Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma
Abstract
1. Introduction
2. Results
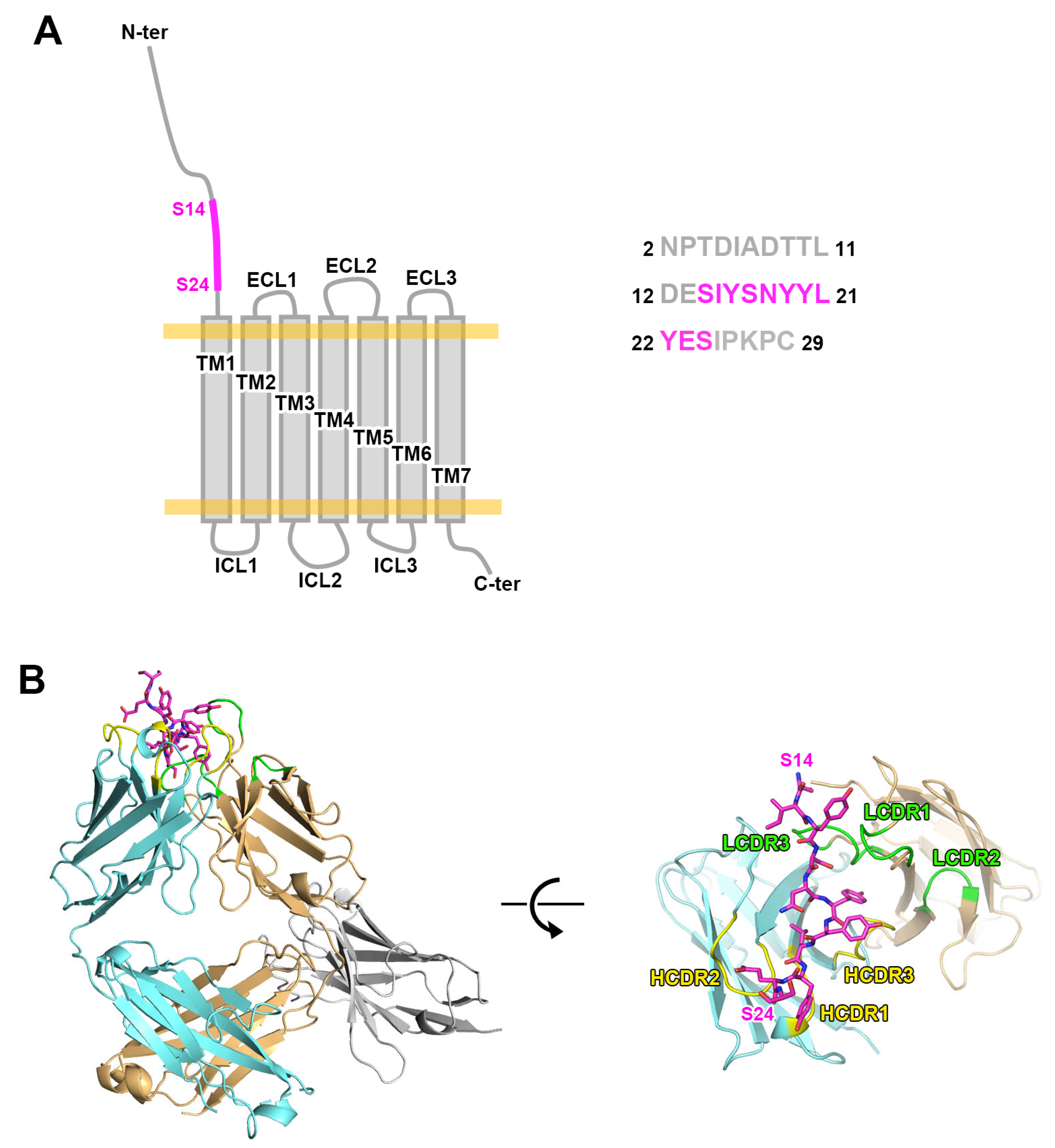
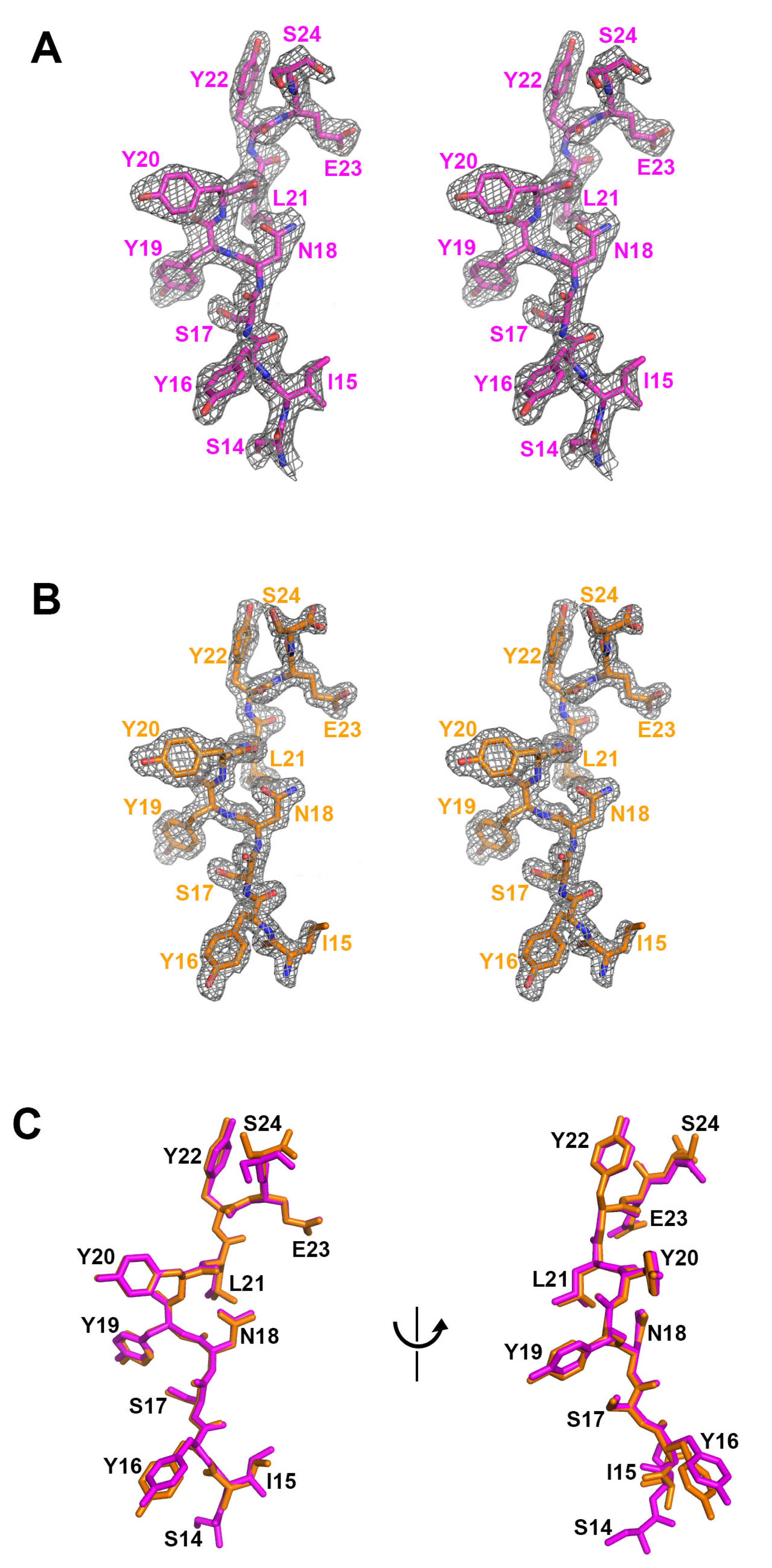
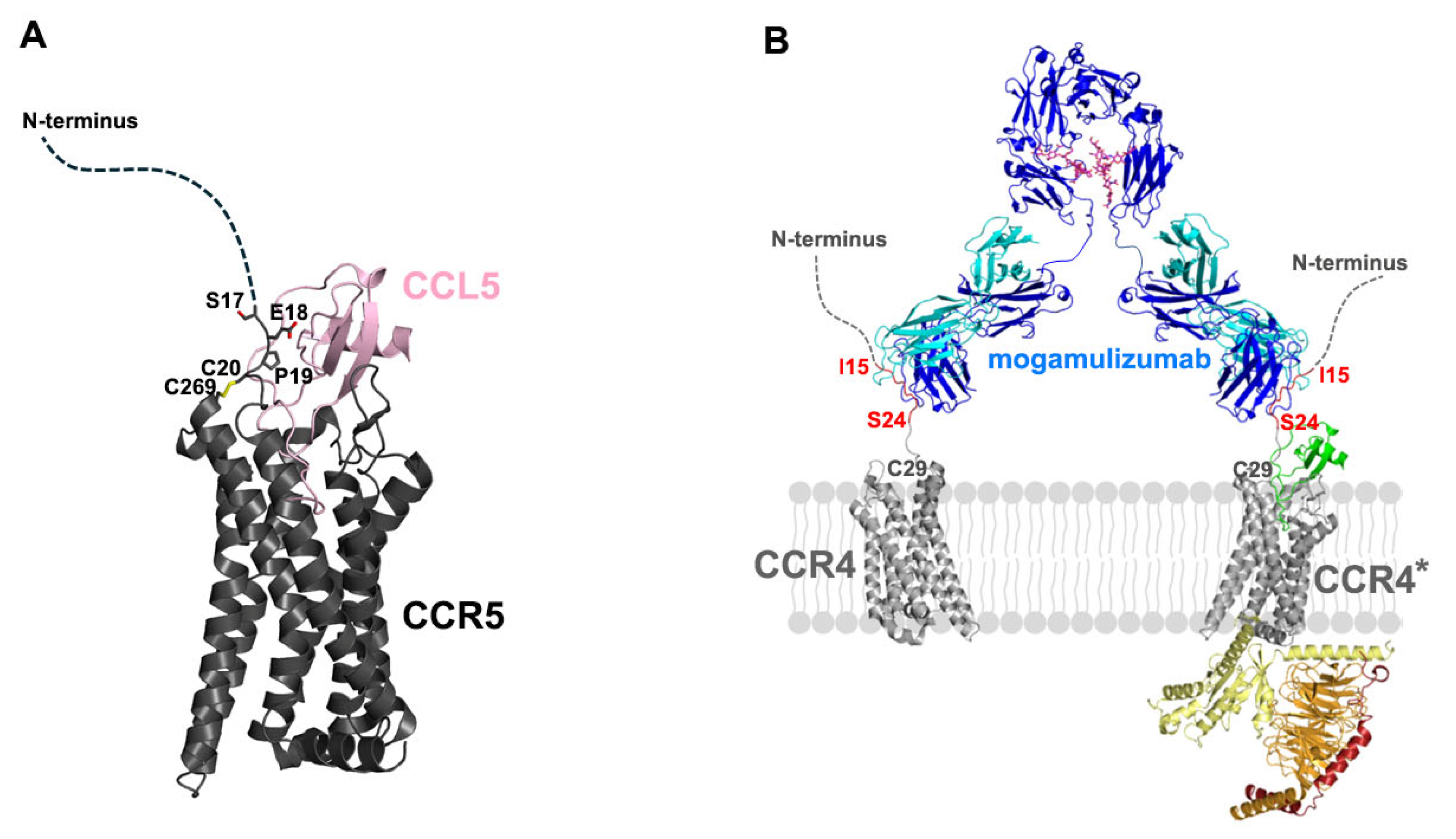
2.1. Structure of Mogamulizumab with Bound CCR4 Peptides
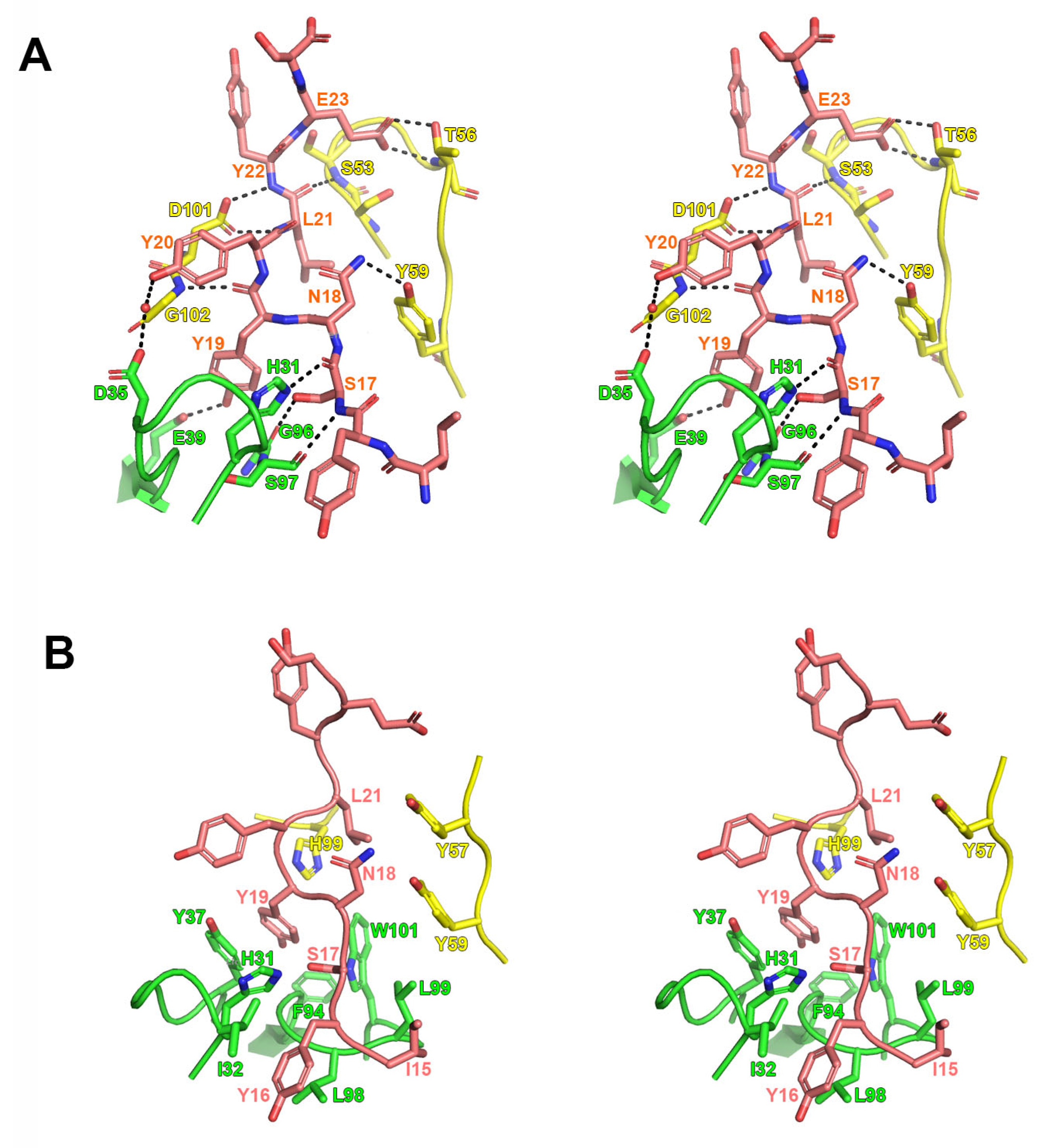
2.2. Interactions Between Mogamulizumab and CCR4
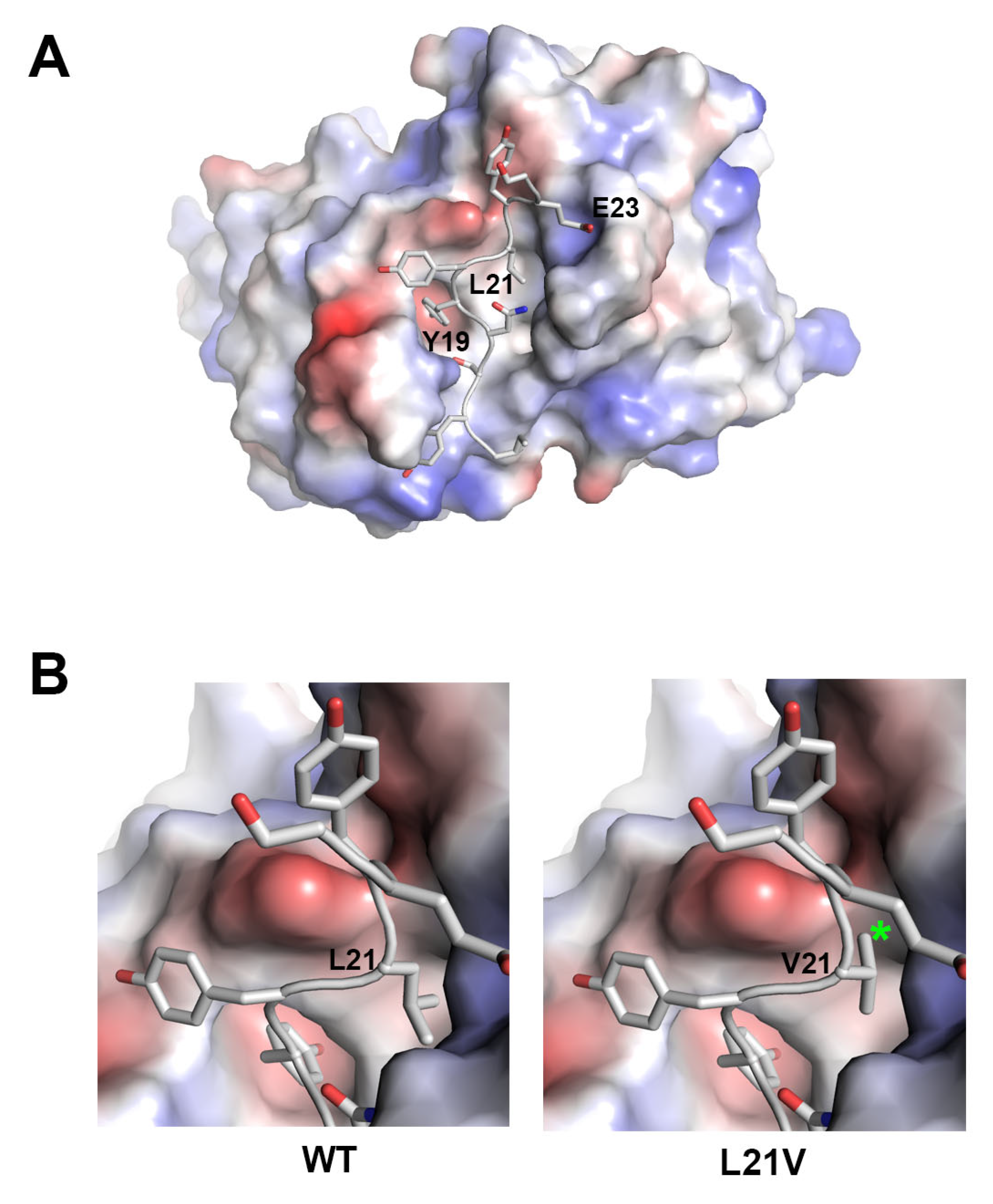
2.3. Structural Insights into Resistance to Mogamulizumab
2.4. Molecular Mechanism of Mogamulizumab ADCC
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Peptide Preparation
4.2. Crystallization of Proteins
4.3. Data Collection and Structure Determination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2014, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C chemokines CCL17 and CCL22 and their receptor CCR4 in