Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice
Abstract
1. Introduction
2. Results
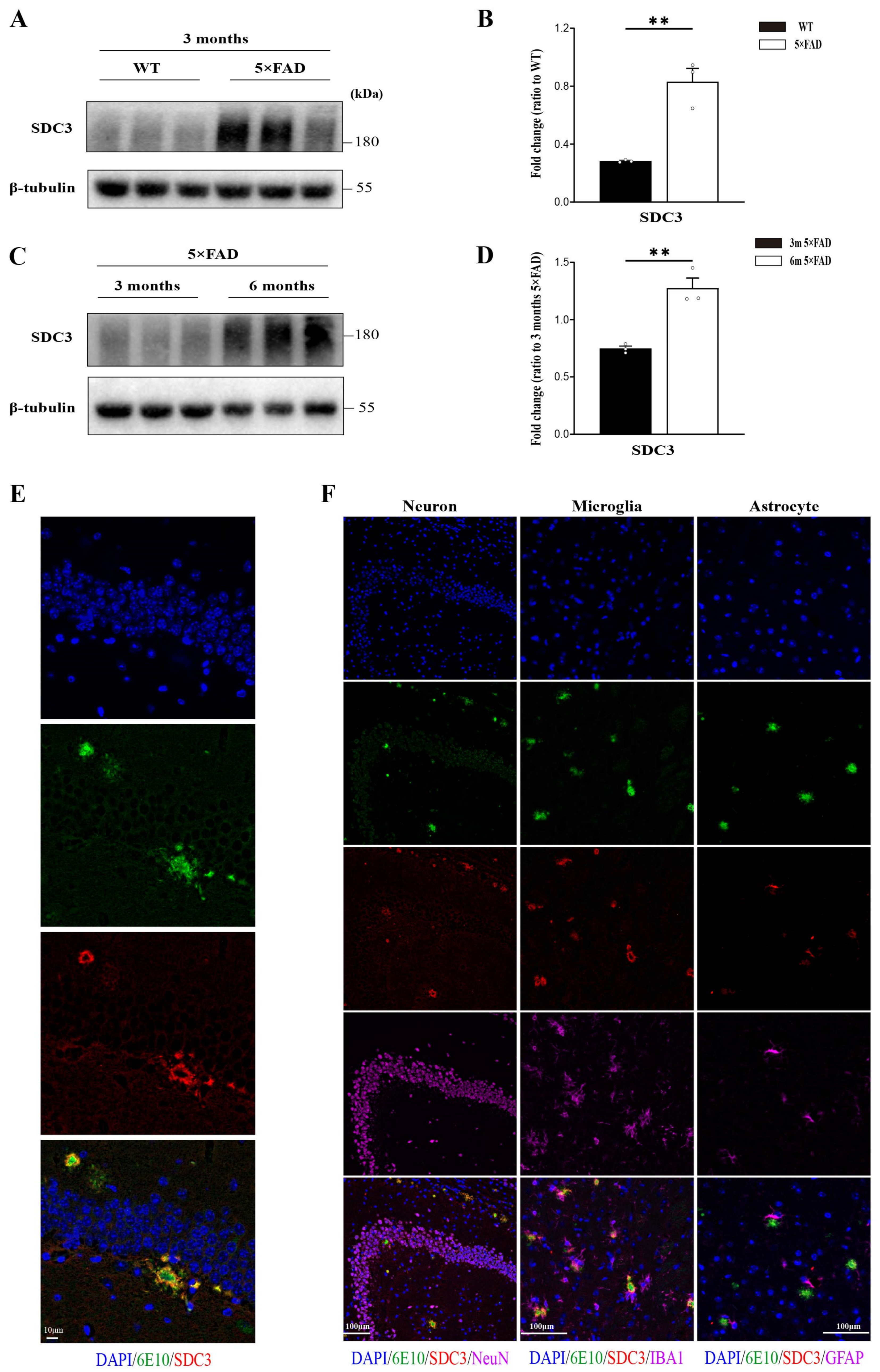
2.1. SDC3 Is Upregulated in the Brains of 5×FAD Mice and Co-Deposits with Aβ
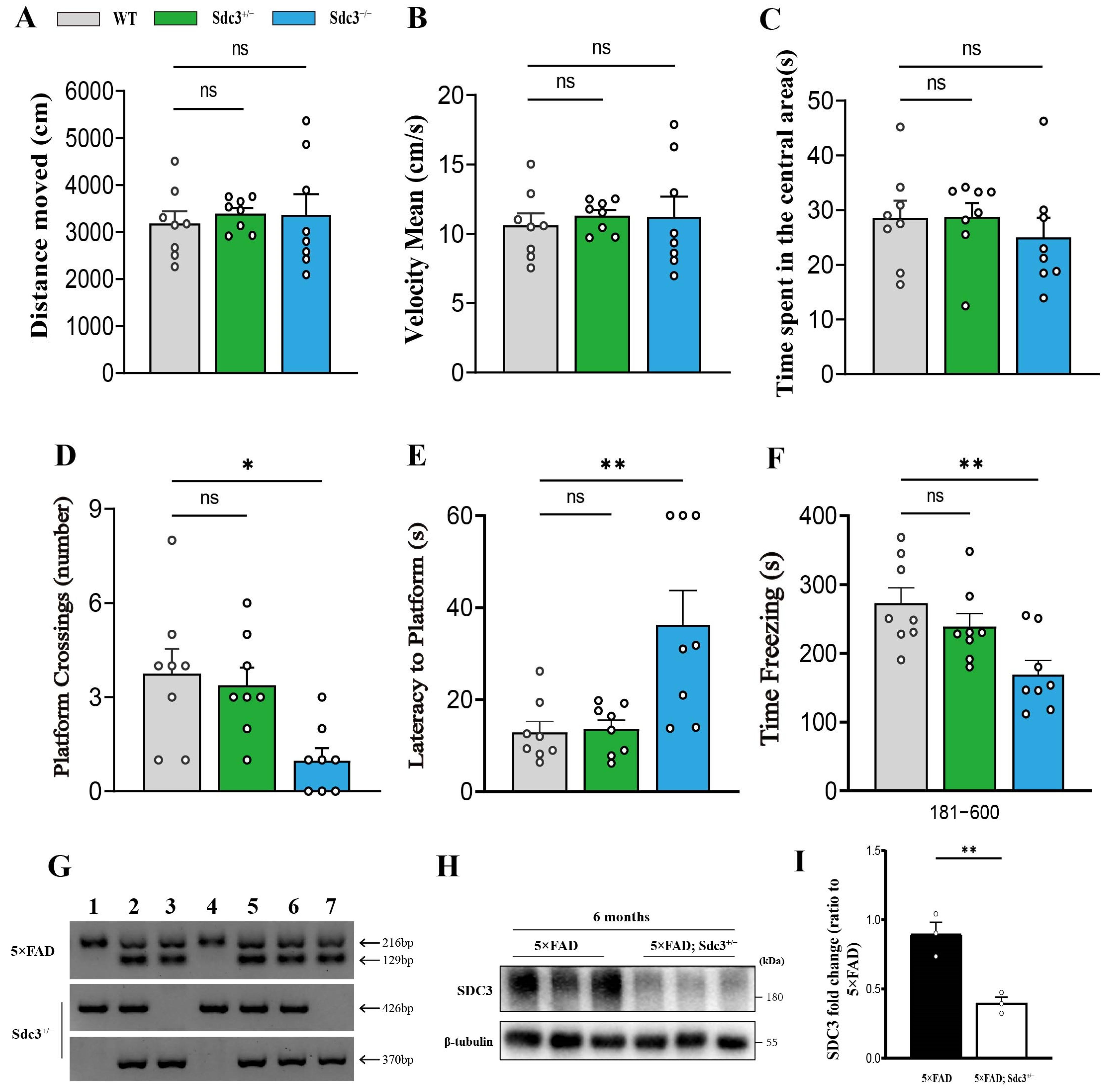
2.2. Sdc3−/− Mice Display Cognitive Deficits, While Sdc3+/− Mice Remain Unaffected
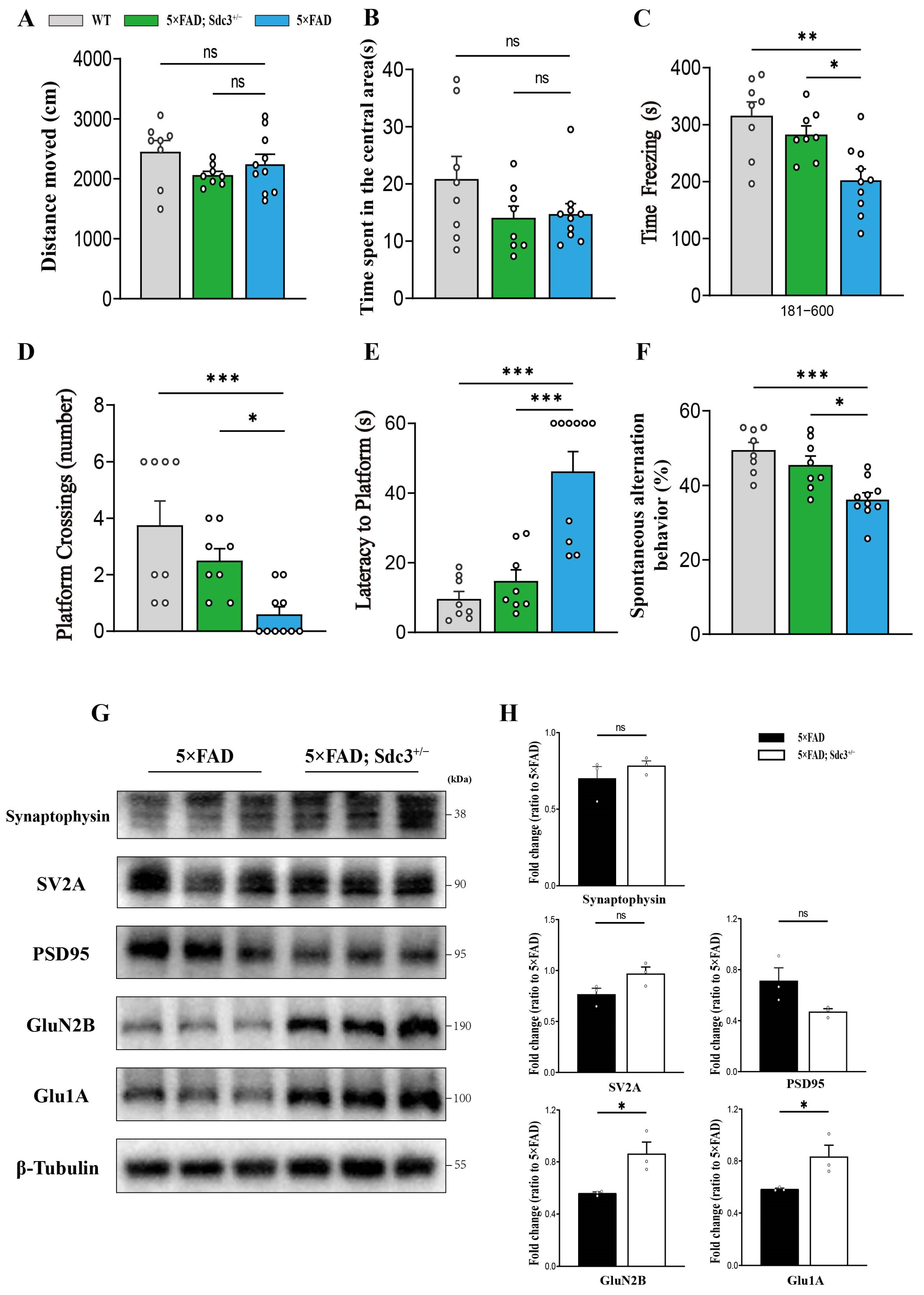
2.3. Downregulation of SDC3 Ameliorates Cognitive Impairment in 5×FAD Mice
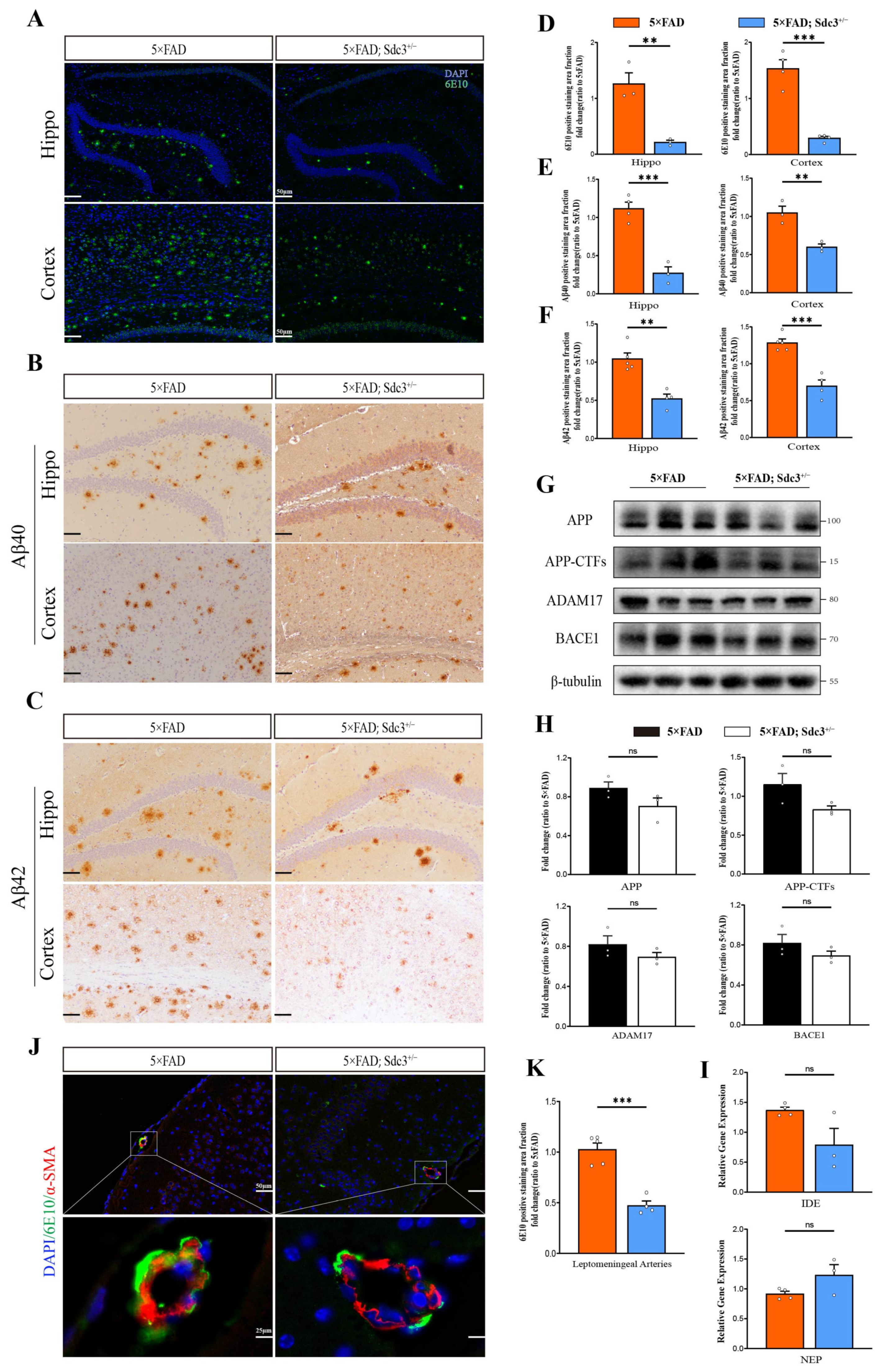
2.4. Downregulation of SDC3 Reduces Aβ Burden in 5×FAD Mice
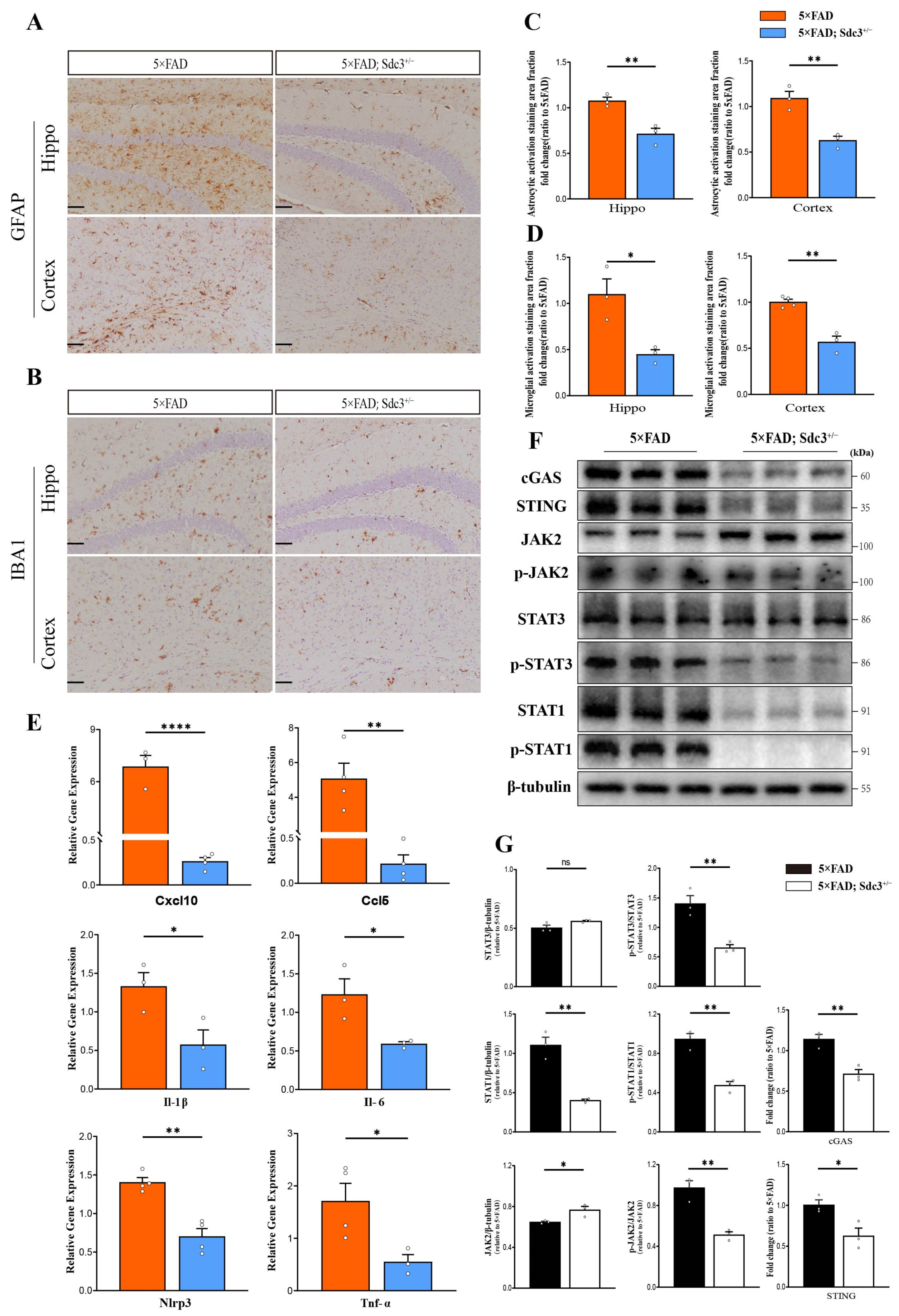
2.5. Downregulation of SDC3 Alleviates Neuroinflammation in the Brains of 5×FAD Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. PCR
4.3. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.4. Behavioral Tests
4.5. Tissue Dissection and Immunohistochemical Methods
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Clark, I.A.; Vissel, B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol. Commun. 2014, 2, 135. [Google Scholar] [CrossRef]
- Hardy, J. The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the “amyloid cascade hypothesis”. FEBS J. 2017, 284, 1040–1044. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Qin, Q.; Teng, Z.; Liu, C.; Li, Q.; Yin, Y.; Tang, Y. TREM2, microglia, and Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111438. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Dokholyan, N.V.; Mohs, R.C.; Bateman, R.J. Challenges and progress in research, diagnostics, and therapeutics in Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2022, 8, e12330. