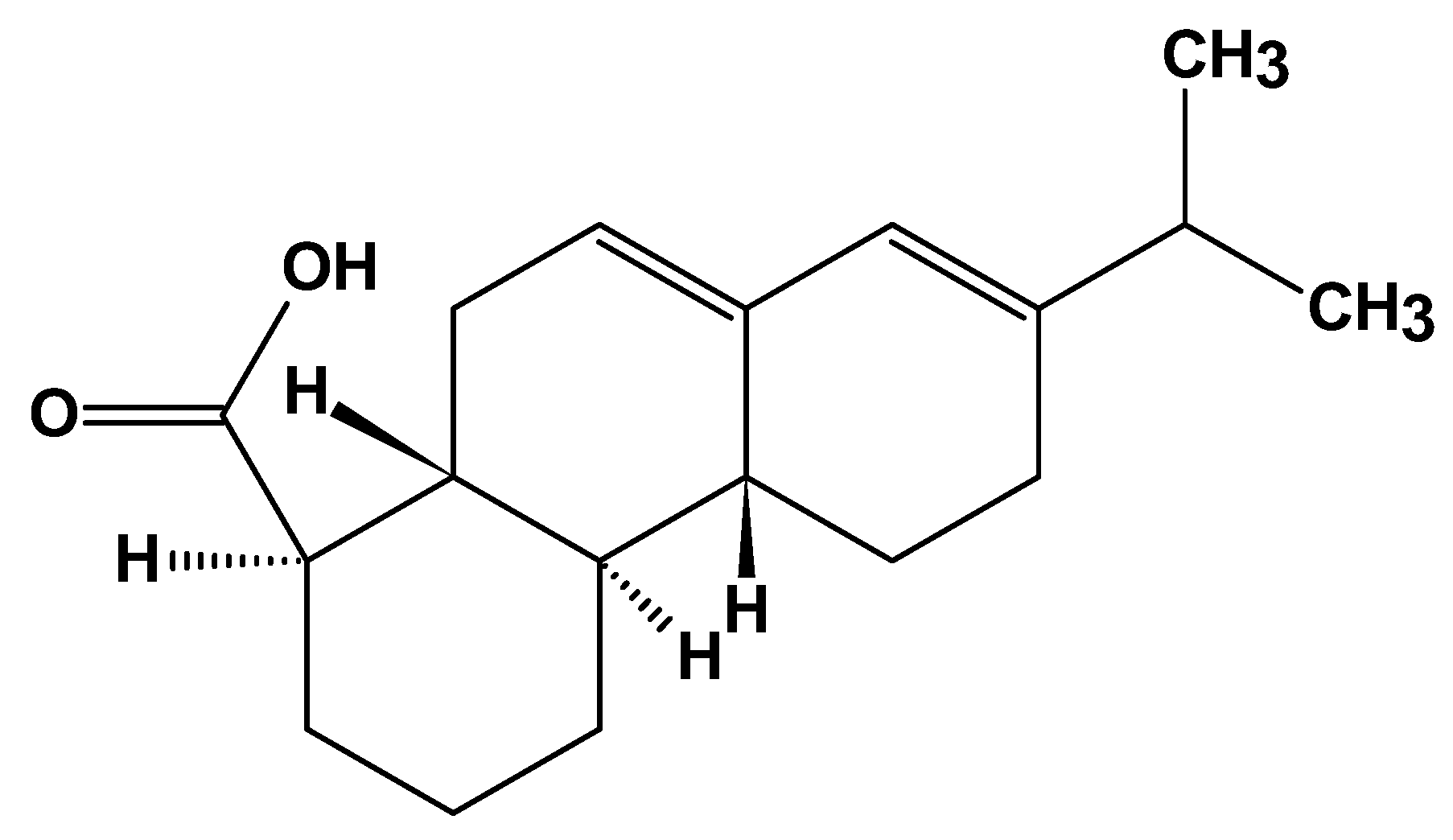
2.3.1. Polyisoprene
2.3.2. Plant Oils
2.3.3. Rosin
2.3.4. Hyaluronic Acid
2.4. Specific Types of the Functional Forms of Natural Polymer-Based Materials
2.4.1. Nanofibers
2.4.2. Nanotubes
2.4.3. Hydrogels
2.4.4. Aerogels
2.4.5. Films/Membranes
2.4.6. Three-Dimensional Porous Scaffolds
2.5. Functional Additives in Natural Polymer-Based Biomaterials
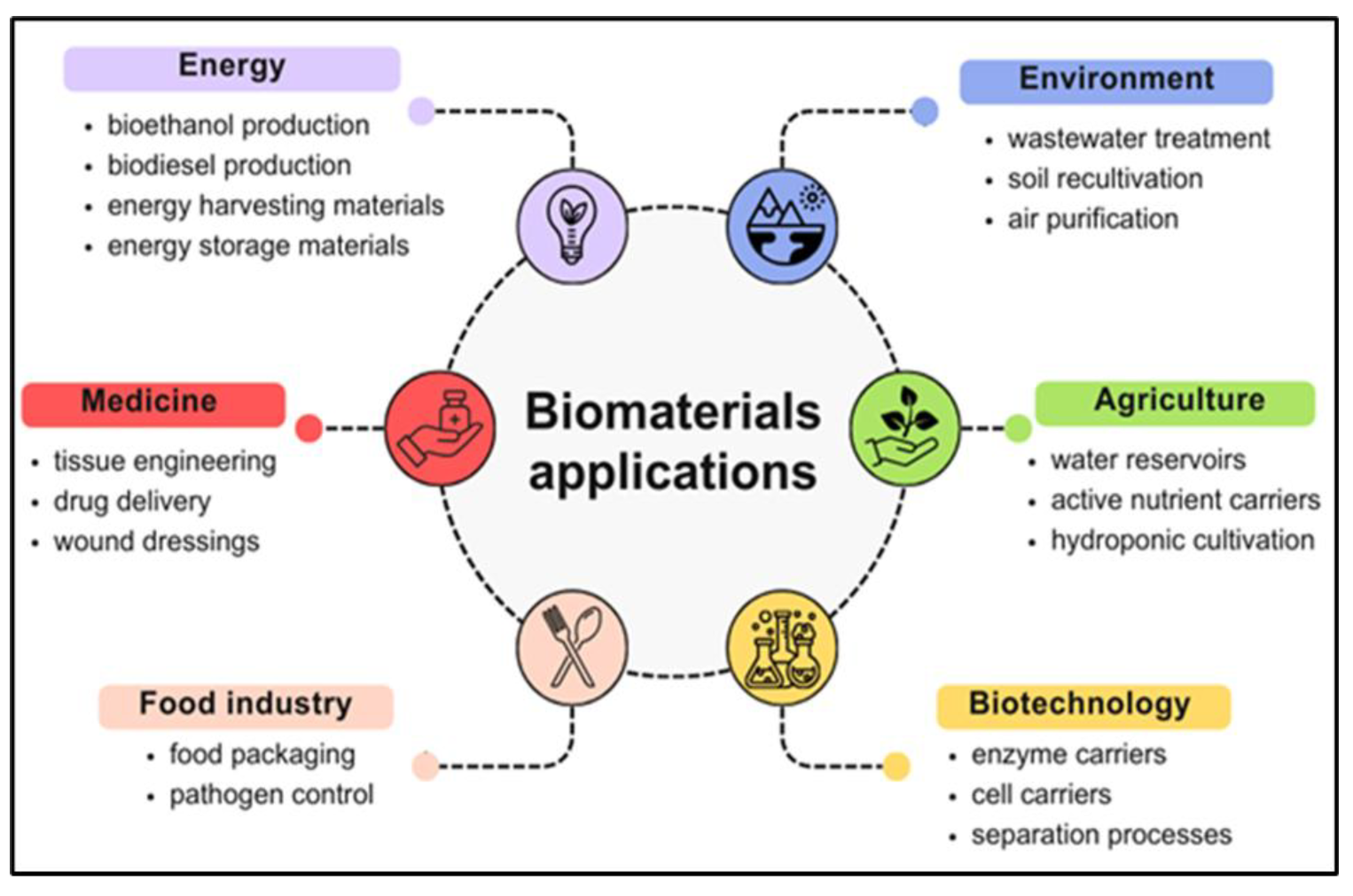
3. Innovations in the Application of Natural-Originated Polymeric Biomaterials
3.1. Energy Sector
3.2. Medical Sector
3.3. Environmental Sector
3.4. Construction Sector
3.5. Textile Sector
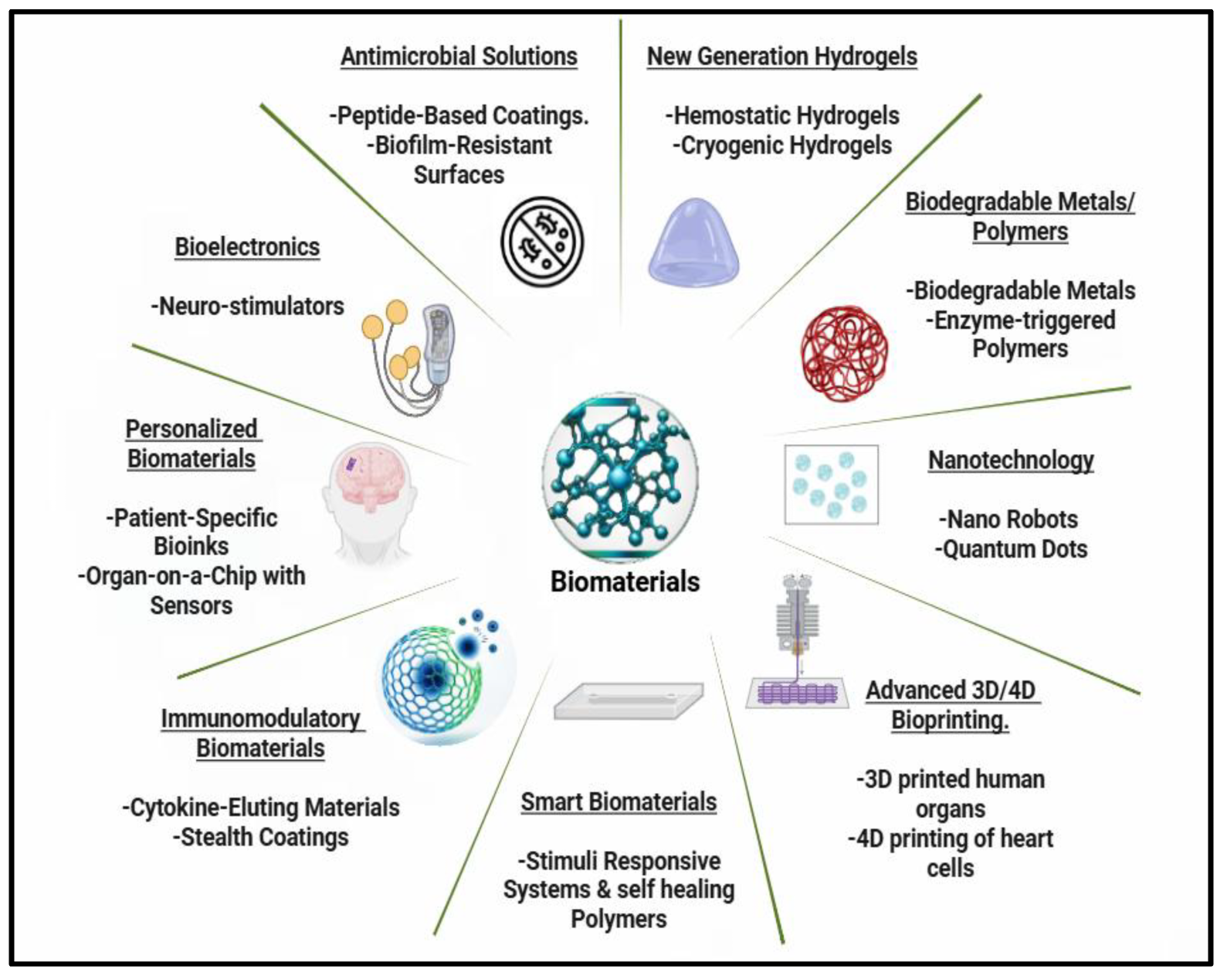
4. Future Development Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme, International Resource Panel. Global Resources Outlook 2024—Bend the Trend: Pathways to a Liveable Planet as Resource Use Spikes; United Nations Environment Programme: Nairobi, Kenya, 2024; Available online: https://wedocs.unep.org/handle/20.500.11822/44901 (accessed on 1 August 2024).
