Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle
Abstract
1. Introduction
2. Results
2.1. Control Muscle Performance
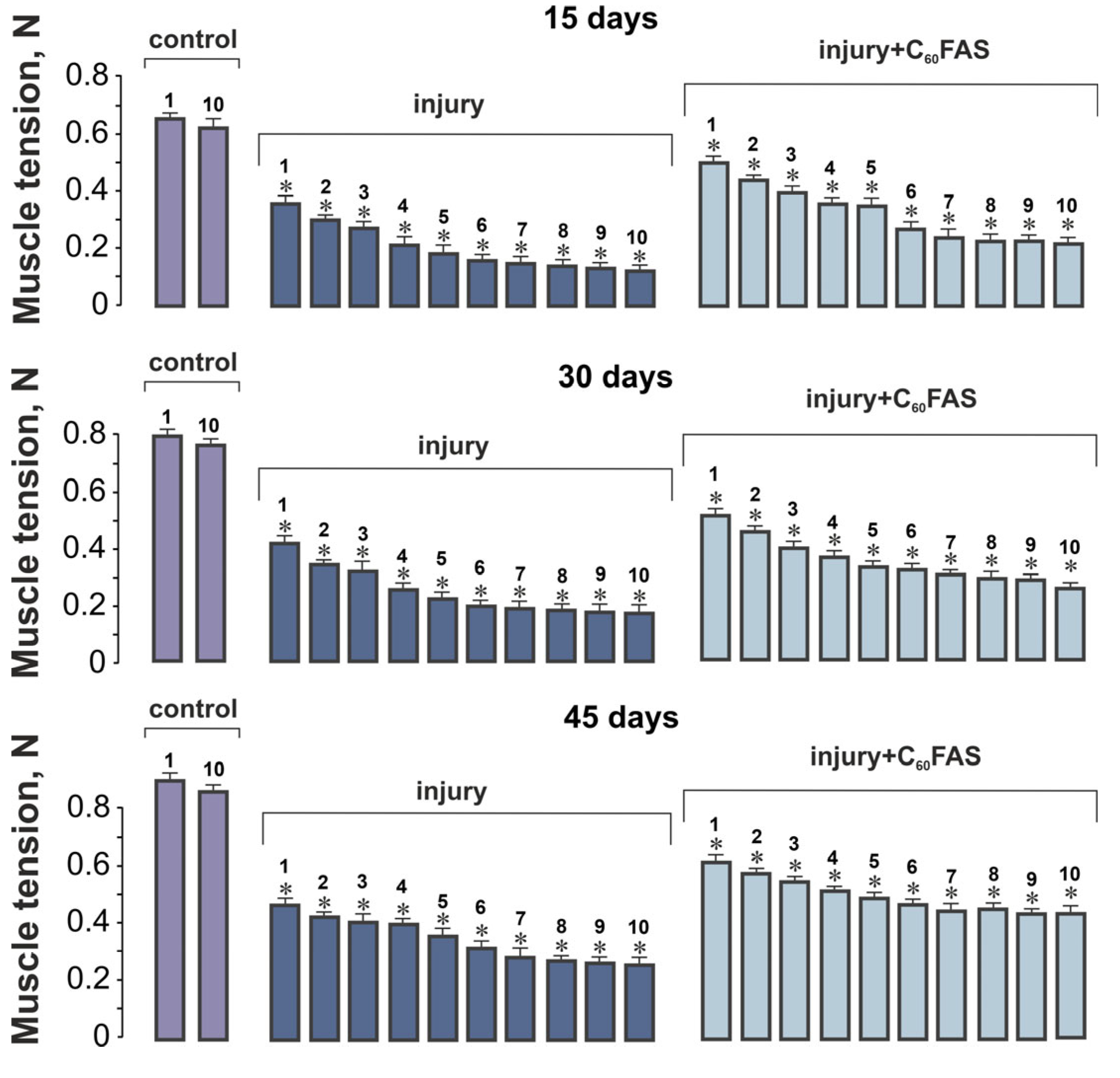
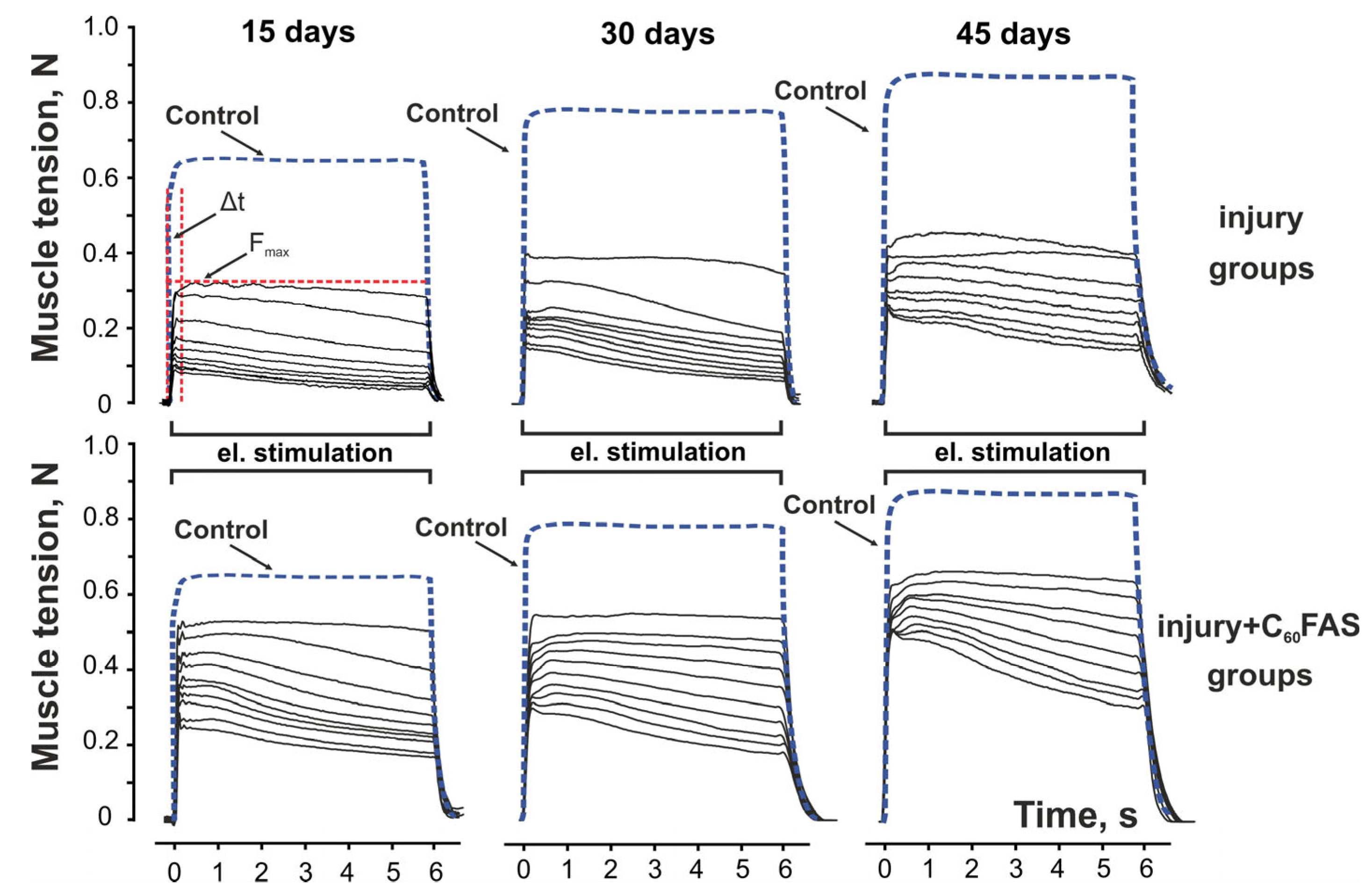
2.2. Muscle Contraction Force Generation
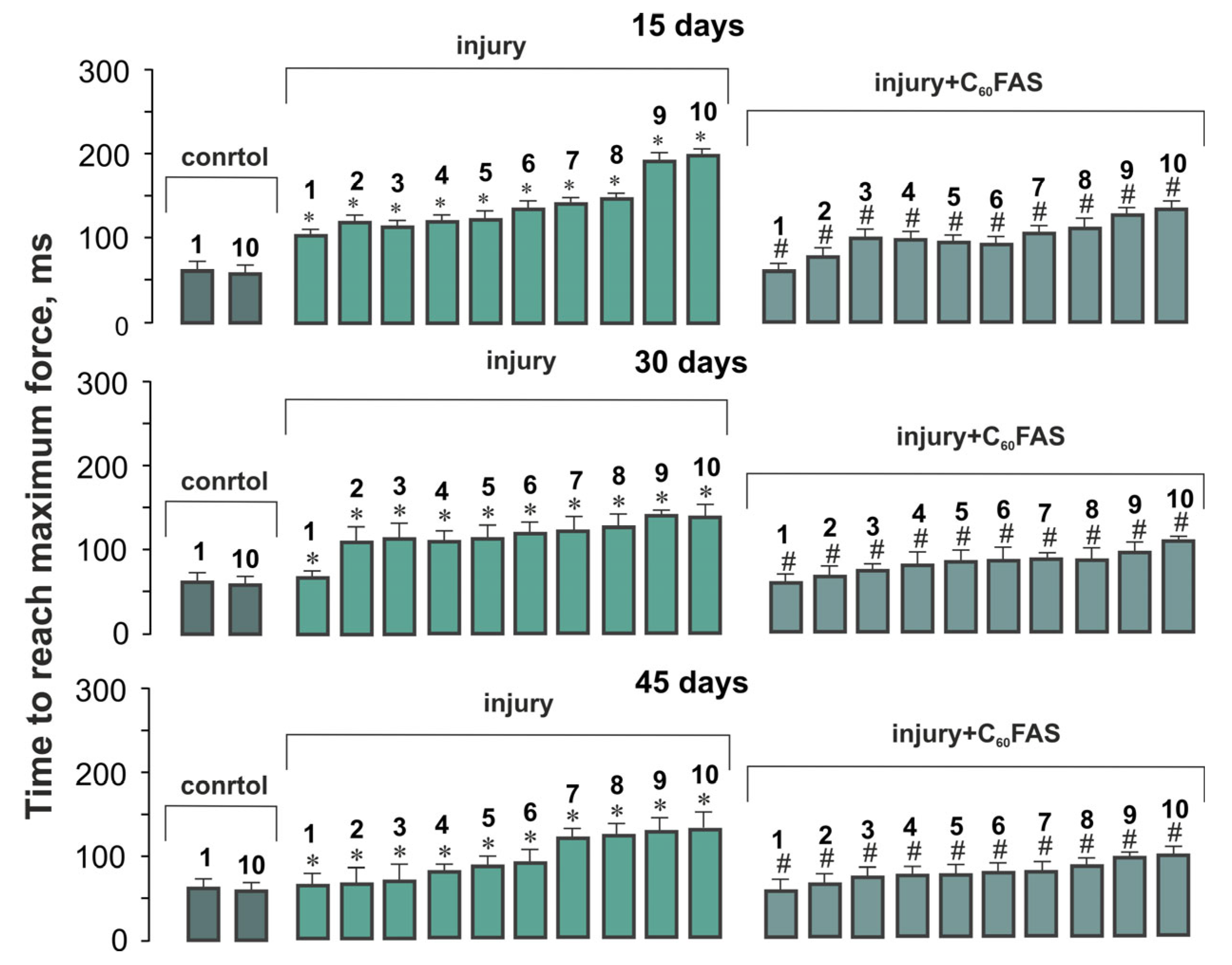
2.3. Temporal Dynamics of Muscle Contraction
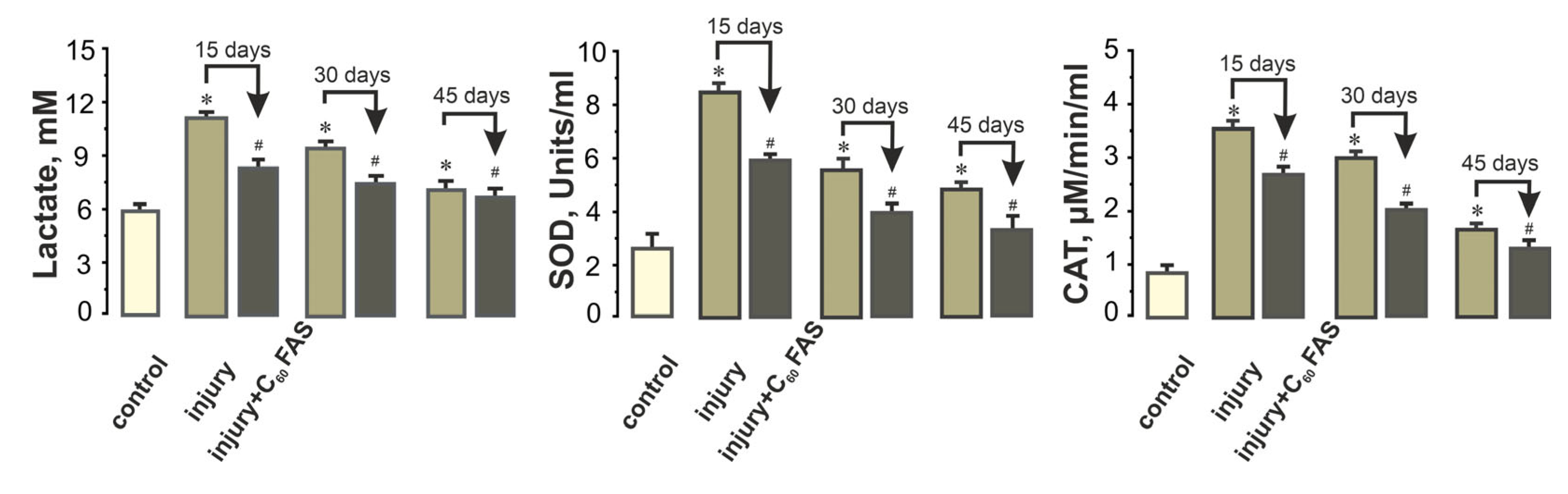
2.4. Systemic Biochemical Markers of Muscle Recovery
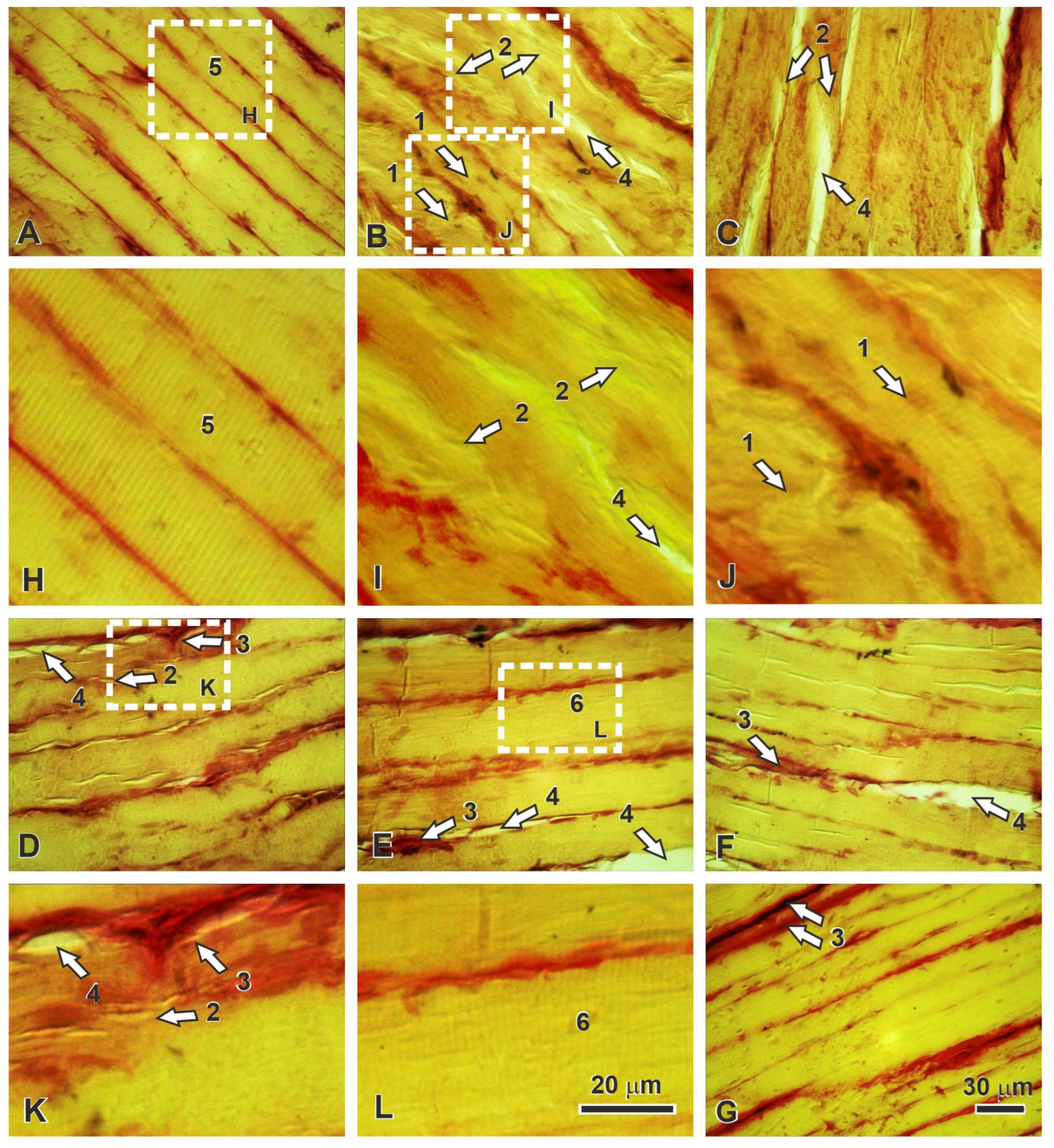
2.5. Histopathological Correlates of Functional Recovery
3. Discussion
4. Materials and Methods
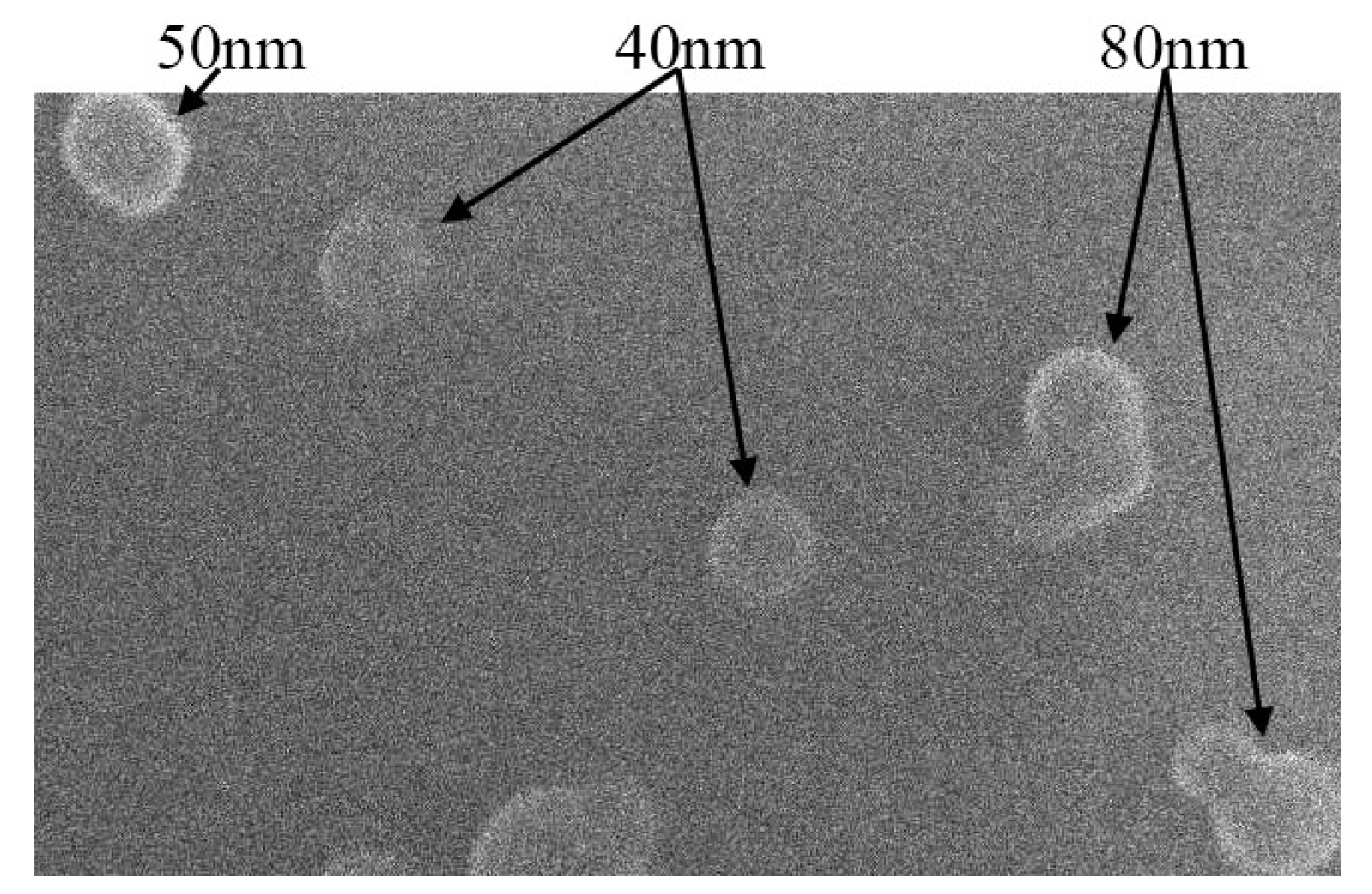
4.1. Material Preparation and Characterization
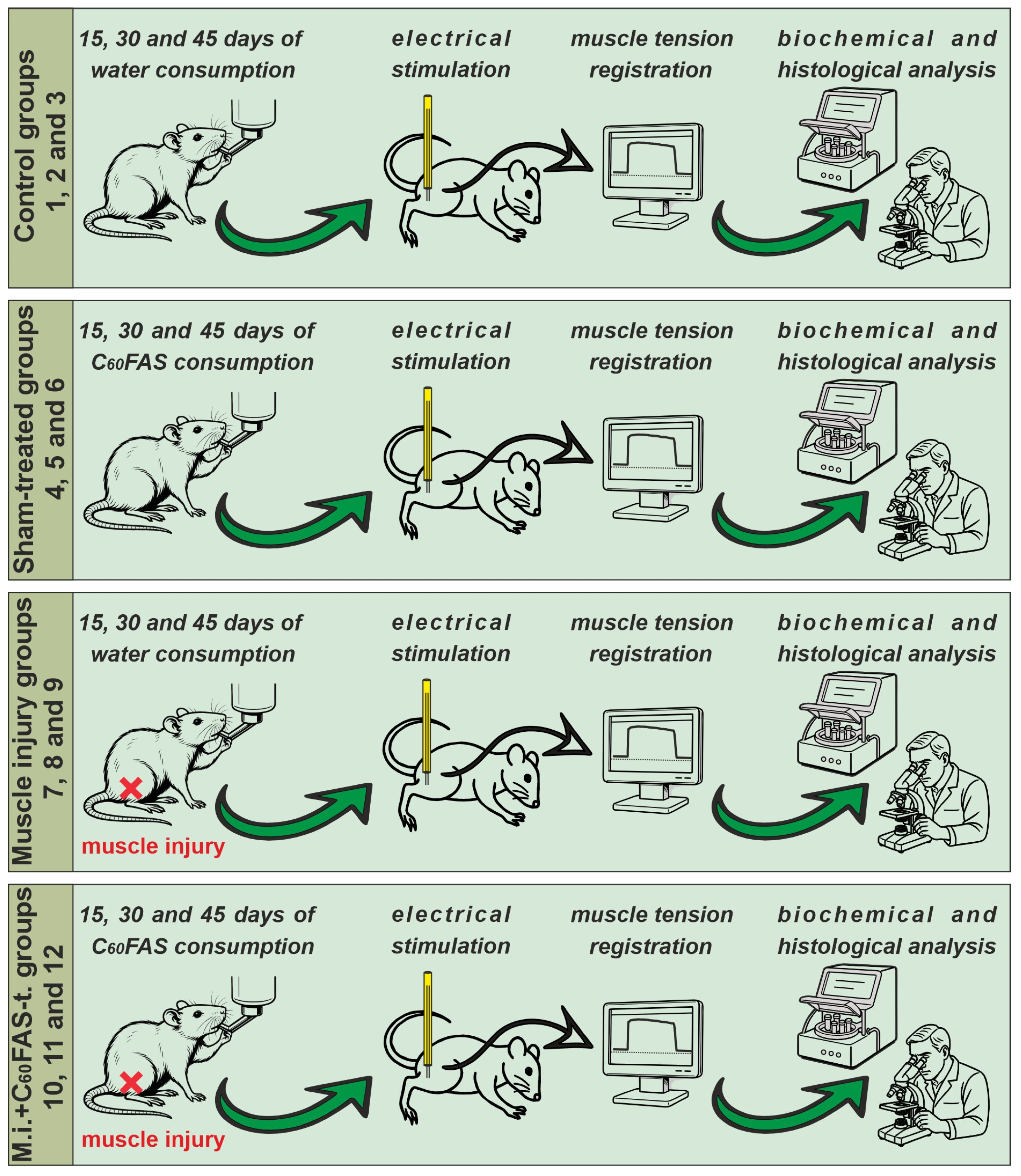
4.2. Procedure and Experimental Animals
4.3. Operations and Electrical Stimulation Protocols
4.4. Biomechanical, Biochemical, and Histological Analyses
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ANOVA | Analysis of variance |
| CAT | Catalase |
| C60FAS | C60 fullerene aqueous solutions |
| DLS | Dynamic light scattering |
| GC/MS | Gas Chromatography/Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| NLRP3 | NOD-like receptor protein 3 |
| NMDA | N-methyl-D-aspartate |