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.J.; Conner, K.; Asundi, V.K.; O’Mahony, D.J.; Stahl, R.C.; Showalter, L.; Cizmeci-Smith, G.; Hartman, J.; Rothblum, L.I. cDNA cloning, genomic organization, and in vivo expression of rat N-syndecan. J. Biol. Chem. 1997, 272, 2873–2879. [Google Scholar] [CrossRef][Green Version]
- Carey, D.J.; Evans, D.M.; Stahl, R.C.; Asundi, V.K.; Conner, K.J.; Garbes, P.; Cizmeci-Smith, G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J. Cell Biol. 1992, 117, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Upholt, W.B.; Kosher, R.A. Syndecan 3: A member of the syndecan family of membrane-intercalated proteoglycans that is expressed in high amounts at the onset of chicken limb cartilage differentiation. Proc. Natl. Acad. Sci. USA 1992, 89, 3271–3275. [Google Scholar] [CrossRef]
- Carey, D.J. N-syndecan: Structure and function of a transmembrane heparan sulfate proteoglycan. Perspect. Dev. Neurobiol. 1996, 3, 331–346. [Google Scholar]
- Kinnunen, A.; Kinnunen, T.; Kaksonen, M.; Nolo, R.; Panula, P.; Rauvala, H. N-syndecan and HB-GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur. J. Neurosci. 1998, 10, 635–648. [Google Scholar] [CrossRef]
- Kinnunen, T.; Raulo, E.; Nolo, R.; Maccarana, M.; Lindahl, U.; Rauvala, H. Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J. Biol. Chem. 1996, 271, 2243–2248. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Sheng, M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 7415–7425. [Google Scholar] [CrossRef] [PubMed]