- Le, D.L.; Salomone, R.; Nguyen, Q.T. Circular bio-based building materials: A literature review of case studies and sustainability assessment methods. Build. Environ. 2023, 244, 110774. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular bioeconomy concepts—A perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Pandit, P.; Nadathur, G.T.; Maiti, S.; Regubalan, B. Functionality and properties of bio-based materials. In Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Springer: Singapore, 2018; pp. 81–103. [Google Scholar] [CrossRef]
- Fusteș-Dămoc, I.; Dinu, R.; Măluțan, T.; Mija, A. Valorisation of chitosan natural building block as a primary strategy for the development of sustainable fully bio-based epoxy resins. Polymers 2023, 15, 4627. [Google Scholar] [CrossRef] [PubMed]
- Nwabike, A.A.; Elemike, E.E.; Akpeji, H.B.; Amitaye, E.; Hossain, I.; Mbonu, J.I.; Aziza, A.E. Chitosan: A Sustainable Biobased Material for Diverse Applications. J. Environ. Chem. Eng. 2024, 12, 113208. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- Fijoł, N.; Aguilar-Sánchez, A.; Mathew, A.P. 3D-Printable Biopolymer-Based Materials for Water Treatment: A Review. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Andrew, J.J.; Dhakal, H.N. Sustainable Biobased Composites for Advanced Applications: Recent Trends and Future Opportunities—A Critical Review. Compos. Part C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Mahmud, M.Z.A.; Rabbi, S.M.F.; Islam, M.D.; Hossain, N. Synthesis and Applications of Natural Fiber-Reinforced Epoxy Composites: A Comprehensive Review. SPE Polym. 2024, 5, e12345. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Zhang, H.; Liu, Q.; Li, R.; Li, B.; Wang, J. Three-Dimensional Hierarchical and Interconnected Honeycomb-Like Porous Carbon Derived from Pomelo Peel for High Performance Supercapacitors. J. Solid State Chem. 2018, 257, 64–71. [Google Scholar] [CrossRef]
- Capêto, A.P.; Amorim, M.; Sousa, S.; Costa, J.R.; Uribe, B.; Guimarães, A.S.; Pintado, M.; Oliveira, A.L.S. Fire-Resistant Bio-Based Polyurethane Foams Designed with Two By-Products Derived from Sugarcane Fermentation Process. Waste Biomass Valorization 2024, 15, 2045–2059. [Google Scholar] [CrossRef]
- Kore, S.D.; Sudarsan, J.S. Hemp Concrete: A Sustainable Green Material for Conventional Concrete. J. Build. Mater. Sci. 2021, 3, 2. [Google Scholar] [CrossRef]
- Weiss, M.; Haufe, J.; Carus, M.; Brandão, M.; Bringezu, S.; Hermann, B.; Patel, M.K. A Review of the Environmental Impacts of Biobased Materials. J. Ind. Ecol. 2012, 16, S169–S181. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Gabryś, T.; Fryczkowska, B.; Fabia, J.; Biniaś, D. Preparation of an Active Dressing by In Situ Biosynthesis of a Bacterial Cellulose–Graphene Oxide Composite. Polymers 2022, 14, 2800. [Google Scholar] [CrossRef]
- Gupta, G.K.; De, S.; Franco, A.; Balu, A.M.; Luque, R. Sustainable Biomaterials: Current Trends, Challenges and Applications. Molecules 2016, 21, 48. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Dalwadi, S.; Goel, A.; Kapetanakis, C.; Salas-de la Cruz, D.; Hu, X. The Integration of Biopolymer-Based Materials for Energy Storage Applications: A Review. Int. J. Mol. Sci. 2023, 24, 3975. [Google Scholar] [CrossRef]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.O. Nanocellulose Derived from Agricultural Biowaste By-Products–Sustainable Synthesis, Biocompatibility, Biomedical Applications, and Future Perspectives: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Tanskul, S.; Amornthatree, K.; Jaturonlak, N. A New Cellulose-Producing Bacterium, Rhodococcus sp. MI 2: Screening and Optimization of Culture Conditions. Carbohydr. Polym. 2013, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber Dimensions, Lignin and Cellulose Content of Various Plant Materials and Their Suitability for Paper Production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; De Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering—A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Betlej, I.; Antczak, A.; Szadkowski, J.; Drożdżek, M.; Krajewski, K.; Radomski, A.; Zawadzki, J.; Borysiak, S. Evaluation of the Hydrolysis Efficiency of Bacterial Cellulose Gel Film after the Liquid Hot Water and Steam Explosion Pretreatments. Polymers 2022, 14, 2104. [Google Scholar] [CrossRef]
- Yassin, M.A.; Gad, A.A.M.; Ghanem, A.F.; Abdel Rehim, M.H. Green Synthesis of Cellulose Nanofibers Using Immobilized Cellulase. Carbohydr. Polym. 2019, 205, 255–260. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and Characterization of Cellulose Nanofibers from Rose Stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural Modification and Characterization of Bacterial Cellulose–Alginate Composite Scaffolds for Tissue Engineering. Carbohydr. Polym. 2015, 132, 146–155. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and Characterization of a Bacterial Cellulose/Chitosan Composite for Potential Biomedical Application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate Based Polyurethanes: A Review of Recent Advances and Perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.H.; De Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an Adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Gadgey, K.K.; Bahekar, A. Investigation on Uses of Crab Based Chitin and Its Derivatives. Int. J. Mech. Eng. Technol. 2017, 8, 456–466. [Google Scholar]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Alemu, D.; Getachew, E.; Mondal, A.K. Study on the Physicochemical Properties of Chitosan and Their Applications in the Biomedical Sector. Int. J. Polym. Sci. 2023, 2023, 5025341. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C. Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study. Polymers 2023, 15, 2816. [Google Scholar] [CrossRef]
- Di Liberto, E.A.; Dintcheva, N.T. Biobased Films Based on Chitosan and Microcrystalline Cellulose for Sustainable Packaging Applications. Polymers 2024, 16, 568. [Google Scholar] [CrossRef]
- Gomes, D.; Batista-Silva, J.P.; Sousa, A.; Passarinha, L.A. Progress and Opportunities in Gellan Gum-Based Materials: A Review of Preparation, Characterization and Emerging Applications. Carbohydr. Polym. 2023, 311, 120732. [Google Scholar] [CrossRef]
- Dev, M.J.; Warke, R.G.; Warke, G.M.; Mahajan, G.B.; Patil, T.A.; Singhal, R.S. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour. Technol. 2022, 359, 127498. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhu, L.; Guo, J.; Sun, Z.; Zhao, M.; Zhan, X. Enhancing biocompatibility and neuronal anti-inflammatory activity of polymyxin B through conjugation with gellan gum. Int. J. Biol. Macromol. 2020, 147, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.A.M.; Forouzandeh-Malati, M.; Hassanzadeh-Afruzi, F.; Noruzi, E.B.; Ganjali, F.; Kashtiaray, A.; Bani, M.S.; Eftekhari, R.B.; Eivazzadeh-Keihan, R.; Maleki, A. Magnetic xanthan gum-silk fibroin hydrogel: A nanocomposite for biological and hyperthermia applications. Int. J. Biol. Macromol. 2023, 253, 127005. [Google Scholar] [CrossRef]
- Das, M.; Giri, T.K. Hydrogels based on gellan gum in cell delivery and drug delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101586. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Lee, C.S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef]
- Tiwari, A. Recent Developments in Bio-Nanocomposites for Biomedical Applications; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Casettari, L.; Bonacucina, G.; Morris, G.A.; Perinelli, D.R.; Lucaioli, P.; Cespi, M.; Palmieri, G.F. Dextran and its potential use as tablet excipient. Powder Technol. 2015, 273, 125–132. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Radke, W.; Held, D.; Bock, H.; Kilz, P. Molecular Weight Determination of Dextran According to USP/EP Monograph; Agilent Technologies: Santa Clara, CA, USA, 2023; Available online: https://lcms.cz/labrulez-bucket-strapi-h3hsga3/an_wingpc_dextran_usp_ep_gpc_sec_5994_5763en_agilent_d1fd4c393f/an-wingpc-dextran-usp-ep-gpc-sec-5994-5763en-agilent.pdf (accessed on 8 August 2024).