| NPs | Nanoparticles |
| ROS | Reactive oxygen species |
| S.E.M. | Standard error mean |
| SEM | Scanning electron microscopy |
| SOD | Superoxide dismutase |
References
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Äärimaa, V.; Vaittinen, S.; Kalimo, H.; Järvinen, M. Muscle injuries: Optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Prylutskyy, Y.; Nozdrenko, D.; Motuziuk, O.; Prylutska, S.; Bogutska, K.; Abramchuk, O.; Morenko, A.; Franskevych, D.; Scharff, P.; Ritter, U. C60 fullerene reduces the development of post-traumatic dysfunction in rat soleus muscle. Int. J. Mol. Sci. 2024, 25, 12206. [Google Scholar] [CrossRef]
- Greising, S.M.; Warren, G.L.; Southern, W.M.; Nichenko, A.S.; Qualls, A.E.; Corona, B.T.; Call, J.A. Early rehabilitation for volumetric muscle loss injury augments endogenous regenerative aspects of muscle strength and oxidative capacity. BMC Musculoskelet. Disord. 2018, 19, 173. [Google Scholar] [CrossRef]
- Prylutskyy, Y.; Nozdrenko, D.; Motuziuk, O.; Prylutska, S.; Vareniuk, I.; Nurishchenko, N.; Franskevych, D.; Soroca, V.; Bogutska, K.; Ritter, U. C60 fullerene promotes post-traumatic recovery of the rat muscle gastrocnemius. Nanomedicine 2025, 20, 571–584. [Google Scholar] [CrossRef]