- Hienola, A.; Tumova, S.; Kulesskiy, E.; Rauvala, H. N-syndecan deficiency impairs neural migration in brain. J. Cell Biol. 2006, 174, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, K.-I.; Hart, M.; Li, L. Alzheimer’s disease and heparan sulfate proteoglycan. Front. Biosci. A J. Virtual library 1998, 3, d327–d337. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H.; Williamson, T.; Reed, G.; Clarris, H.; Beyreuther, K.; Masters, C.L.; Nurcombe, V. The role of heparan sulfate proteoglycans in the pathogenesis of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1996, 777, 316–321. [Google Scholar] [CrossRef]
- van Horssen, J.; Wesseling, P.; van den Heuvel, L.P.; de Waal, R.M.; Verbeek, M.M. Heparan sulphate proteoglycans in Alzheimer’s disease and amyloid-related disorders. Lancet. Neurol. 2003, 2, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ghazizadeh, M.; Sato, S.; Oguro, T.; Kawanami, O. Interaction between beta-amyloid protein and heparan sulfate proteoglycans from the cerebral capillary basement membrane in Alzheimer’s disease. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2009, 16, 277–282. [Google Scholar]
- Bruinsma, I.B.; te Riet, L.; Gevers, T.; ten Dam, G.B.; van Kuppevelt, T.H.; David, G.; Küsters, B.; de Waal, R.M.W.; Verbeek, M.M. Sulfation of heparan sulfate associated with amyloid-beta plaques in patients with Alzheimer’s disease. Acta Neuropathol. 2010, 119, 211–220. [Google Scholar] [CrossRef]
- O’Callaghan, P.; Sandwall, E.; Li, J.P.; Yu, H.; Ravid, R.; Guan, Z.Z.; van Kuppevelt, T.H.; Nilsson, L.N.G.; Ingelsson, M.; Hyman, B.T.; et al. Heparan sulfate accumulation with Abeta deposits in Alzheimer’s disease and Tg2576 mice is contributed by glial cells. Brain Pathol. 2008, 18, 548–561. [Google Scholar] [CrossRef]