- Kavlak, S. Effects of Molecular Weight of Dextran on Dynamic Mechanical Properties in Functional Polymer Blend Systems. Hacet. J. Biol. Chem. 2022, 50, 325–333. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, G.L.; Jia, S.L.; Chi, Z.; Hu, Z.; Chi, Z.M. Pullulan biosynthesis and its regulation in Aureobasidium spp. Carbohydr. Polym. 2021, 251, 117020. [Google Scholar] [CrossRef]
- De Souza, C.K.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Hoffmann, T.G.; Purohit, S.D.; Han, S.S. Pullulan as a sustainable biopolymer for versatile applications: A review. Mater. Today Commun. 2023, 36, 105469. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid. Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 55–60. [Google Scholar] [CrossRef]
- Thangavel, P.; Vilvanathan, S.P.; Kuttalam, I.; Lonchin, S. Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int. J. Biol. Macromol. 2020, 149, 395–403. [Google Scholar] [CrossRef]
- Autissier, A.; Letourneur, D.; Le Visage, C. Pullulan-based hydrogel for smooth muscle cell culture. J. Biomed. Mater. Res. A 2007, 82, 336–342. [Google Scholar] [CrossRef]
- Morimoto, N.; Endo, T.; Iwasaki, Y.; Akiyoshi, K. Design of hybrid hydrogels with self-assembled nanogels as cross-linkers: Interaction with proteins and chaperone-like activity. Biomacromolecules 2005, 6, 1829–1834. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Control. Release 2011, 149, 209–224. [Google Scholar] [CrossRef]
- Nilsson, K.; Scheirer, W.; Merten, O.W.; Östberg, L.; Liehl, E.; Katinger, H.W.D.; Mosbach, K. Entrapment of animal cells for production of monoclonal antibodies and other biomolecules. Nature 1983, 302, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Karoubi, G.; Ormiston, M.L.; Stewart, D.J.; Courtman, D.W. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials 2009, 30, 5445–5455. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Chen, Q.; Pei, Y.; Tang, K.; Albu-Kaya, M.G. Structure, Extraction, Processing, and Applications of Collagen as an Ideal Component for Biomaterials—A Review. Collagen Leather 2023, 5, 20. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Zou, Y.; Lu, G.; Ronca, A.; D’Amora, U.; Chen, F.; Chen, Z.; Lin, H.; Fan, Y. Advanced Application of Collagen-Based Biomaterials in Tissue Repair and Restoration. J. Leather Sci. Eng. 2022, 4, 15. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Valcarcel, J.; Fraguas, J.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Vázquez, J.A. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Mar. Drugs 2021, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-Based Biomaterials and Gelatin as an Additive for Chronic Wound Repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-Based Adhesive Hydrogel with Self-Healing, Hemostasis, and Electrical Conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef]
- Chaudhary, J.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, V.K. Hydrogel of Gelatin in the Presence of Graphite for the Adsorption of Dye: Towards the Concept for Water Purification. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Marciano, J.S.; Ferreira, R.R.; de Souza, A.G.; Barbosa, R.F.S.; de Moura Junior, A.J.; Rosa, D.S. Biodegradable Gelatin Composite Hydrogels Filled with Cellulose for Chromium (VI) Adsorption from Contaminated Water. Int. J. Biol. Macromol. 2021, 181, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sahi, A.; Gundu, S.; Kumari, P.; Klepka, T.; Sionkowska, A. Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications. Biomimetics 2023, 8, 55. [Google Scholar] [CrossRef]
- Le, B.H.T.; Minh, T.; Nguyen, D.; Minh, D. Naturally Derived Biomaterials: Preparation and Application. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Saikia, S.; Saikia, M. Physical and Chemical Properties of Indian Silk Fibres. Pharma Innov. J. 2022, 11, 732–738. [Google Scholar]
- Gamboa-Martínez, T.C.; Gómez Ribelles, J.L.; Gallego Ferrer, G. Fibrin Coating on Poly (L-lactide) Scaffolds for Tissue Engineering. J. Bioact. Compat. Polym. 2011, 26, 464–477. [Google Scholar] [CrossRef]
- Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 14789. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Barker, T.H. Fibrin-Based Biomaterials: Modulation of Macroscopic Properties through Rational Design at the Molecular Level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef]
- Wein, S.; Schemmer, C.; Al Enezy-Ulbrich, M.A.; Jung, S.A.; Rütten, S.; Kühnel, M.; Jonigk, D.; Jahnen-Dechent, W.; Pich, A.; Neuss, S. Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments. Gels 2024, 10, 182. [Google Scholar] [CrossRef]
- Bencherif, S.A. Fibrin: An Underrated Biopolymer for Skin Tissue Engineering. J. Regen. Med. 2017, 6, 1–3. [Google Scholar]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Prado-Audelo, M.L.; Mendoza-Muñoz, N.; Escutia-Guadarrama, L.; Giraldo-Gomez, D.M.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gomez, G. Recent Advances in Elastin-Based Biomaterials. J. Pharm. Pharm. Sci. 2020, 23, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Meng, X.; Guo, Z. Elastin Structure, Synthesis, Regulatory Mechanism and Relationship with Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 596622. [Google Scholar] [CrossRef] [PubMed]
- Tarakanova, A.; Chang, S.W.; Buehler, M.J. Computational Materials Science of Bionanomaterials: Structure, Mechanical Properties and Applications of Elastin and Collagen Proteins. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 941–962. [Google Scholar] [CrossRef]
- Moscarelli, P.; Boraldi, F.; Bochicchio, B.; Pepe, A.; Salvi, A.M.; Quaglino, D. Structural Characterization and Biological Properties of the Amyloidogenic Elastin-Like Peptide (VGGVG)3. Matrix Biol. 2014, 36, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, S.; Jing, D.; Liu, N.; Luo, X. The Construction of Elastin-Like Polypeptides and Their Applications in Drug Delivery System and Tissue Repair. J. Nanobiotechnology 2023, 21, 12. [Google Scholar] [CrossRef]
- Tansaz, S.; Boccaccini, A.R. Biomedical Applications of Soy Protein: A Brief Overview. J. Biomed. Mater. Res. A 2016, 104, 553–569. [Google Scholar] [CrossRef]
- Chien, K.B.; Aguado, B.A.; Bryce, P.J.; Shah, R.N. In Vivo Acute and Humoral Response to Three-Dimensional Porous Soy Protein Scaffolds. Acta Biomater. 2013, 9, 8983–8990. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, M.; Dai, S.; Song, J.; Ni, X.; Fang, Y.; Corke, H.; Jiang, F. Interactions Between Carboxymethyl Konjac Glucomannan and Soy Protein Isolate in Blended Films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Shankar, A.; Seyam, A.F.M.; Hudson, S.M. Electrospinning of Soy Protein Fibers and Their Compatibility with Synthetic Polymers. J. Text. Appar. Technol. Manag. 2013, 8, 1–14. [Google Scholar]
- Peles, Z.; Binderman, I.; Berdicevsky, I.; Zilberman, M. Soy Protein Films for Wound-Healing Applications: Antibiotic Release, Bacterial Inhibition and Cellular Response. J. Tissue Eng. Regen. Med. 2013, 7, 401–412. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, J.; Fei, X.; Shao, Z.; Chen, X. An Antimicrobial Film by Embedding In Situ Synthesized Silver Nanoparticles in Soy Protein Isolate. Mater. Lett. 2013, 95, 142–144. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Yolou, F.S.; Sarr, S.O.; Atheba, P.G.; Amin, C.N.; Ake, M. Whey Proteins Analysis in Aqueous Medium and in Artificial Gastric and Intestinal Fluids. Int. J. Biol. Chem. Sci. 2012, 6, 1828–1837. [Google Scholar] [CrossRef]
- Tang, C.; Xi, T.; Zheng, J.; Cui, X. Chemical Properties of Whey Protein in Protein Powders and Its Impact on Muscle Growth in Athletes: A Review. Nat. Prod. Commun. 2025, 20, 1–17. [Google Scholar] [CrossRef]
- Soumati, B.; Atmani, M.; Benabderrahmane, A.; Benjelloun, M. Whey Valorization—Innovative Strategies for Sustainable Development and Value-Added Product Creation. J. Ecol. Eng. 2023, 24, 86–104. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Kumar, H.; Kumar, N.; Ranvir, S.; Jana, A.; Buttar, H.S.; Telessy, I.G.; Awuchi, C.G.; Okpala, C.O.R.; Korzeniowska, M.; et al. Whey Proteins Processing and Emergent Derivatives: An Insight Perspective from Constituents, Bioactivities, Functionalities to Therapeutic Applications. J. Funct. Foods 2021, 87, 104760. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and Whey Proteins—From “Gutter-to-Gold”. Int. Dairy. J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Pillai, A.T.; Morya, S.; Kasankala, L.M. Emerging Trends in Bioavailability and Pharma-Nutraceutical Potential of Whey Bioactives. J. Nutr. Metab. 2024, 2024, 8455666. [Google Scholar] [CrossRef]
- Goyal, C.; Dhyani, P.; Rai, D.C.; Tyagi, S.; Dhull, S.B.; Sadh, P.K.; Duhan, J.S.; Saharan, B.S. Emerging Trends and Advancements in the Processing of Dairy Whey for Sustainable Biorefining. J. Food Process. Preserv. 2023, 2023, 6626513. [Google Scholar] [CrossRef]