CNS autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.S.; Wedgeworth, E.; Crichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M.; et al. Survival Outcomes and prognostic Factors in Mycosis Fungoides/Sézary Syndrome: Validation of the Revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging Proposal. J. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef]
- Dobos, G.; Pohrt, A.; Ram-Wolff, C.; Lebbé, C.; Bouaziz, J.-D.; Battistella, M.; Bagot, M.; De Masson, A. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Latzka, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Guenova, E.; Gniadecki, R.; Hodak, E.; Jonak, C.; Klemke, C.-D.; et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2023. Eur. J. Cancer 2023, 195, 113343. [Google Scholar] [CrossRef]
- Duvic, M.; Evans, M.; Wang, C. Mogamulizumab for the treatment of cutaneous T-cell lymphoma: Recent advances and clinical potential. Ther. Adv. Hematol. 2016, 7, 171–174. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.; Teal, E.; Beers, S.; Cragg, M. FC-Engineering for Modulated Effector Functions—Improving antibodies for cancer treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018, 10, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Langridge, T.; Duvic, M. Depletion of regulatory T cells by targeting CC chemokine receptor type 4 with mogamulizumab. OncoImmunology 2015, 4, e1011524. [Google Scholar] [CrossRef]
- Whiteside, T. The role of regulatory T cells in cancer immunology. ImmunoTargets Ther. 2015, 2015, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Jorgensen, J.L.; Goswami, M.; Challagundla, P.; Decker, W.K.; Kim, Y.H.; Duvic, M.A. Reduction