Omega-3 Fatty Acids Mitigate Long-Lasting Disruption of the Endocannabinoid System in the Adult Mouse Hippocampus Following Adolescent Binge Drinking
Abstract
1. Introduction
2. Results
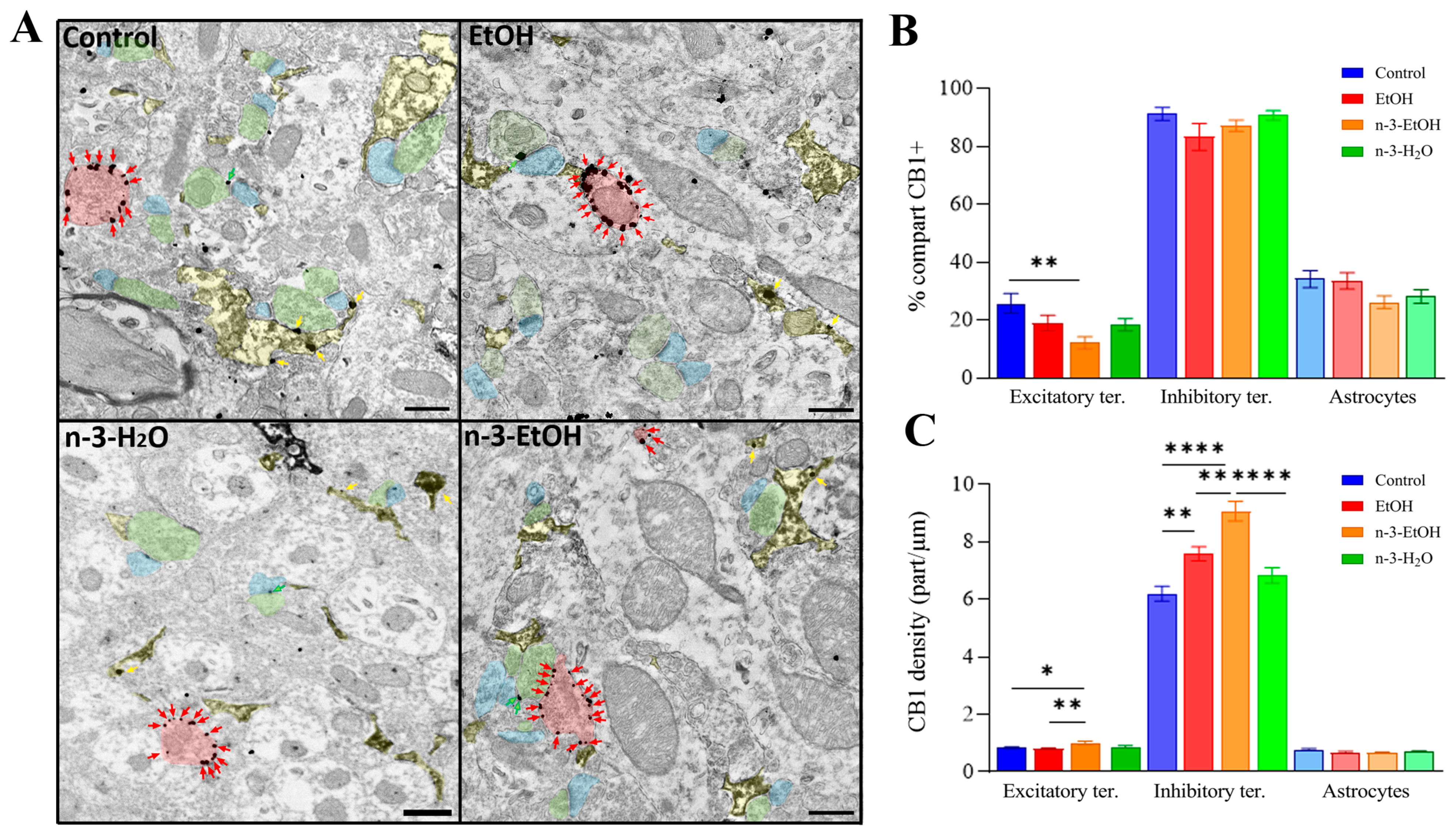
2.1. Immunolocalization of CB1 Receptors in DG Neurons and Astrocytes
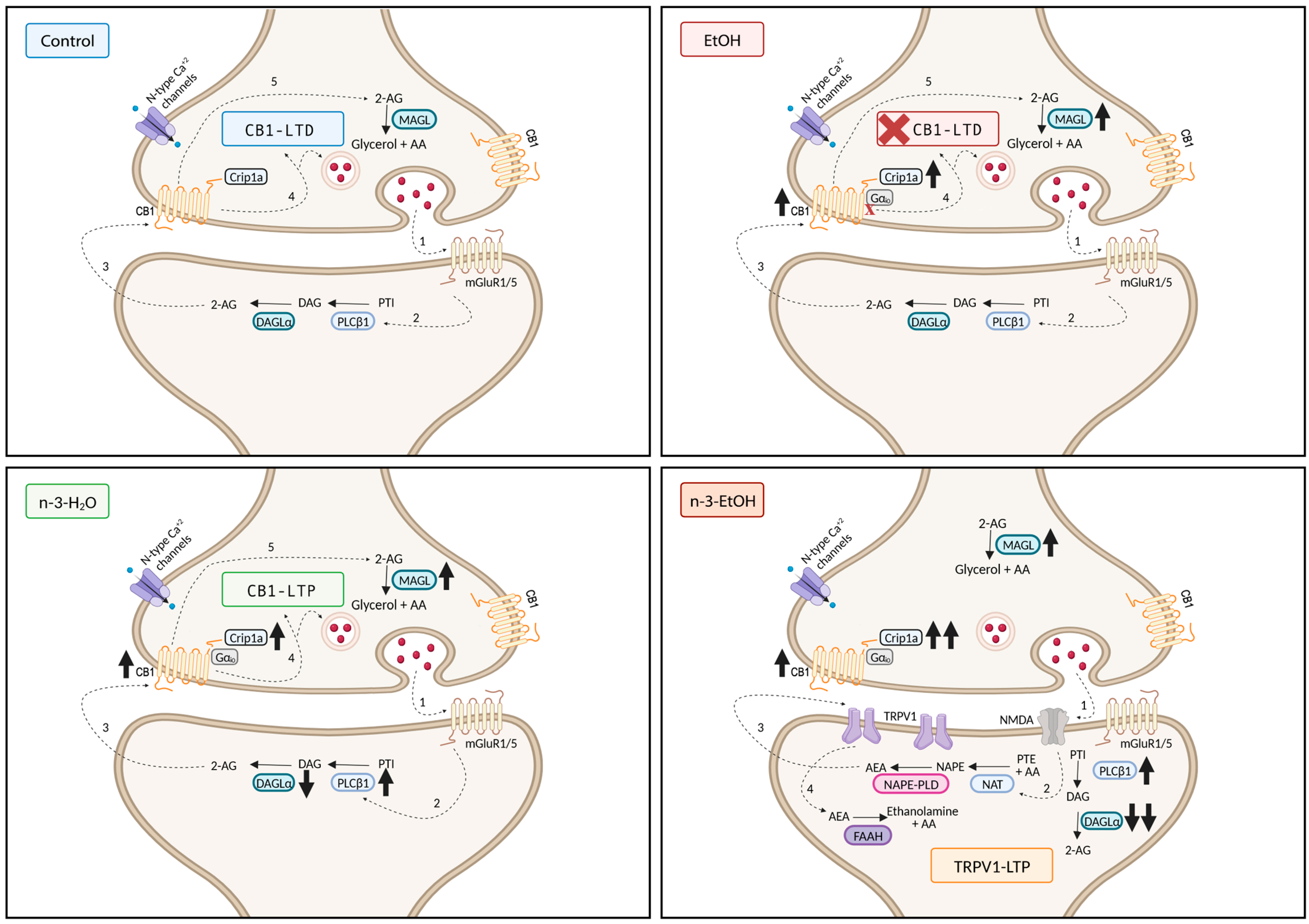
2.2. Expression and CB1 Receptor Coupling to Gαi/o Proteins in Hippocampal Synaptosomes
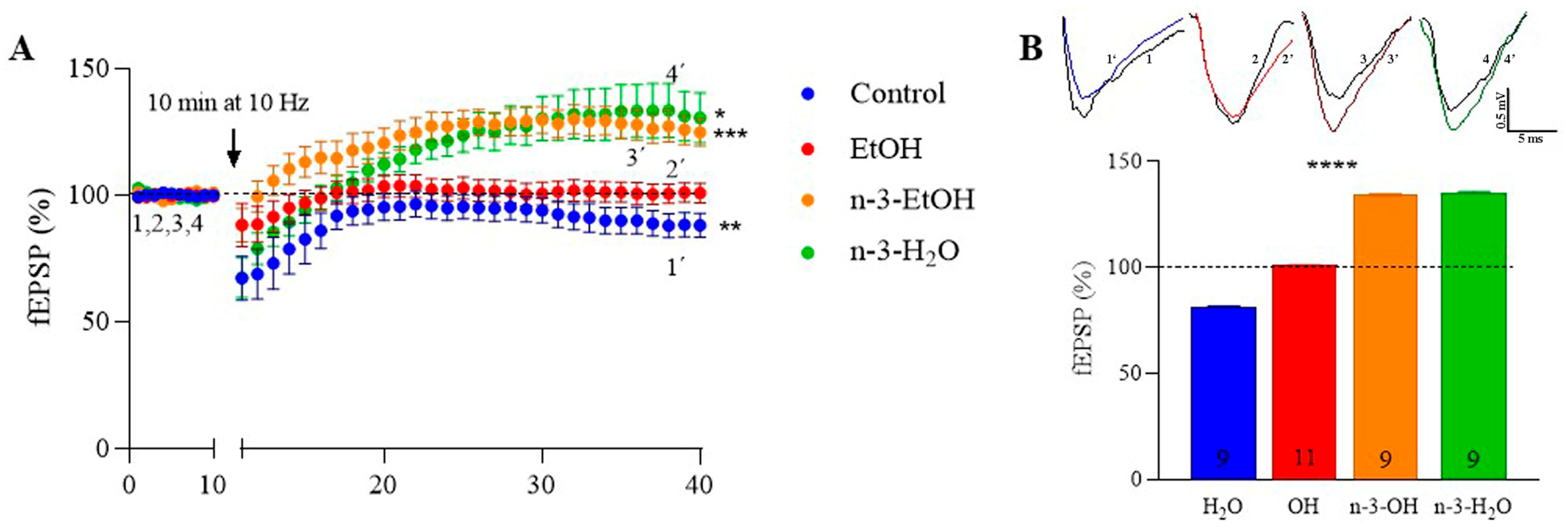
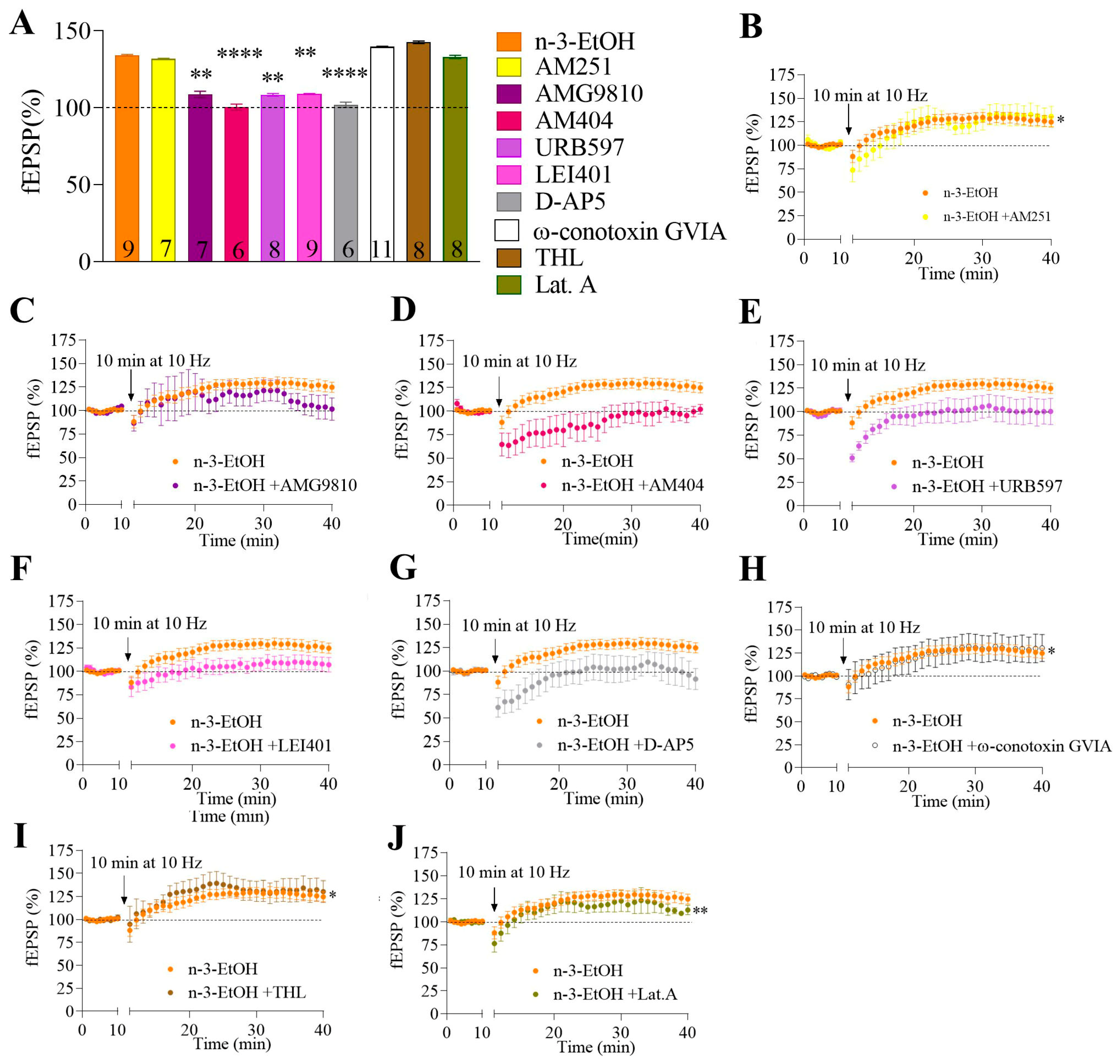
2.3. MPP Excitatory Synaptic Transmission and Plasticity
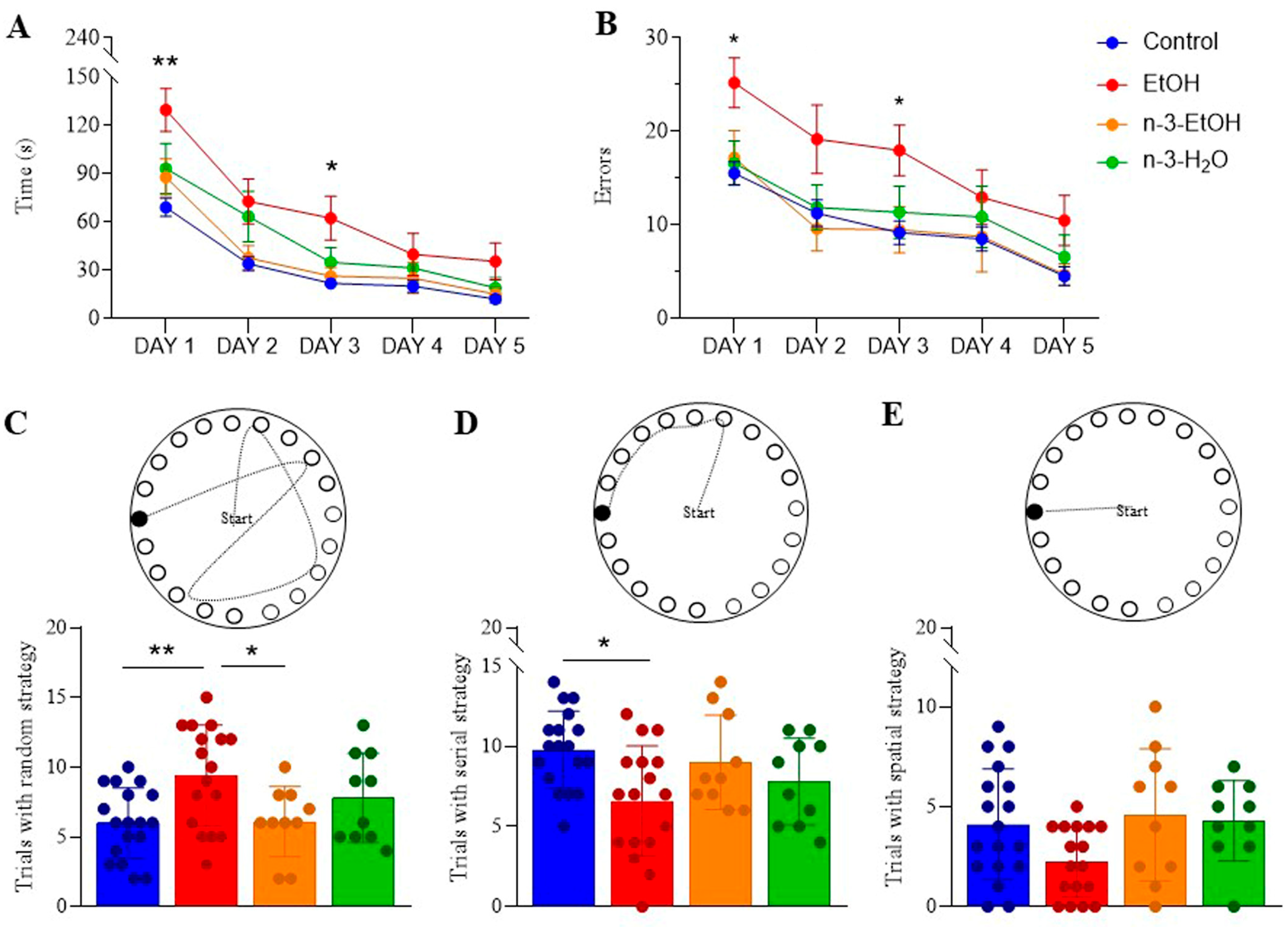
2.4. Learning and Memory in the Barnes Maze Test
3. Discussion
3.1. Omega-3 Supplementation During Abstinence Alters ECS and CB1 Receptor Signaling Molecules Following Adolescent Binge Drinking
3.2. Omega-3 Supplementation During Abstinence Mitigates Long-Lasting Cognitive Deficits Caused by Adolescent Binge Drinking
3.3. Experimental Limitations
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Treatment
4.3. Pre-Embedding Immunogold and Immunoperoxidase for Electron Microscopy
4.4. Synaptosomal Fractionation
4.5. Western Blot Analysis
4.6. [35S]GTPγS Binding Assays
4.7. Slice Preparation and Extracellular Field Recording
4.8. Barnes Maze Test
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cservenka, A.; Brumback, T. The burden of binge and heavy drinking on the brain: Effects on adolescent and young adult neural structure and function. Front. Psychol. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Yun, B.; Cha, J.; Suk, H.I.; Shin, E.K. Neurodevelopmental imprints of sociomarkers in adolescent brain connectomes. Sci. Rep. 2024, 14, 20921. [Google Scholar] [CrossRef] [PubMed]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Fontaine, C.J.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; Rodríguez de Fonseca, F.; Suárez, J.; Barrondo, S.; et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 2020, 45, 309–318. [Google Scholar] [CrossRef]
- Kunos, G. Interactions between alcohol and the endocannabinoid system. Alcohol Clin. Exp. Res. 2020, 44, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; García-Gutiérrez, M.S.; Gasparyan, A.; Navarro, D.; López-Picón, F.; Morcuende, A.; Femenía, T.; Manzanares, J. Biomarkers of the endocannabinoid system in substance use disorders. Biomolecules 2022, 12, 396. [Google Scholar] [CrossRef]