- Li, L.; Kiick, K.L. Resilin-Based Materials for Biomedical Applications. ACS Macro Lett. 2013, 2, 635–640. [Google Scholar] [CrossRef]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous Protein-Based Hydrogels for Cell Encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Su, R.S.C.; Kim, Y.; Liu, J.C. Resilin: Protein-Based Elastomeric Biomaterials. Acta Biomater. 2014, 10, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, T. Estimation of C-MGARCH Models Based on the MBP Method. Stat. Probab. Lett. 2013, 83, 665–673. [Google Scholar] [CrossRef]
- Renner, J.N.; Cherry, K.M.; Su, R.S.C.; Liu, J.C. Characterization of Resilin-Based Materials for Tissue Engineering Applications. Biomacromolecules 2012, 13, 3678–3685. [Google Scholar] [CrossRef]
- Truong, M.Y.; Dutta, N.K.; Choudhury, N.R.; Kim, M.; Elvin, C.M.; Hill, A.J.; Thierry, B.; Vasilev, K. A pH-Responsive Interface Derived from Resilin-Mimetic Protein Rec1-Resilin. Biomaterials 2010, 31, 4434–4446. [Google Scholar] [CrossRef]
- Salehi, M.; Cornish, K.; Bahmankar, M.; Naghavi, M.R. Natural Rubber-Producing Sources, Systems, and Perspectives for Breeding and Biotechnology Studies of Taraxacum kok-saghyz. Ind. Crops Prod. 2021, 170, 113747. [Google Scholar] [CrossRef]
- Nurchi, C.; Buonvino, S.; Arciero, I.; Melino, S. Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 12345. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.S.; Yoo, J.E.; Shin, M.S.; Yamabe, N.; Kim, S.-N.; Lee, S.; Kim, K.H.; Lee, H.-J.; et al. Abietic Acid Isolated from Pine Resin (Resina Pini) Enhances Angiogenesis in HUVECs and Accelerates Cutaneous Wound Healing in Mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y. Rosin: A Comprehensive Review on Traditional Uses, Phytochemistry, and Pharmacology. Fitoterapia 2024, 177, 105321. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical Properties and Application of Hyaluronic Acid: A Systematic Review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Lovely, B.; Kim, Y.T.; Huang, H.; Zink-Sharp, A.; Roman, M. Impacts of Cycles of a Novel Low-Pressure Homogenization Process on Cellulose Nanofibrils (CNF) as a Sustainable Packaging Film Material. Carbohydr. Polym. Technol. Appl. 2025, 9, 100739. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Zhang, Z.; Hou, T.; Xu, J.; Wang, Y.; Ye, H.; Yang, B. Bio-Based and Bio-Degradable Nanofiber Materials: A Sustainable Platform for Energy, Environmental, and Biomedical Applications. Chem. Eng. J. 2024, 491, 152105. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as New-Generation Materials: From Spinning and Nano-Spinning Fabrication Techniques to Emerging Applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Wu, Y.; Bai, F.; Wang, H.; Si, S.; Lu, Y.; Li, X.; Wang, S. Preparation of Cellulose Nanofibers from Bagasse by Phosphoric Acid and Hydrogen Peroxide Enables Fibrillation via a Swelling, Hydrolysis, and Oxidation Cooperative Mechanism. Nanomaterials 2020, 10, 2227. [Google Scholar] [CrossRef]
- Gao, H.; Liu, G.; Guan, J.; Wang, X.; Yu, J.; Ding, B. Biodegradable Hydro-Charging Polylactic Acid Melt-Blown Nonwovens with Efficient PM0.3 Removal. Chem. Eng. J. 2023, 458, 141412. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.C.; Wang, F.; Liu, C.; Mei, C. 3D Printing of Cellulose Nanofiber Monoliths for Thermal Insulation and Energy Storage Applications. Addit. Manuf. 2022, 59, 103124. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298. [Google Scholar] [CrossRef]
- MKR, K.; MN, H.; AB, S.; HA, B. A Study on Microfluidic Spinning Technology (MST) Used for Micro Fibre Fabrication. Adv. Res. Text. Eng. 2021, 6, 1065. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an Intelligent Film Based on Chitosan/Oxidized Chitin Nanocrystals Incorporating Black Rice Bran Anthocyanins for Seafood Spoilage Monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Fan, Y.; Yu, J. Direct Ink Writing of a Bio-Based Ink Made of Low Concentration Cellulose Nanofiber Crosslinked with Poly (Ethylene Glycol) via Hydroxyl-Yne Click Chemistry. Int. J. Biol. Macromol. 2025, 306, 141267. [Google Scholar] [CrossRef]
- Audette, G.F.; Yaseen, A.; Bragagnolo, N.; Bawa, R. Protein Nanotubes: From Bionanotech towards Medical Applications. Biomedicines 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Shi, X.; Li, J.; Ma, Y.; Wang, Y.; Li, T.; Zhang, J.; Zhao, R.; Yu, Z.; et al. A SiRNA-Induced Peptide Co-Assembly System as a Peptide-Based SiRNA Nanocarrier for Cancer Therapy. Mater. Horiz. 2018, 5, 745–752. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Ji, Z.; Sheng, H.; Liu, H. Planting Multiwalled Carbon Nanotubes onto Epoxidized Soybean Oil-Based Vitrimer to Construct a Biobased Photothermal Superhydrophobic Coating with Self-Healing and Closed-Loop Recyclability for Anti/Deicing. ACS Sustain. Chem. Eng. 2024, 12, 7147–7157. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-Based Hydrogel Beads: Preparations, Modifications and Applications in Food and Agriculture Sectors—A Review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, R.; Chahal, G.K. Synthesis of Lignin-Based Hydrogels and Their Applications in Agriculture: A Review. Chem. Pap. 2021, 75, 4465–4478. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Ramírez Martínez, C. Synthesis and Characterization of a Natural-Based Hydrogel for Biomedical Applications A Dissertation Presented By; Instituto Tecnológico y de Estudios Superiores de Monterrey: Monterey City, Mexico, 2022. [Google Scholar]
- Rajanna, G.A.; Manna, S.; Singh, A.; Babu, S.; Singh, V.K.; Dass, A.; Chakraborty, D.; Patanjali, N.; Chopra, I.; Banerjee, T.; et al. Biopolymeric Superabsorbent Hydrogels Enhance Crop and Water Productivity of Soybean–Wheat System in Indo-Gangetic Plains of India. Sci. Rep. 2022, 12, 11955. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhao, Y.; Lei, C.; Wang, Y.; Wu, K.; Yu, G. From Scraps to Sips: Biomass-Based Hydrogel Pulls Drinking Water from Thin Air. Available online: https://phys.org/news/2025-02-scraps-biomass-based-hydrogel-thin.html (accessed on 30 May 2025).
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Mandić, V.; Bafti, A.; Panžić, I.; Radovanović-Perić, F. Bio-Based Aerogels in Energy Storage Systems. Gels 2024, 10, 438. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of Supercritical Fluid Technology for the Production of Tailor-Made Aerogel Particles for Delivery Systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Sun, Y.; Lu, Y.; Chen, S.; Wang, B.; Zhang, H.; Xia, Y.; Yang, D. Turning Gelidium Amansii Residue into Nitrogen-Doped Carbon Nanofiber Aerogel for Enhanced Multiple Energy Storage. Carbon 2018, 137, 31–40. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Krause Bierhalz, A.C. Biopolymer Films and Composite Coatings: Applications. In Handbook of Biopolymers; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 1229–1261. ISBN 9789811907104. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Y.; Chen, L.; Zhang, H.; An, C.; Li, C.; Wang, Q.; Song, J.; He, W.; Wang, H. Glycine/Alginate-Based Piezoelectric Film Consisting of a Single, Monolithic β-Glycine Spherulite towards Flexible and Biodegradable Force Sensor. Regen. Biomater. 2024, 11, rbae047. [Google Scholar] [CrossRef]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and Its Derivatives for Lithium Ion Battery Separators: A Review on the Processing Methods and Properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Xu, S.; Yue, Z.; Zhang, Z.; Chen, M.; Zhou, H.; Jin, W. Pressure-Assisted Vacuum Filtration Fabrication of Polymer Membranes with Enhanced Selectivity for the Separation of an Ethanol/Carbon Dioxide Mixture. J. Memb. Sci. 2024, 701, 122765. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Prazeres Mazur, L.; Reis Ferreira, R.; Felix da Silva Barbosa, R.; Henrique Santos, P.; Barcelos da Costa, T.; Gurgel Adeodato Vieira, M.; da Silva, A.; dos Santos Rosa, D.; Helena Innocentini Mei, L. Development of Novel Biopolymer Membranes by Electrospinning as Potential Adsorbents for Toxic Metal Ions Removal from Aqueous Solution. J. Mol. Liq. 2024, 395, 123782. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Sayeb, S.; Ounif, I.; Ferhi, M.; Karima, H.N.; Ennigrou, D.J. Preparation and Characterization of Acetate Cellulose Electrospun Nanofibers Membrane: Potential Application on Wastewater Treatment. Heliyon 2024, 10, e32552. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Ebrahimi, B.; Awlqadr, F.H.; Ov, N.S.; Ebdali, H.; Sarlak, Z.; Bahrami, L.; Mohammadi, R. Gelatin-Chitosan Based Composite Films Enriched with Satureja Kermanshahensis Jamzad Essential Oil Nanoemulsion and Carbon Dots: Characterization and Functional Properties. J. Polym. Environ. 2024, 33, 1292–1307. [Google Scholar] [CrossRef]
- Hastuti, N.; Setiawan, H.; Kanomata, K.; Kitaoka, T. Cellulose nanofibers of oil palm biomass in alginate-based membranes for water-ethanol mixture separation. Cellul. Chem. Technol. 2022, 56, 737–747. [Google Scholar] [CrossRef]