- Hudák, A.; Letoha, A.; Vizler, C.; Letoha, T. Syndecan-3 as a Novel Biomarker in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3407. [Google Scholar] [CrossRef]
- Letoha, T.; Hudák, A.; Kusz, E.; Pettkó-Szandtner, A.; Domonkos, I.; Jósvay, K.; Hofmann-Apitius, M.; Szilák, L. Contribution of syndecans to cellular internalization and fibrillation of amyloid-β(1–42). Sci. Rep. 2019, 9, 1393. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhao, N.; Yamaguchi, Y.; Cirrito, J.R.; Kanekiyo, T.; Holtzman, D.M.; Bu, G. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 332ra344. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Gea, L.; García, B.; Martín, C.; Ordiales, H.; García-Suárez, O.; Piña-Batista, K.M.; Merayo-Lloves, J.; Quirós, L.M.; Fernández-Vega, I. Heparan Sulfate Proteoglycans Undergo Differential Expression Alterations in Alzheimer Disease Brains. J. Neuropathol. Exp. Neurol. 2020, 79, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhang, L.; Zhang, Y.; Sun, X.; Wang, J.; Li, X.; Zhang, L.; Wang, X.; Morahan, G.; Qin, C. Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource. Int. J. Mol. Sci. 2022, 24, 682. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; Otte-Höller, I.; David, G.; Maat-Schieman, M.L.; van den Heuvel, L.P.; Wesseling, P.; de Waal, R.M.; Verbeek, M.M. Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol. 2001, 102, 604–614. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Otte-Höller, I.; van den Born, J.; van den Heuvel, L.P.; David, G.; Wesseling, P.; de Waal, R.M. Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer’s disease brain. Am. J. Pathol. 1999, 155, 2115–2125. [Google Scholar] [CrossRef]