- Duchesne, E.; Dufresne, S.S.; Dumont, N.A. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: From fundamental research to the clinic. Phys. Ther. 2017, 97, 807–817. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60] fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Prylutskyy, Y.; Nozdrenko, D.; Motuziuk, O.; Prylutska, S.; Nurishchenko, N.; Franskevych, D.; Cherepanov, V.; Kalinin, I.; Korzhyk, O.; Ritter, U. C60 fullerene improves the contractile activity of the injured rat muscle gastrocnemius. Nanotechnology 2025, 36, 125101. [Google Scholar] [CrossRef]
- Jin, L.; Ding, M.; Oklopcic, A.; Aghdasi, B.; Xiao, L.; Li, Z.; Jevtovic-Todorovic, V.; Li, X. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine 2017, 13, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Nozdrenko, D.; Abramchuk, O.; Prylutska, S.; Vygovska, O.; Soroca, V.; Bogutska, K.; Khrapatyi, S.; Prylutskyy, Y.; Scharff, P.; Ritter, U. Analysis of biomechanical parameters of muscle soleus contraction and blood biochemical parameters in rat with chronic glyphosate intoxication and therapeutic use of C60 fullerene. Int. J. Mol. Sci. 2021, 22, 4977. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Li, C.W.; Li, L.L.; Chen, S.; Zhang, J.X.; Lu, W.L. Antioxidant Nanotherapies for the Treatment of Inflammatory Diseases. Front. Bioeng. Biotechnol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Lao, F.; Chen, L.; Li, W.; Ge, C.; Qu, Y.; Sun, Q.; Zhao, Y.; Han, D.; Chen, C. Fullerene nanoparticles selectively enter oxidation-damaged cerebral microvessel endothelial cells and inhibit jnk-related apoptosis. ACS Nano 2009, 3, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.; Luh, T.Y.; Choi, D.W.; Lin, T.S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Dugan, L.L. Sod activity of carboxyfullerenes predicts their neuroprotective efficacy: A structure-activity study. Nanomed. Nanotechnol. 2008, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Su-Feng, F.; Shin-Zong, L.; Cheng-Kong, C.; Tieng-Yau, L.; Low-Tone, H. Local carboxyfullerene protects cortical infarction in rat brain. Neurosci. Res. 2002, 43, 317–321. [Google Scholar] [CrossRef]