- De Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-Based Polymer Electrolyte Membranes for Sustainable Aqueous Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560. [Google Scholar] [CrossRef]
- Yodsanga, S.; Poeaim, S.; Chantarangsu, S.; Swasdison, S. Investigation of Biodegradation and Biocompatibility of Chitosan–Bacterial Cellulose Composite Scaffold for Bone Tissue Engineering Applications. Cells 2025, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Aldhaher, A.; Shahabipour, F.; Shaito, A.; Al-Assaf, S.; Elnour, A.A.M.; Sallam, E.B.; Teimourtash, S.; Elfadil, A.A. 3D Hydrogel/Bioactive Glass Scaffolds in Bone Tissue Engineering: Status and Future Opportunities. Heliyon 2023, 9, e17050. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, H.; Kong, Q.; Feng, X.; Li, Z.; Xue, C. Food-Grade 3D-Printable Porous Scaffolds with Advanced Stem Cell Microenvironments Enabled by Bilayer Emulgel. Food Hydrocoll. 2025, 166, 111373. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Biopolymer Based Three-Dimensional Biomimetic Micro/Nanofibers Scaffolds with Porous Structures via Tailored Charge Repulsions for Skin Tissue Regeneration. Polym. Adv. Technol. 2021, 32, 3535–3548. [Google Scholar] [CrossRef]
- Koumentakou, I.; Michopoulou, A.; Noordam, M.J.; Terzopoulou, Z.; Bikiaris, D.N. 3D-Printed Scaffolds for Tissue Engineering Applications Using Thermosensitive Hydrogels Based on Biopolymer Blends. J. Mater. Sci. 2024, 59, 9021–9041. [Google Scholar] [CrossRef]
- Spessot, E.; Passuello, S.; Shah, L.V.; Maniglio, D.; Motta, A. Nanocomposite Methacrylated Silk Fibroin-Based Scaffolds for Bone Tissue Engineering. Biomimetics 2024, 9, 218. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kungumaraj, K.K.; Mani, D.; Mani, P. Chitosan/Guar Gum—Graphene Oxide Porous Scaffolds for Tissue Engineering Applications. Mater. Today Commun. 2024, 41, 110288. [Google Scholar] [CrossRef]
- Beltran-Vargas, N.E.; Peña-Mercado, E.; Sánchez-Gómez, C.; Garcia-Lorenzana, M.; Ruiz, J.C.; Arroyo-Maya, I.; Huerta-Yepez, S.; Campos-Terán, J. Sodium Alginate/Chitosan Scaffolds for Cardiac Tissue Engineering: The Influence of Its Three-Dimensional Material Preparation and the Use of Gold Nanoparticles. Polymers 2022, 14, 3233. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Polkowska, I.; Małek, M.; Kluczyński, J.; Paździor-Czapula, K.; Wekwejt, M.; Michno, A.; Ronowska, A.; Pałubicka, A.; Nowicka, B.; et al. The Characterization of Collagen-Based Scaffolds Modified with Phenolic Acids for Tissue Engineering Application. Sci. Rep. 2023, 13, 9966. [Google Scholar] [CrossRef]
- Makar, L.E.; Nady, N.; Shawky, N.; Kandil, S.H. Genipin versus Ferric Chloride Cross-Linked Unmodified Gum Arabic/Chitosan/Nano-Hydroxyapatite Nanocomposite Hydrogels as Potential Scaffolds for Bone Regeneration. Sci. Rep. 2023, 13, 14402. [Google Scholar] [CrossRef]
- Petit, N.; Gomes, A.; Chang, Y.Y.J.; Da Silva, J.; Leal, E.C.; Carvalho, E.; Gomes, P.; Browne, S.; Browne, S.; Browne, S. Development of a Bioactive Hyaluronic Acid Hydrogel Functionalised with Antimicrobial Peptides for the Treatment of Chronic Wounds. Biomater. Sci. 2025. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Guo, B.; Wu, D.; Yang, F.; Ding, Y. Advances of Natural Hydrogel-Based Vascularization Strategies for Soft Tissue Repair. Front. Mater. 2024, 11, 1446035. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Parkstam, A.; Karlsson, A.; Quakkelaar, L.; Askari, M.; Ahmed, S.S.; Eriksson, T.; Salazar Alvarez, G. Exploration of Bioactive Additives for Hyaluronan Based Hydrogels A Literature Study in Collaboration with Galderma; MAT-VET 22005; Uppsala University: Uppsala, Sweden, 2022. [Google Scholar]
- Peng, N.; Huang, D.; Gong, C.; Wang, Y.; Zhou, J.; Chang, C. Controlled Arrangement of Nanocellulose in Polymeric Matrix: From Reinforcement to Functionality. ACS Nano 2020, 14, 16169–16179. [Google Scholar] [CrossRef]
- Erezuma, I.; Eufrasio-da-Silva, T.; Golafshan, N.; Deo, K.; Mishra, Y.K.; Castilho, M.; Gaharwar, A.K.; Leeuwenburgh, S.; Dolatshahi-Pirouz, A.; Orive, G. Nanoclay Reinforced Biomaterials for Mending Musculoskeletal Tissue Disorders. Adv. Healthc. Mater. 2021, 10, 2100217. [Google Scholar] [CrossRef]
- Lagarón, J.M.; López-Rubio, A.; José Fabra, M. Bio-Based Packaging. J. Appl. Polym. Sci. 2016, 133, 2. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Bengoechea, C.; Pérez, E.; Guerrero, A. Effect of Different Polyols as Plasticizers in Soy Based Bioplastics. Ind. Crops Prod. 2020, 153, 112522. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Islam, M.S.; Nassar, M.; Elsayed, M.A.; Jameel, D.B.; Ahmad, T.T.; Rahman, M.M. In Vitro Optical and Physical Stability of Resin Composite Materials with Different Filler Characteristics. Polymers 2023, 15, 2121. [Google Scholar] [CrossRef]
- Gayathri, V.; Khan, T.; Gowtham, M.; Balan, R.; Sebaey, T.A. Functionalized Conductive Polymer Composites for Tissue Engineering and Biomedical Applications- a Mini Review. Front. Bioeng. Biotechnol. 2025, 13, 1533944. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jiang, Z.; Wu, T. The Development and Application of Contemporary Phosphorus Flame Retardants: A Review. Front. Mater. 2025, 12, 1508000. [Google Scholar] [CrossRef]
- Guo, Y.; Kleemann, J.; Sijbesma, R.P.; Tomović, Ž. Phosphorus-Containing Polyisocyanurate Elastomers for Flame Retardant Application. ACS Appl. Polym. Mater. 2023, 5, 4689–4697. [Google Scholar] [CrossRef]
- Arnold, W.A.; Blum, A.; Branyan, J.; Bruton, T.A.; Carignan, C.C.; Cortopassi, G.; Datta, S.; Dewitt, J.; Doherty, A.C.; Halden, R.U.; et al. Quaternary Ammonium Compounds: A Chemical Class of Emerging Concern. Environ. Sci. Technol. 2023, 57, 7645–7665. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, S.H.; Lee, S.; Bharadwaj, A.V.S.L.S.; Lee, Y.S.; Yoo, C.G.; Kim, T.H. A Review of Biomass-Derived UV-Shielding Materials for Bio-Composites. Energies 2023, 16, 2311. [Google Scholar] [CrossRef]
- Costa, T.; Sampaio-Marques, B.; Neves, N.M.; Aguilar, H.; Fraga, A.G. Antimicrobial Properties of Hindered Amine Light Stabilizers in Polymer Coating Materials and Their Mechanism of Action. Front. Bioeng. Biotechnol. 2024, 12, 1390513. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Uthman, T.O. A Review of History, Properties, Classification, Applications and Challenges of Natural and Synthetic Dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef]
- Navarro-Segarra, M.; Tortosa, C.; Ruiz-Díez, C.; Desmaële, D.; Gea, T.; Barrena, R.; Sabaté, N.; Esquivel, J.P. A Plant-Like Battery: A Biodegradable Power Source Ecodesigned for Precision Agriculture. Energy Environ. Sci. 2022, 15, 2900–2915. [Google Scholar] [CrossRef]
- Huang, X.; Hou, H.; Yu, B.; Bai, J.; Guan, Y.; Wang, L.; Chen, K.; Wang, X.; Sun, P.; Deng, Y.; et al. Fully Biodegradable and Long-Term Operational Primary Zinc Batteries as Power Sources for Electronic Medicine. ACS Nano 2023, 17, 5727–5739. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Xiao, M.; Wang, S.; Huang, S.; Han, D.; Meng, Y. Polymeric Binders Used in Lithium Ion Batteries: Actualities, Strategies and Trends. ChemElectroChem 2024, 11, e202400123. [Google Scholar] [CrossRef]
- Huang, S.; Yang, D.; Zhang, W.; Qiu, X.; Li, Q.; Li, C. Dual-Templated Synthesis of Mesoporous Lignin-Derived Honeycomb-Like Porous Carbon/SiO2 Composites for High-Performance Li-Ion Battery. Microporous Mesoporous Mater. 2021, 317, 111004. [Google Scholar] [CrossRef]
- Lahtinen, P. Berry Extracts and Nanocellulose as Bioactive Skin Sprays and Surgical Dressings to Prevent the Growth of Dangerous Microbes|VTT Finland. VTT Research. 2024. Available online: https://www.vttresearch.com/en/news-and-ideas/berry-extracts-and-nanocellulose-bioactive-skin-sprays-and-surgical-dressings (accessed on 30 August 2024).
- Bioretec Ltd. Renewing Orthopedics, ActivTM. Bioretec. 2024. Available online: https://bioretec.com/products/activa (accessed on 30 August 2024).
- Emulate Inc. Culture of Innovation Leveraging Human Biology + Technology to Ignite a New Era in Human Health. Emulate Bio. 2025. Available online: https://emulatebio.com/about/ (accessed on 10 September 2024).
- Chen, J.; Zhao, C.; Liu, H.; Wang, Z.; Ma, L.; Zhang, J.; Xu, N.; Hu, K.; Duan, L. Integrated Micro/Nano Drug Delivery System Based on Magnetically Responsive Phase-Change Droplets for Ultrasound Theranostics. Front. Bioeng. Biotechnol. 2024, 12, 1323056. [Google Scholar] [CrossRef] [PubMed]
- Fouly, A.; Daoush, W.M.; Elqady, H.I.; Abdo, H.S. Fabrication of PMMA Nanocomposite Biomaterials Reinforced by Cellulose Nanocrystals Extracted from Rice Husk for Dental Applications. Friction 2024, 12, 2808–2825. [Google Scholar] [CrossRef]
- SmartReactors Inc. Cell Membrane: Mimicking the Human Lung. SmartReactors. 2025. Available online: https://www.smartreactors.com/cellmembrane (accessed on 13 September 2024).