- Sánchez-Marín, L.; Flores-López, M.; Pastor, A.; Gavito, A.L.; Suárez, J.; de la Torre, R.; Pavón, F.J.; Rodríguez de Fonseca, F.; Serrano, A. Acute stress and alcohol exposure during adolescence result in an anxious phenotype in adulthood: Role of altered glutamate/endocannabinoid transmission mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110460. [Google Scholar] [CrossRef]
- Wolfe, S.; Vozella, V.; Roberto, M. The synaptic interactions of alcohol and the endogenous cannabinoid system. Alcohol Res. Curr. Rev. 2022, 42, 03. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Winters, N.D.; Bedse, G.; Astafyev, A.A.; Patrick, T.A.; Altemus, M.; Morgan, A.J.; Mukerjee, S.; Johnson, K.D.; Mahajan, V.R.; Uddin, M.J.; et al. Targeting diacylglycerol lipase reduces alcohol consumption in preclinical models. J. Clin. Investig. 2021, 131, e146861. [Google Scholar] [CrossRef]
- Elliott, G.O.; Petrie, G.N.; Kroll, S.L.; Roche, D.J.O.; Mayo, L.M. Changes in peripheral endocannabinoid levels in substance use disorders: A review of clinical evidence. Am. J. Drug Alcohol Abus. 2025, 51, 152–164. [Google Scholar] [CrossRef]
- Serrano, A.; Natividad, L.A. Alcohol-endocannabinoid interactions: Implications for addiction-related behavioral processes. Alcohol Res. Curr. Rev. 2022, 42, 09. [Google Scholar] [CrossRef]
- Hungund, B.L.; Szakall, I.; Adam, A.; Basavarajappa, B.S.; Vadasz, C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003, 84, 698–704. [Google Scholar] [CrossRef]
- Maccioni, P.; Colombo, G.; Carai, M.A.M. Blockade of the cannabinoid CB1 receptor and alcohol dependence: Preclinical evidence and preliminary clinical data. CNS Neurol. Disord. Drug Targets 2010, 9, 55–59. [Google Scholar] [CrossRef]
- Rico-Barrio, I.; Peñasco, S.; Lekunberri, L.; Serrano, M.; Egaña-Huguet, J.; Mimenza, A.; Soria-Gomez, E.; Ramos, A.; Buceta, I.; Gerrikagoitia, I.; et al. Environmental enrichment rescues endocannabinoid-dependent synaptic plasticity lost in young adult male mice after ethanol exposure during adolescence. Biomedicines 2021, 9, 825. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food. Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importance of a balanced omega-6/omega-3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [PubMed]