- Snow, A.D.; Sekiguchi, R.T.; Nochlin, D.; Kalaria, R.N.; Kimata, K. Heparan sulfate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer’s disease brain. Am. J. Pathol. 1994, 144, 337–347. [Google Scholar]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. USA 2021, 118, e2011226118. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Turnbull, J.; Powell, A.; Guimond, S. Heparan sulfate: Decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001, 11, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.B.; DeVos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [PubMed]
- Hudák, A.; Kusz, E.; Domonkos, I.; Jósvay, K.; Kodamullil, A.T.; Szilák, L.; Hofmann-Apitius, M.; Letoha, T. Contribution of syndecans to cellular uptake and fibrillation of α-synuclein and tau. Sci. Rep. 2019, 9, 16543. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Bansal, R.; Frederickson, R.C.; Brunden, K.R. Proteoglycan-mediated inhibition of A beta proteolysis. A potential cause of senile plaque accumulation. J. Biol. Chem. 1995, 270, 18666–18671. [Google Scholar] [CrossRef]
- Kehoe, O.; Kalia, N.; King, S.; Eustace, A.; Boyes, C.; Reizes, O.; Williams, A.; Patterson, A.; Middleton, J. Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis Res. Ther. 2014, 16, R148. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef]
- Xie, Z.; Li, L.; Hou, W.; Fan, Z.; Zeng, L.; He, L.; Ji, Y.; Zhang, J.; Wang, F.; Xing, Z.; et al. Critical role of Oas1g and STAT1 pathways in neuroinflammation: Insights for Alzheimer’s disease therapeutics. J. Transl. Med. 2025, 23, 182. [Google Scholar] [CrossRef]