- Synakis Corp. Innovative Biomaterial-Based Treatments for Ocular Injuries and Diseases. Synakis. 2024. Available online: https://www.ventureradar.com/organisation/Synakis/6667d61a-8eed-4f55-9b61-b60b62d6b543 (accessed on 13 September 2024).
- Lin, X.; Zhang, D.; Chen, X.; Liu, M. Self-Cross-Linked Oxidized Sodium Alginate/Gelatin/Halloysite Hydrogel as Injectable, Adhesive, Antibacterial Dressing for Hemostasis. ACS Sustain. Chem. Eng. 2024, 12, 11444–11840. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Luo, R.; Li, L.; Zheng, T.; Huang, K.; Qin, Y.; Yang, X.; Zhang, X.; Wang, Y. A Drug-Free Cardiovascular Stent Functionalized with Tailored Collagen Supports In-Situ Healing of Vascular Tissues. Nat. Commun. 2024, 15, 11234. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Tyagi, R.D.; Zhang, X. Carbon dioxide and methane as carbon source for the production of polyhydroxyalkanoates and concomitant carbon fixation. Bioresour. Technol. 2024, 391, 129977. [Google Scholar] [CrossRef] [PubMed]
- Dhivagar, R.; Suraparaju, S.K.; Atamurotov, F.; Kannan, K.G.; Opakhai, S.; Omara, A.A.M. Performance analysis of snail shell biomaterials in solar still for clean water production: Nature-inspired innovation for sustainability. Water Sci. Technol. 2024, 89, 3325–3343. [Google Scholar] [CrossRef]
- Vu, P.V.; Doan, T.D.; Tu, G.C.; Do, N.H.N.; Le, K.A.; Le, P.K. A novel application of cellulose aerogel composites from pineapple leaf fibers and cotton waste: Removal of dyes and oil in wastewater. J. Porous Mater. 2022, 29, 1137–1147. [Google Scholar] [CrossRef]
- Ughetti, A.; Roncaglia, F.; Anderlini, B.; D’Eusanio, V.; Russo, A.L.; Forti, L. Integrated carbonate-based CO2 capture-biofixation through cyanobacteria. Appl. Sci. 2023, 13, 10779. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Gürsoy, B. Mycelium-based composite graded materials: Assessing the effects of time and substrate mixture on mechanical properties. Biomimetics 2022, 7, 48. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, B.; Lee, S.H.; Lum, W.C.; Ren, Y.; Zhou, X.; Wang, H.; Zhou, B.; Zhang, J. Fabrication of rigid flame retardant foam using bio-based sucrose-furanic resin for building material applications. Chem. Eng. J. 2024, 495, 153614. [Google Scholar] [CrossRef]
- Wachsmann, S.B.; Ruf, M.; Prinz, C.; Oehlsen, N.; Zhou, X.; Dyballa, M.; Arweiler, C.; Leistner, P.; Steeb, H.; Garrecht, H.; et al. Chitin/chitosan biocomposite foams with chitins from different organisms for sound absorption. ACS Sustain. Chem. Eng. 2024, 12, 11841–12269. [Google Scholar] [CrossRef]
- Hahn, J. TômTex™: Leather Alternative Biomaterial from Seafood Shells and Coffee Grounds. Dezeen. 2020. Available online: https://www.dezeen.com/2020/08/22/tomtex-leather-alternative-biomaterial-seafood-shells-coffee/ (accessed on 11 October 2024).
- Ochoa, C.; Iszauk, E. Bringing Life to Materials to Replace Plastics and Leather. Extantia Capital Blog. 2025. Available online: https://medium.com/extantia-capital/bringing-life-to-materials-to-replace-plastics-and-leather-why-we-invested-in-modern-synthesis-cb4a2c022fe9 (accessed on 11 October 2024).
- MYCO WorkTM. Mushroom-Based ‘Leather’ Is Now a Scalable Alternative to Animal Leathers, Poised for Market Disruption. MycoWorks. 2025. Available online: https://www.mycoworks.com/mushroom-based-leather-is-now-a-scalable-alternative-to-animal-leathers-poised-for-market-disruption (accessed on 15 October 2024).
- Walker, K.T.; Keane, J.; Goosens, V.J.; Song, W.; Lee, K.Y.; Ellis, T. Self-pigmenting textiles grown from cellulose-producing bacteria with engineered tyrosinase expression. Nat. Biotechnol. 2024, 43, 345–354. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M.; Zhang, X. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100778. [Google Scholar] [CrossRef]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-chip meets artificial intelligence in drug evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Deng, L.; Yong, Y.Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Zhou, X.-G.; Wu, A.-G. The application of biomaterials in spinal cord injury. Int. J. Mol. Sci. 2023, 24, 816. [Google Scholar] [CrossRef] [PubMed]
- Swansea University. ProColl: Characterisation of Recombinant Human Procollagen. Swansea University. 2024. Available online: https://www.swansea.ac.uk/medicine/enterprise-and-innovation/business-support-projects/accelerate-healthcare-technology-centre/procoll/ (accessed on 13 January 2025).
- ProColl. The Synthetic Biology Company Producing Best-in-Class Bovine and Animal-Free Collagen. ProColl. 2025. Available online: https://www.procoll.co.uk/ (accessed on 13 January 2025).
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 82. [Google Scholar] [CrossRef]
- Mango Materials. The Future is Biodegradable. Mango Materials. 2025. Available online: https://www.mangomaterials.com/ (accessed on 18 January 2025).
- Vorländer, M. Auralization; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- European Parliament. The Impact of Textile Production and Waste on the Environment (Infographics). European Parliament. 2024. Available online: https://www.europarl.europa.eu/topics/en/article/20201208STO93327/the-impact-of-textile-production-and-waste-on-the-environment-infographics (accessed on 16 February 2025).
- Skoczinski, P.; Carus, M.; Tweddle, G.; Ruiz, P.; Hark, N.; Zhang, A.; de Guzman, D.; Ravenstijn, J.; Käb, H.; Raschka, A. Bio-Based Building Blocks and Polymers—Global Capacities, Production and Trends 2023–2028. Renewable Carbon. 2024. Available online: https://renewable-carbon.eu/publications/product/bio-based-building-blocks-and-polymers-global-capacities-production-and-trends-2023-2028/ (accessed on 23 February 2025).
- Resonant Link Medical. What Are Bioelectronic Devices and How Are They Fueling Healthcare Innovation. Resonant Link Medical. 2025. Available online: https://www.rlmedical.com/resource/bioelectronic-devices-fueling-healthcare-innovation (accessed on 26 February 2025).
- Wakefield, E. New Bioprinting Development May Lead to 3D Printed Human Organs. VoxelMatters. 2025. Available online: https://www.voxelmatters.com/new-bioprinting-development-may-lead-to-3d-printed-human-organs/ (accessed on 27 February 2025).
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 96. [Google Scholar] [CrossRef]
- Oluwole, S.A.; Weldu, W.D.; Jayaraman, K.; Barnard, K.A.; Agatemor, C. Design Principles for Immunomodulatory Biomaterials. ACS Appl. Bio Mater. 2024, 7, 8059–8075. [Google Scholar] [CrossRef]
- Negut, I.; Albu, C.; Bita, B. Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings 2024, 14, 282. [Google Scholar] [CrossRef]
- Abbasi, I. What Are Biodegradable Metallic Materials? AZoM. 2024. Available online: https://www.azom.com/article.aspx?ArticleID=23752 (accessed on 1 March 2025).