- Cutuli, D.; Pagani, M.; Caporali, P.; Galbusera, A.; Laricchiuta, D.; Foti, F.; Neri, C.; Spalleta, G.; Caltagirone, C.; Petrosini, L.; et al. Effects of omega-3 fatty acid supplementation on cognitive functions and neural substrates: A voxel-based morphometry study in aged mice. Front. Aging Neurosci. 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Morrow, J.D.; Picklo, M.J. Elevated oxidation of docosahexaenoic acid, 22:6 (n-3), in brain regions of rats undergoing ethanol withdrawal. Neurosci. Lett. 2006, 405, 172–174. [Google Scholar] [CrossRef]
- Tajuddin, N.; Moon, K.H.; Marshall, S.A.; Nixon, K.; Neafsey, E.J.; Kim, H.Y.; Collins, M.A. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS ONE 2014, 9, e101223. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- Patten, A.; Sickmann, H.M.; Dyer, R.A.; Innis, S.M.; Christie, B.R. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci. Lett. 2013, 551, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Rico-Barrio, I.; Grandes, P. The effect of omega-3 fatty acids on alcohol-induced damage. Front. Nutr. 2023, 10, 1068343. [Google Scholar] [CrossRef] [PubMed]
- Cardona, D.; Carvajal, F.; Lerma-Cabrera, J.M.; Sánchez-Gil, A.; Rueda-Ruzafa, L. Impact of omega-3 polyunsaturated fatty acids on alcohol use and negative consequences: A systematic review. Nutr. Rev. 2025, 26, nuaf036. [Google Scholar]
- Manduca, A.; Bara, A.; Larrieu, T.; Lassalle, O.; Joffre, C.; Layé, S.; Manzoni, O.J. Amplification of mGlu5-endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. J. Neurosci. 2017, 37, 6851–6868. [Google Scholar] [CrossRef]
- Thomazeau, A.; Bosch-Bouju, C.; Manzoni, O.; Layé, S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex 2017, 27, 2571–2579. [Google Scholar] [CrossRef]
- Martín-Llorente, A.; Serrano, M.; Bonilla-Del Río, I.; Lekunberri, L.; Ocerin, G.; Puente, N.; Ramos, A.; Rico-Barrio, I.; Gerrikagoitia, I.; Grandes, P. Omega-3 recovers cannabinoid 1 receptor expression in the adult mouse brain after adolescent binge drinking. Int. J. Mol. Sci. 2023, 24, 17316. [Google Scholar] [CrossRef]