- Liu, M.; Pan, J.; Li, X.; Zhang, X.; Tian, F.; Li, M.; Wu, X.; Zhang, L.; Qin, C. Interleukin-6 deficiency reduces neuroinflammation by inhibiting the STAT3-cGAS-STING pathway in Alzheimer’s disease mice. J. Neuroinflammation 2024, 21, 282. [Google Scholar] [CrossRef]
- Chen, K.; Lai, C.; Su, Y.; Bao, W.D.; Yang, L.N.; Xu, P.P.; Zhu, L.Q. cGAS-STING-mediated IFN-I Response in Host Defense and Neuroinflammatory Diseases. Curr. Neuropharmacol. 2022, 20, 362–371. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Yu, L.; Qiao, G.; Qin, D.; Law, B.Y.-K.; Ren, F.; Wu, J.; Wu, A. Mitophagy and cGAS-STING crosstalk in neuroinflammation. Acta Pharm. Sinica. B 2024, 14, 3327–3361. [Google Scholar] [CrossRef]
- Arakawa, H.; Iguchi, Y. Ethological and multi-behavioral analysis of learning and memory performance in laboratory rodent models. Neurosci. Res. 2018, 135, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, J.; Huang, L.; Wang, T.J.; Huang, Y.; Li, Z.; He, J.; Sun, C.; Wang, J.; Chen, X.; et al. The pros and cons of motor, memory, and emotion-related behavioral tests in the mouse traumatic brain injury model. Neurol. Res. 2022, 44, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, D.; Sun, H.; Qu, Y.; Su, X. Behavioral tests for evaluating the characteristics of brain diseases in rodent models: Optimal choices for improved outcomes (Review). Mol. Med. Rep. 2022, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef] [PubMed]
| Target Primer | Oligonucleotide Sequence (5′ → 3′) |