- Bourassa, D.J. An alternate view of fullerene material’s antioxidant activity (NOLF 4 in series: “will nanocarbon onion-like fullerenes (NOLFs) play a decisive role in the future of molecular medicine?”). J. Nanomed. Res. 2020, 8, 1–5. [Google Scholar]
- Hou, W.; Shen, L.; Zhu, Y.; Wang, X.; Du, T.; Yang, F.; Zhu, Y. Fullerene Derivatives for Tumor Treatment: Mechanisms and Application. Int. J. Nanomed. 2024, 2024, 9771–9797. [Google Scholar] [CrossRef] [PubMed]
- Nedzvetsky, V.S.; Sukharenko, E.V.; Baydas, G.; Andrievsky, G.V. Water-soluble C60 fullerene ameliorates astroglial reactivity and TNFa production in retina of diabetic rats. Regul. Mech. Biosyst. 2019, 10, 513–519. [Google Scholar] [CrossRef]
- Burke, R. Spinal motoneurons. In Neuroscience in the 21st Century: From Basic to Clinical; Springer International Publishing: Cham, Switzerland, 2022; pp. 1437–1473. [Google Scholar]
- Mahapatra, C.; Thakkar, R.; Kumar, R. Modulatory impact of oxidative stress on action potentials in pathophysiological states: A comprehensive review. Antioxidants 2024, 13, 1172. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, T.; Cheng, K.; Chen, M.; Wang, Y.; Jiang, Y.; Yang, P. Carboxylic Acid Fullerene (C60) derivatives attenuated neuroinflammatory responses by modulating mitochondrial dynamics. Nanoscale Res. Lett. 2015, 10, 953. [Google Scholar] [CrossRef]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Halestrap, A.P. Lactate transport in skeletal muscle—Role and regulation of the monocarboxylate transporter. J. Physiol. 1999, 517 Pt 3, 633–642. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar] [CrossRef]
- Crevecoeur, F.; Thonnard, J.L.; Lefèvre, P.; Scott, S.H. Long-latency feedback coordinates upper-limb and hand muscles during object manipulation Tasks. eNeuro. 2016, 3, ENEURO.0129-15.2016. [Google Scholar] [CrossRef]
- Vlasenko, O.V.; Pilyavskii, A.I.; Maiskii, V.A.; Maznichenko, A. Fos immunoreactivity and NADPH-d reactivity in the brain cortex of rats realizing motivated stereotyped movements by the forelimb. Neurophysiology 2008, 40, 295–303. [Google Scholar] [CrossRef]
- Tal’nov, A.N.; Tomiak, T.; Maznychenko, A.V.; Dovgalets, G.V.; Kostyukov, A.I. Firing patterns of human biceps brachii motor units during isotorque ramp-and-hold movements in the elbow joint. Neurophysiology 2014, 46, 212–220. [Google Scholar] [CrossRef]