- Bonilla-Del Río, I.; Puente, N.; Peñasco, S.; Rico, I.; Gutiérrez-Rodríguez, A.; Elezgarai, I.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; Christie, B.R.; et al. Adolescent ethanol intake alters cannabinoid type-1 receptor localization in astrocytes of the adult mouse hippocampus. Addict. Biol. 2019, 24, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Saumell-Esnaola, M.; Ocerin, G.; García del Caño, G.; Puente, N.; Sallés, J.; Rodríguez de Fonseca, F.; Rodríguez-Arias, M.; Gerrikagoitia, I.; Grandes, P. Impact of omega-3 on endocannabinoid system expression and function, enhancing cognition and behavior in male mice. Nutrients 2024, 16, 4344. [Google Scholar] [CrossRef]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Achicallende, S.; Ramos, A.; Reguero, L.; Elezgarai, I.; et al. Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology 2019, 153, 32–40. [Google Scholar] [CrossRef]
- Serrano, A.; Pavon, F.J.; Buczynski, M.W.; Schlosburg, J.; Natividad, L.A.; Polis, I.Y.; Stouffer, D.G.; Zorrilla, E.P.; Roberto, M.; Cravatt, B.F.; et al. Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology 2018, 43, 1840–1850. [Google Scholar] [CrossRef]
- Stopponi, S.; Fotio, Y.; Domi, A.; Borruto, A.M.; Natividad, L.; Roberto, M.; Ciccocioppo, R.; Canella, N. Inhibition of fatty acid amide hydrolase in the central amygdala alleviates co-morbid expression of innate anxiety and excessive alcohol intake. Addict. Biol. 2018, 23, 1223–1232. [Google Scholar] [CrossRef]
- Fucich, E.A.; Mayeux, J.P.; McGinn, M.A.; Gilpin, N.W.; Edwards, S.; Molina, P.E. A novel role for the endocannabinoid system in ameliorating motivation for alcohol drinking and negative behavioral affect after traumatic brain injury in rats. J. Neurotrauma 2019, 36, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- García-Baos, A.; Alegre-Zurano, L.; Cantacorps, L.; Martín-Sánchez, A.; Valverde, O. Role of cannabinoids in alcohol-induced neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110054. [Google Scholar] [CrossRef]
- Borgonetti, V.; Vozella, V.; Ware, T.; Cruz, B.; Bullard, R.; Cravatt, B.F.; Galeotti, N.; Roberto, M. Excessive alcohol intake produces persistent mechanical allodynia and dysregulates the endocannabinoid system in the lumbar dorsal root ganglia of genetically-selected Marchigian Sardinian alcohol-preferring rats. Pharmacol. Res. 2024, 209, 107462. [Google Scholar] [CrossRef] [PubMed]