|---|---|
| PCR | |
| 5×FAD | CGGGCCTCTTCGCTATTAC |
| ACCCCCATGTCAGAGTTCCT | |
| TATACAACCTTGGGGGATGG | |
| SDC3 F1 | TGGTTGTAAGACACAAATGCCCTAG |
| SDC3 R1 | AAAGGTTGGGTGTTAGCAGGCTAG |
| SDC3 F2 | TGATAGGGAGAAGACTAGGGAAGCC |
| SDC3 R2 | ATTGTAGCCCTACCTCAACCTCAGC |
| qRT-PCR | |
| Il-6 F | TCGTGGAAATGAGAAAAGAGTTG |
| Il-6 R | GACCACAGTGAGGAATGTCCAC |
| Tnf-α F | CCAGACCCTCACACTCAGATCAT |
| Tnf-α R | CGGCAGAGAGGAGGTTGACT |
| Nlrp3 F | GATCTTCGCTGCGATCAACAG |
| Nlrp3 R | CGTGCATTATCTGAACCCCAC |
| Il-1β F | TACCTATGTCTTGCCCGTGG |
| Il-1β R | TAGCAGGTCGTCATCCC |
| Ccl5 F | ACTCCCTGCTGCTTTGCCTAC |
| Ccl5 R | GAGGTTCCTTCGAGTGACA |
| Cxcl10 F | CCAAGTGCTGCCGTCATTTTC |
| Cxcl10 R | TCCCTAAGGCCCTCATTCTCA |
| Nep-F | GAGCCCCTTACTAGGCCTGTGT |
| Nep-R | CTCGATTCAGACATAGGCTTTCTAAA |
| Ide-F | CCGGCCATCCAGAGAATAGAA |
| Ide-R | ACGGTATTCCCGTTTGTCTTCA |
| Gapdh F | ATTCAACGGCACAGTCAAGG |
| Gapdh R | GCATTAGCTTCAGATTTACGG |
| Antibodies | Source | Identifier |
|---|---|---|
| Western blot | ||
| goat anti-Syndecan-3 | R&D Systems | AF2734 |
| rabbit anti-APP | CST | E4H1U |
| rabbit anti-APP-CTFs | Abcam | AB32136Y188 |
| rabbit anti-ADAM17 | Abcam | AB2051 |
| rabbit anti-BACE1 | CST | D10E5 |
| mouse anti-Synaptophysin | Abcam | AB309493 |
| rabbit anti-SV2A | CST | D1L8S |
| rabbit anti-PSD95 | CST | AB18258 |
| rabbit anti-GluN2B | CST | Cat# 4207 |
| rabbit anti-Glu1A | CST | Cat# 13,185 |
| rabbit anti-STAT3 | CST | Cat# 12,640 |
| rabbit anti-phospho-STAT3 | CST | Cat# 9131 |
| rabbit anti-STAT1 | CST | Cat# 9172 |
| rabbit anti-phospho-STAT1 | CST | Cat# 9167 |