- Mao, G.D.; Thomas, P.D.; Lopaschuk, G.D.; Poznansky, M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 1993, 268, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, M.; Yang, G.; Wang, L.; Gao, Q.; Zhang, H. Muscle injury associated elevated oxidative stress and abnormal myogenesis in patients with idiopathic scoliosis. Int. J. Biol. Sci. 2019, 15, 2584–2595. [Google Scholar] [CrossRef]
- Wight, T.N.; Potter-Perigo, S. The extracellular matrix: An active or passive player in fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G950–G955. [Google Scholar] [CrossRef]
- Ritter, U.; Prylutskyy, Y.u.I.; Evstigneev, M.P.; Davidenko, N.A.; Cherepanov, V.V.; Senenko, A.I.; Marchenko, O.A.; Naumovets, A.G. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fuller. Nanotubes Carbon Nanostruct. 2015, 23, 530–534. [Google Scholar] [CrossRef]
- Keykhosravi, S.; Rietveld, I.B.; Couto, D.; Tamarit, J.L.; Barrio, M.; Céolin, R.; Moussa, F. [60] Fullerene for medicinal purposes, a purity criterion towards regulatory considerations. Materials 2019, 12, 2571. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 584. ISBN 9780702068645. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozdrenko, D.; Prylutskyy, Y.; Sulyma, O.; Kozik, Y.; Vareniuk, I.; Ritter, U.; Abramovych, T.; Sokolowska, I.; Maznychenko, A. Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle. Int. J. Mol. Sci. 2025, 26, 5511. https://doi.org/10.3390/ijms26125511
Nozdrenko D, Prylutskyy Y, Sulyma O, Kozik Y, Vareniuk I, Ritter U, Abramovych T, Sokolowska I, Maznychenko A. Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle. International Journal of Molecular Sciences. 2025; 26(12):5511. https://doi.org/10.3390/ijms26125511
Chicago/Turabian StyleNozdrenko, Dmytro, Yuriy Prylutskyy, Oleksii Sulyma, Yevhenii Kozik, Igor Vareniuk, Uwe Ritter, Tetiana Abramovych, Inna Sokolowska, and Andriy Maznychenko. 2025. "Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle" International Journal of Molecular Sciences 26, no. 12: 5511. https://doi.org/10.3390/ijms26125511
APA StyleNozdrenko, D., Prylutskyy, Y., Sulyma, O., Kozik, Y., Vareniuk, I., Ritter, U., Abramovych, T., Sokolowska, I., & Maznychenko, A. (2025). Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle. International Journal of Molecular Sciences, 26(12), 5511. https://doi.org/10.3390/ijms26125511