- Mitrirattanakul, S.; López-Valdés, H.E.; Liang, J.; Matsuka, Y.; Mackie, K.; Faull, K.F.; Spigelman, I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin. Exp. Res. 2007, 31, 855–867. [Google Scholar] [CrossRef]
- Kawamura, Y.; Fukaya, M.; Maejima, T.; Yoshida, T.; Miura, E.; Watanabe, M.; Ohno-Shosaku, T.; Kano, M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006, 26, 2991–3001. [Google Scholar] [CrossRef]
- Pava, M.; Woodward, J. A Review of the interactions between alcohol and the endocannabinoid system: Implications for alcohol dependence and future directions for research. Alcohol 2012, 46, 185–204. [Google Scholar] [CrossRef]
- Liput, D.J.; Pauly, J.R.; Stinchcomb, A.L.; Nixon, K. Binge alcohol exposure transiently changes the endocannabinoid system: A potential target to prevent alcohol-induced neurodegeneration. Brain Sci. 2017, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.Y.; Maccioni, P.; Garcia-Gutierrez, M.S.; Femenia, T.; Xie, S.; Carai, M.A.M.; Manzanares, J.; Cooper, T.B.; Hungund, B.L.; Colombo, G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict. Biol. 2012, 17, 62–75. [Google Scholar] [CrossRef]
- Oliver, E.E.; Hughes, E.K.; Puckett, M.K.; Chen, R.; Lowther, W.T.; Howlett, A.C. Cannabinoid receptor interacting protein 1a (CRIP1a) in health and disease. Biomolecules 2020, 10, 1609. [Google Scholar] [CrossRef]
- Sarkar, P.; Chattopadhyay, A. Cholesterol in GPCR Structures: Prevalence and relevance. J. Membr. Biol. 2022, 255, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Jakubik, J.; El-Fakahany, E.E. Allosteric modulation of GPCRs of class A by cholesterol. Int. J. Mol. Sci. 2021, 22, 1953. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.; Sánchez-Gil, A.; Cardona, D.; Rincón-Cervera, M.A.; Lerma-Cabrera, J.M. The effect of very-long-chain n-3 polyunsaturated fatty acids in the central nervous system and their potential benefits for treating alcohol use disorder: Reviewing pre-clinical and clinical data. Nutrients 2023, 15, 2993. [Google Scholar] [CrossRef]
- Li, H.B.; Mao, R.R.; Zhang, J.C.; Yang, Y.; Cao, J.; Xu, L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol. Psychiatry 2008, 64, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kössl, M.; Holsboer, F.; Zieglgänsberger, W.; Landgraf, R.; Lutz, B.; Wotjak, C.T. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J. Neurosci. 2007, 27, 832–839. [Google Scholar] [CrossRef]