- Weerarathna, I.N.; Kumar, P.; Dzoagbe, H.Y.; Kiwanuka, L. Advancements in Micro/Nanorobots in Medicine: Design, Actuation, and Transformative Application. ACS Omega 2025, 10, 11234–11246. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A. Advancements in 3D-Printed Biomaterials for Personalized Medicine: A Review. Prem. J. Sci. 2024, 4, 53. Available online: https://premierscience.com/pjs-24-282/ (accessed on 1 March 2025). [CrossRef]
- Park, J.E.; Kim, D.H. Advanced Immunomodulatory Biomaterials for Therapeutic Applications. Adv. Healthc. Mater. 2024, 13, 2304125. [Google Scholar] [CrossRef]
- Bu, N.; Li, L.; Hu, X. Recent Trends in Natural Polymer-Based Hydrogels for Biomedical Applications. Biofunct. Mater. 2024, 2, 1. [Google Scholar] [CrossRef]
- Hu, T.; Fang, J.; Shen, Y.; Li, M.; Wang, B.; Xu, Z.; Hu, W. Advances of Naturally Derived Biomedical Polymers in Tissue Engineering. Front. Chem. 2024, 12, 1469183. [Google Scholar] [CrossRef]
- Barhoum, A.; Sadak, O.; Ramirez, I.A.; Iverson, N. Stimuli-Bioresponsive Hydrogels as New Generation Materials for Implantable, Wearable, and Disposable Biosensors for Medical Diagnostics: Principles, Opportunities, and Challenges. Adv. Colloid. Interface Sci. 2023, 317, 123–147. [Google Scholar] [CrossRef]
- Hong, Y.; Dai, G.; Xu, C.; Lee, W.-H. Biodegradable Elastic Hydrogels for Bioprinting. U.S. Patent US11884765B2, 13 December 2023. [Google Scholar]



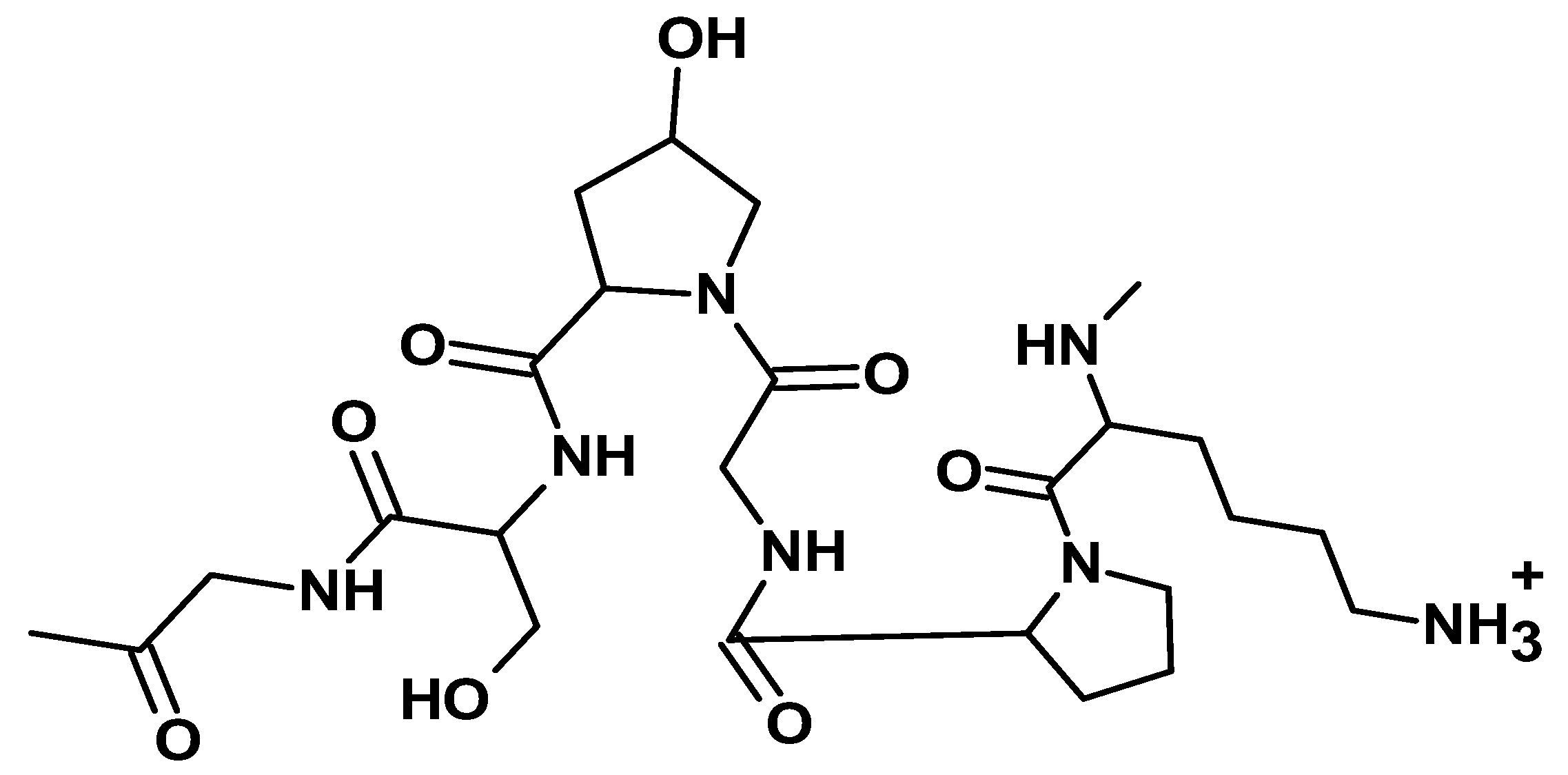
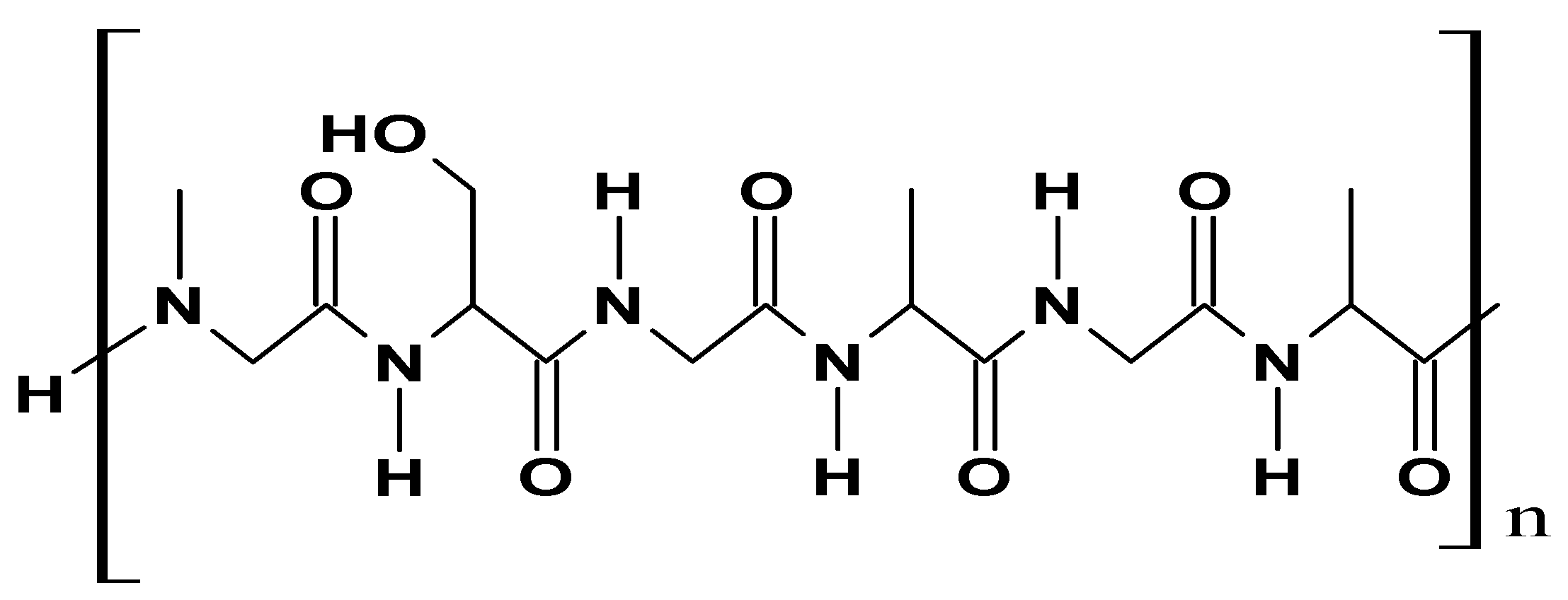
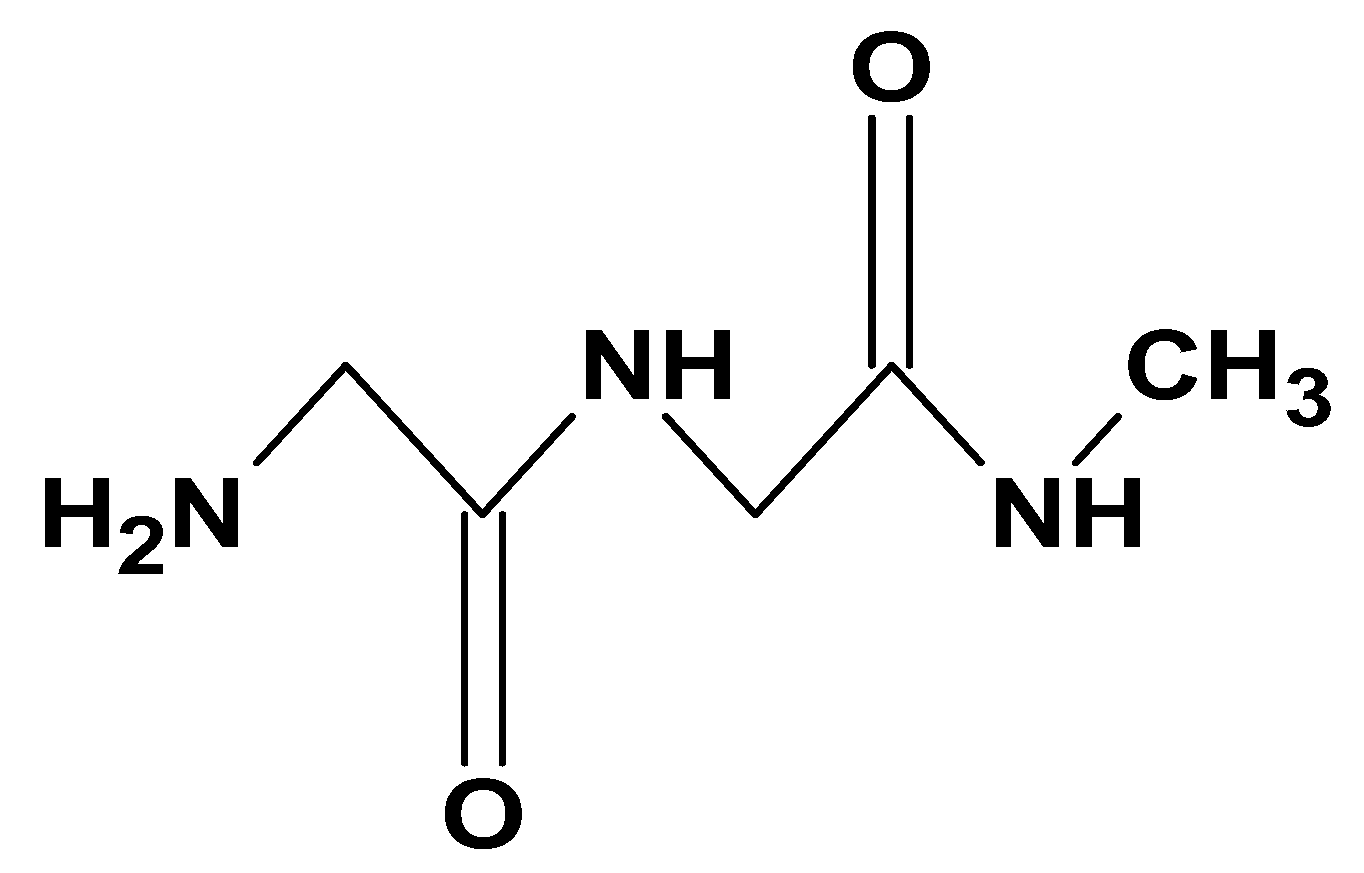
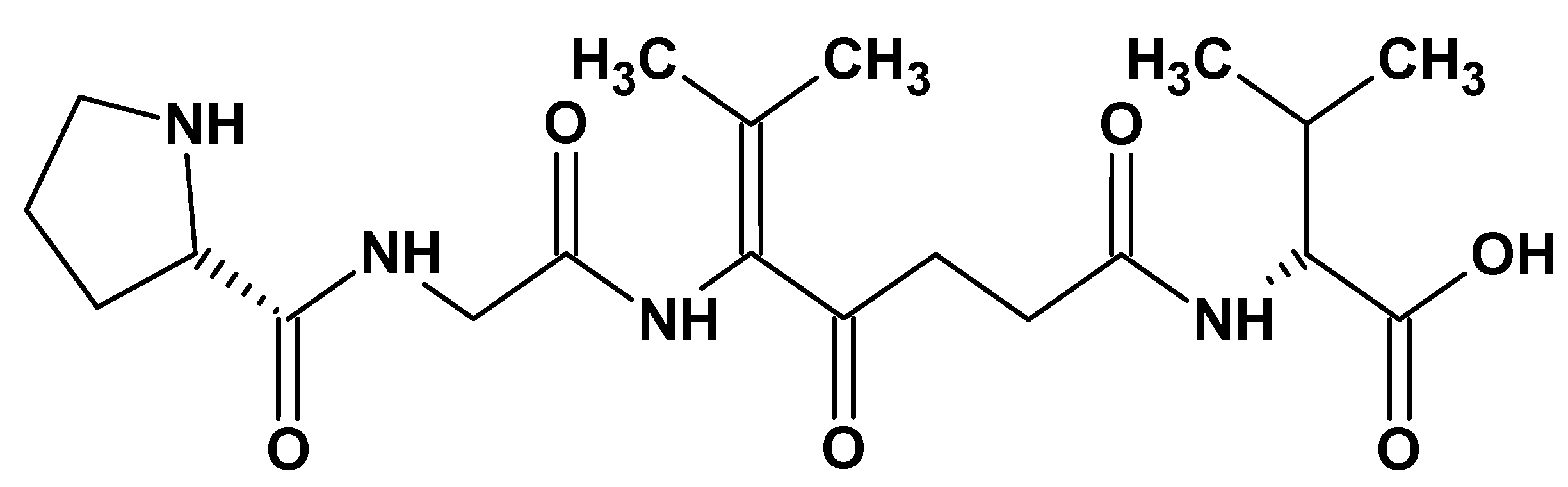

| Additive Type | Examples | Specific Role | Outcome | Reference |
|---|---|---|---|---|
| Crosslinkers | Glutaraldehyde Genipin Epichlorohydrin 1,4-Butanediol diglycidyl ether (BDDE) N,N′-methylenebisacrylamide Ethylene glycol dimethacrylate Poly (ethylene glycol) diacrylates | Formation of chemical or covalent bonds between polymer chains to enhance stability and mechanical strength. | Enhance structural integrity, tunable degradation rates, reduce rapid turnover. Toxicity concerns with synthetic crosslinkers. | [162,163] |
| Bioactive molecules | Polynucleotides (PN) Mannitol Growth factors Antimicrobial peptides Retinol Ascorbic acid Ferulic acid | Stimulate biological responses such as antioxidation, infection control, drug release, etc. | Enhanced tissue regeneration, antioxidative protection, infection control, vascularization, and prolonged biomaterial life. | [164] |
| Reinforcing agents | Nanocellulose Nanoclay Nanoparticles | Increase mechanical strength, stiffness, surface area, biocompatibility, water retention. | Improved load-bearing capacity, stress resistance, hydrophilicity, and durability. | [165,166] |
| Plasticizers | Glycerol, ethylene glycol (EG) Diethylene glycol (DEG) Triethylene glycol (TEG) Triethanolamine (TEA) Sorbitol | Reduce brittleness and increase flexibility. | Enhanced elasticity, processability, mechanical and barrier properties. | [167,168] |
| Fillers | Hydroxyapatite (HA) Tricalcium phosphate (TPA) Bioactive glass Silica nanoparticles | Modify texture, mechanical properties, and bioactivity. | Increased hardness, density, compressibility, osteoconductivity, and bioactivity. HA improves bone metabolism and has antibacterial effects. | [169,170] |
| Conductive agents | Polypyrrole Polyaniline Polythiophene Graphene oxide Carbon black | Impart electrical conductivity into the material. | Enable stimulus-responsive behavior and biosensing applications. | [171] |
| Flame retardants | Phosphates (e.g., Diethyl(hydroxymethyl)phosphonate and triphenyl phosphate (TPHP)) Halogenated compounds (Polybrominated diphenyl ethers (PBDEs)) | Improve thermal stability and reduce flammability. | Enhance processing and application safety for wearable sensors and textiles. | [172,173] |
| Antistatic agents | Quaternary ammonium compounds (benzylalkyldimethyl ammonium compounds (BACs), Alkyltrimethyl ammonium compounds (ATMACs) Dialkyldimethyl ammonium compounds (DADMACs)) | Reduce static charge accumulation in biomaterials. | Enhance handling and comfort in wearable applications. | [174] |
| UV stabilizers | Hindered amine light stabilizers (HALS) Benzophenone derivatives (Benzophenone-3 (BP-3), Benzophenone-12 (BP-12)) | Benzophenones absorb harmful UV light and dissipate as harmless thermal energy. HALS functions by scavenging free radicals. | Prolonged material lifespan and stability under sunlight exposure. | [175,176] |