| HRP Anti-β Tubulin | Abcam | ab21058 |
| Immunostaining | ||
| rabbit anti-IBA1 | Fujifilm Wako | Cat# 019-19741 |
| mouse anti-GFAP | Cell Signaling | Cat# 3670 |
| Purified anti-β-Amyloid, 1–16 | BioLegend | Cat# 803,001 |
| Aβ(1–40) Anti-Human | IBL America | Cat# 18,580 |
| Aβ(1–42) Anti-Human | IBL America | Cat# 18,582 |
| Rabbit anti-α-SMA | proteintech | 55135-1-AP |
| rabbit IgG (Alexa Fluor 647) | Abcam | ab150075 |
| mouse IgG (Alexa Fluor 488) | Abcam | ab150113 |
| goat IgG (Alexa Fluor 555) | Abcam | ab150134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Li, M.; Liu, M.; Wu, X.; Tian, F.; Gong, Y.; Cao, Y.; Zhang, J.; Zhang, X.; Qin, C.; et al. Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice. Int. J. Mol. Sci. 2025, 26, 5502. https://doi.org/10.3390/ijms26125502
Ye F, Li M, Liu M, Wu X, Tian F, Gong Y, Cao Y, Zhang J, Zhang X, Qin C, et al. Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice. International Journal of Molecular Sciences. 2025; 26(12):5502. https://doi.org/10.3390/ijms26125502
Chicago/Turabian StyleYe, Fan, Mingfeng Li, Min Liu, Xinghan Wu, Fan Tian, Yanju Gong, Yan Cao, Jingtai Zhang, Xueling Zhang, Chuan Qin, and et al. 2025. "Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice" International Journal of Molecular Sciences 26, no. 12: 5502. https://doi.org/10.3390/ijms26125502
APA StyleYe, F., Li, M., Liu, M., Wu, X., Tian, F., Gong, Y., Cao, Y., Zhang, J., Zhang, X., Qin, C., & Zhang, L. (2025). Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice. International Journal of Molecular Sciences, 26(12), 5502. https://doi.org/10.3390/ijms26125502