- Bialecki, J.; Werner, A.; Weilinger, N.L.; Tucker, C.M.; Vecchiarelli, H.A.; Egaña, J.; Mendizabal-Zubiaga, J.L.; Grandes, P.; Hill, M.N.; Thompson, R.J. suppression of presynaptic glutamate release by postsynaptic metabotropic NMDA receptor signalling to pannexin-1. J. Neurosci. 2020, 40, 729–742. [Google Scholar] [CrossRef]
- Matta, J.A.; Miyares, R.L.; Ahern, G.P. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007, 578, 397–411. [Google Scholar] [CrossRef]
- Lovinger, D.M.; Abrahao, K.P. Synaptic plasticity mechanisms common to learning and alcohol use disorder. Learn Mem. 2018, 25, 425–434. [Google Scholar] [CrossRef]
- Renteria, R.; Jeanes, Z.M.; Morrisett, R.A. Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPV1 receptors. Alcohol Clin. Exp. Res. 2014, 38, 2763–2769. [Google Scholar] [CrossRef]
- Subbanna, S.; Shivakumar, M.; Psychoyos, D.; Xie, S.; Basavarajappa, B.S. Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J. Neurosci. 2013, 33, 6350–6366. [Google Scholar] [CrossRef]
- Drissi, I.; Deschamps, C.; Fouquet, G.; Alary, R.; Peineau, S.; Gosset, P.; Sueur, H.; Marcq, I.; Debuysscher, S.; Naassila, M.; et al. Memory and plasticity impairment after binge drinking in adolescent rat hippocampus: GluN2A/GluN2B NMDA receptor subunits imbalance through HDAC2. Addict. Biol. 2020, 25, e12760. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.; Collingridge, G.L. Persistent memories of long-term potentiation and the N-methyl-D-aspartate receptor. Brain Neurosci. Adv. 2019, 3, 2398212819848213. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Kimura, S.; Akaike, N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J. Physiol. 1994, 475, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Patten, A.R.; Brocardo, P.S.; Christie, B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J. Nutr. Biochem. 2013, 24, 760–769. [Google Scholar] [CrossRef]
- Isaac, A.R.; Coelho de Velasco, P.; Dias Fraga, K.Y.; Tavares-do-Carmo, M.G.; Pereira Campos, R.M.; Iannotti, F.A.; Verde, R.; Gondim Martins, D.B.; Aragao Santos, T.; Klippel Ferreira, B.; et al. Maternal omega-3 intake differentially affects the endocannabinoid system in the progeny’s neocortex and hippocampus: Impact on synaptic markers. J. Nutr. Biochem. 2021, 96, 108782. [Google Scholar] [CrossRef]