| Colorants | Natural dyes (anthocyanins, Indigofera tinctoria, Rubia tinctorum, Curcuma longa, Lawsonia inermis, Haematoxylum campechianum, etc.) Synthetic dyes (Tartrazine (E102), Indigo Carmine (E132), Allura Red (E129), Sunset Yellow (E110), etc.) | Provides aesthetic appeal and visualization. | Improved product appearance and visualization. | [177] |
| Sector | Biomaterial Type | Innovation | Reference |
|---|---|---|---|
| Energy | Cellulose | A plant-like battery, also called a biodegradable battery. | [178] |
| Gelatin hydrogel | Biodegradable primary zinc–molybdenum (Zn−Mo) battery with gelatin hydrogel electrolyte. | [179] | |
| Algal biopolymers | Usage as electrodes, binders, electrolytes, and separators in batteries (green battery cycle—LixC6 and LiFePO4). | [180] | |
| Lignin-derived carbon | Mesoporous lignin-derived honeycomb-like porous carbon/SiO2 composites for high-performance Li-ion battery. | [181] | |
| Medicine | Wild berry extract and nanocellulose | Antimicrobial skin sprays and surgical dressings. | [182] |
| Poly L-lactide-co-glycolide copolymer (PLGA) | Production of polylactic acid (PLA) and polyglycolic acid (PGA) for the synthesis of bioabsorbable implants applied in orthopedics, cardiovascular interventions, and tissue engineering to provide temporary support while facilitating tissue regeneration. | [183] | |
| Collagen, fibrin, and glycoproteins | Integrated into the development of organs-on-chips (OoCs), which have great implications for drug testing, screening, disease modeling, and personalized medicine. | [184] | |
| Lipids-based membrane shells | Integrated micro-/nano-drug delivery system based on magnetically responsive phase-change droplets for ultrasound theranostics. | [185] | |
| Cellulose nanocrystals from rice husks | Reinforced polymethyl methacrylate (PMMA) nanocomposites for dental application. | [186] | |
| Nanocellulose | Nanocellulose membrane that propels artificial lung devices. | [187] | |
| Hyaluronan | Advanced hydrogel designed for precise ocular delivery. | [188] | |
| Alginate and gelatin | Oxidized sodium alginate/gelatin/halloysite hydrogel used as an injectable, adhesive, and antibacterial dressing for hemostasis. | [189] | |
| Collagen | Formulation of drug-free coating functionalized with tailored collagen supports for vascular tissue healing. | [190] | |
| Environment | Polyhydroxyalkanoate (PHA) from methanogens | Concurrent carbon capture (CO2 and CH4) and utilization by methanogens for PHA production. | [191] |
| Snail shells | Snail shell biomaterials in solar still for clean water production. | [192] | |
| Cellulose | Cellulose from pineapple leaf fibers and cotton waste are used in making aerogel composites for the removal of dyes and oil in wastewater. | [193] | |
| Microalgae biomass | Integrated carbonate-based carbon capture and algae biofixation systems. | [194] | |
| Construction | Mycelium based composite (MBC) | MBC as load-bearing masonry components in construction. | [195] |
| Sucrose | Rigid flame-retardant foam as a building material synthesized from bio-based sucrose–furanic resin. | [196] | |
| Chitin and chitosan | Chitin/chitosan composite foam for sound absorption. | [197] | |
| Textile | Chitosan from mushroom and shrimp shells; bacterial cellulose; mycelium | Animal and petrochemical-free biotextiles as an alternative to conventional leather and textiles. | [198,199,200] |
| Melanated bacterial cellulose from genetically engineered Komagataeibacter rhaeticus | Used as a self-pigmented biotextile. | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amponsah, O.; Nopuo, P.S.A.; Manga, F.A.; Catli, N.B.; Labus, K. Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends. Int. J. Mol. Sci. 2025, 26, 5518. https://doi.org/10.3390/ijms26125518
Amponsah O, Nopuo PSA, Manga FA, Catli NB, Labus K. Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends. International Journal of Molecular Sciences. 2025; 26(12):5518. https://doi.org/10.3390/ijms26125518
Chicago/Turabian StyleAmponsah, Oscar, Prince Sungdewie Adama Nopuo, Felista Adrehem Manga, Nicole Bianca Catli, and Karolina Labus. 2025. "Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends" International Journal of Molecular Sciences 26, no. 12: 5518. https://doi.org/10.3390/ijms26125518
APA StyleAmponsah, O., Nopuo, P. S. A., Manga, F. A., Catli, N. B., & Labus, K. (2025). Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends. International Journal of Molecular Sciences, 26(12), 5518. https://doi.org/10.3390/ijms26125518