- Puente, N.; Reguero, L.; Elezgarai, I.; Canduela, M.J.; Mendizabal-Zubiaga, J.; Ramos-Uriarte, A.; Fernández-Espejo, E.; Grandes, P. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct. Funct. 2015, 220, 1187–1194. [Google Scholar] [CrossRef]
- Gamoh, S.; Hashimoto, M.; Sugioka, K.; Hossain, M.S.; Hata, N.; Misawa, Y.; Masumura, S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 1999, 93, 237–241. [Google Scholar] [CrossRef]
- Fama, R.; Le Berre, A.P.; Sullivan, E.V. Alcohol’s unique effects on cognition in women: A 2020 (re)view to envision future research and treatment. Alcohol Res. 2020, 40, 3. [Google Scholar] [CrossRef]



| Control | EtOH | n-3-EtOH | n-3-H2O | |
|---|---|---|---|---|
| CB1 receptor | 100.00 ± 6.58 | 138.16 ± 11.72 * | 152.86 ± 3.04 *** | 137.05 ± 14.28 * |
| Crip1a | 100.00 ± 9.44 | 142.91 ± 13.27 * | 194.56 ± 19.08 *** | 149.96 ± 12.53 *** |
| Gαo | 100.00 ± 1.75 | 92.88 ± 2.48 | 84.53 ± 4.98 | 67.22 ± 8.86 * |
| Gαi1 | 100.00 ± 9.10 | 98.56 ± 6.81 | 91.00 ± 7.07 | 84.68 ± 5.59 |
| Gαi2 | 100.00 ± 10.94 | 128.07 ± 15.63 | 91.78 ± 1.05 | 102.10 ± 6.52 |
| Gαi3 | 100.00 ± 0.11 | 93.88 ± 5.60 | 97.63 ± 3.98 | 74.49 ± 10.65 |
| Control | EtOH | n-3-EtOH | n-3-H2O | |
|---|---|---|---|---|
| PLCβ1 | 100.00 ± 5.52 | 108.81 ± 12.243 | 138.29 ± 9.55 ** | 129.69 ± 7.92 ** |
| DAGLα | 100.00 ± 3.68 | 106.82 ± 3.43 | 20.42 ± 1.93 ***ϕϕ | 47.88 ± 10.29 ***ϕϕϕϯϯ |
| DAGLβ | 100.00 ± 12.11 | 88.45 ± 16.12 | 147.39 ± 15.31 | 135.91 ± 16.12 |
| MAGL | 100.00 ± 8.78 | 150.46 ± 14.23 * | 150.73± 15.47 * | 132.74 ± 7.41 * |
| NAPE-PLD | 100.00 ± 6.60 | 118.94 ± 11.82 | 87.31 ± 11.61 | 108.81 ± 18.54 |
| FAAH | 100.00 ± 9.50 | 113.29 ± 11.01 | 106.84 ± 13.41 | 99.02 ± 4.09 |
| Standard Diet | N-3 Enriched Diet | |||
|---|---|---|---|---|
| Fats (%) | 4.0 | 5.9 | ||
| mg/kg | % of total fats | mg/kg | % of total fats | |
| SFA | 6000 | 15.0 | 11,867 | 20.1 |
| PUFA | 21,000 | 52.5 | 31,129 | 52.8 |
| Omega-3 | 1000 | 2.5 | 5437 | 9.2 |
| Omega-6 | 20,000 | 50.0 | 25,679 | 43.5 |
| Ratio omega-6/omega-3 | - | 20.0 | - | 4.7 |
| EPA | 0 | 0 | 1325 | 2.2 |
| DHA | 0 | 0 | 899 | 1.5 |
| Drug | Action | Concentration (μM) |
|---|---|---|
| AM251 | CB1 antagonist | 4 |
| AMG9810 | TRPV1 antagonist | 3 |
| AM404 | AEA reuptake inhibitor | 30 |
| URB597 | FAAH inhibitor | 2 |
| LEI401 | NAPE-PLD inhibitor | 10 |
| D-AP5 | NMDA antagonist | 50 |
| Lat.A | Actin assembly inhibitor | 500 |
| ω-conotoxin GVIA | N-type Ca2+ channels blockade | 1 |
| THL | DGL inhibitor | 10 |
| WIN-2 | CB1 agonist | 5 |
| Capsaicin | TRPV1 agonist | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, M.; Saumell-Esnaola, M.; Ocerin, G.; García del Caño, G.; Soria-Gómez, E.; Mimenza, A.; Puente, N.; Bonilla-Del Río, I.; Ramos-Uriarte, A.; Reguero, L.; et al. Omega-3 Fatty Acids Mitigate Long-Lasting Disruption of the Endocannabinoid System in the Adult Mouse Hippocampus Following Adolescent Binge Drinking. Int. J. Mol. Sci. 2025, 26, 5507. https://doi.org/10.3390/ijms26125507
Serrano M, Saumell-Esnaola M, Ocerin G, García del Caño G, Soria-Gómez E, Mimenza A, Puente N, Bonilla-Del Río I, Ramos-Uriarte A, Reguero L, et al. Omega-3 Fatty Acids Mitigate Long-Lasting Disruption of the Endocannabinoid System in the Adult Mouse Hippocampus Following Adolescent Binge Drinking. International Journal of Molecular Sciences. 2025; 26(12):5507. https://doi.org/10.3390/ijms26125507
Chicago/Turabian StyleSerrano, Maitane, Miquel Saumell-Esnaola, Garazi Ocerin, Gontzal García del Caño, Edgar Soria-Gómez, Amaia Mimenza, Nagore Puente, Itziar Bonilla-Del Río, Almudena Ramos-Uriarte, Leire Reguero, and et al. 2025. "Omega-3 Fatty Acids Mitigate Long-Lasting Disruption of the Endocannabinoid System in the Adult Mouse Hippocampus Following Adolescent Binge Drinking" International Journal of Molecular Sciences 26, no. 12: 5507. https://doi.org/10.3390/ijms26125507
APA StyleSerrano, M., Saumell-Esnaola, M., Ocerin, G., García del Caño, G., Soria-Gómez, E., Mimenza, A., Puente, N., Bonilla-Del Río, I., Ramos-Uriarte, A., Reguero, L., Christie, B. R., Rodríguez de Fonseca, F., Rodríguez-Arias, M., Gerrikagoitia, I., & Grandes, P. (2025). Omega-3 Fatty Acids Mitigate Long-Lasting Disruption of the Endocannabinoid System in the Adult Mouse Hippocampus Following Adolescent Binge Drinking. International Journal of Molecular Sciences, 26(12), 5507. https://doi.org/10.3390/ijms26125507