of Regulatory T Cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sézary Syndrome. Clin. Cancer Res. 2014, 21, 274–285. [Google Scholar] [CrossRef]
- Ogura, M.; Ishida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tobinai, K.; Fujimoto, K.; Yamamoto, K.; Miyamoto, T.; Uike, N.; et al. Multicenter Phase II study of mogamulizumab (KW-0761), a defucosylated Anti-CC chemokine receptor 4 antibody, in patients with relapsed peripheral T-Cell lymphoma and cutaneous T-Cell lymphoma. J. Clin. Oncol. 2014, 32, 1157–1163. [Google Scholar] [CrossRef]
- PharmD, S.W.; Bcps, J.B.M.P. Mogamulizumab-KPKC: A novel therapy for the treatment of cutaneous T-Cell lymphoma. J. Adv. Pract. Oncol. 2019, 10, 883–888. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Albrecht, J.D.; Alberti-Violetti, S.; Berti, E. CCR4 in cutaneous T-cell lymphoma: Therapeutic targeting of a pathogenic driver. Eur. J. Immunol. 2021, 51, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Beygi, S.; Duran, G.E.; Fernandez-Pol, S.; Rook, A.H.; Kim, Y.H.; Khodadoust, M.S. Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma. Blood 2022, 139, 3732–3736. [Google Scholar] [CrossRef]
- Cleary, K.L.S.; Chan, H.T.C.; James, S.; Glennie, M.J.; Cragg, M.S. Antibody Distance from the Cell Membrane Regulates Antibody Effector Mechanisms. J. Immunol. 2017, 198, 3999–4011. [Google Scholar] [CrossRef]
- Yoshie, O. CCR4 as a therapeutic target for cancer immunotherapy. Cancers 2021, 13, 5542. [Google Scholar] [CrossRef]
- Choi, S.B.; Gu, N.; Jang, Y.-J.; Park, U.B.; Jeong, T.J.; Lee, H.T.; Heo, Y.-S. Crystal structure of cobolimab, an investigational antibody against Tim-3 for cancer immunotherapy, in complex with anti-kappa nanobody. Korean Soc. Struct. Biol. 2022, 10, 46–50. [Google Scholar] [CrossRef]
- Ereño-Orbea, J.; Sicard, T.; Cui, H.; Carson, J.; Hermans, P.; Julien, J.-P. Structural basis of enhanced crystallizability induced by a molecular chaperone for antibody Antigen-Binding fragments. J. Mol. Biol. 2017, 430, 322–336. [Google Scholar] [CrossRef]
- Querfeld, C. Mechanisms of resistance to mogamulizumab. Blood 2022, 139, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Shen, Q.; Yao, B.; Mao, C.; Chen, L.-N.; Zhang, H.; Shen, D.-D.; Zhang, C.; Li, W.; Du, X.; et al. Identification and mechanism of G protein-biased ligands for chemokine receptor CCR1. Nat. Chem. Biol. 2021, 18, 264–271. [Google Scholar] [CrossRef]
- Shao, Z.; Tan, Y.; Shen, Q.; Hou, L.; Yao, B.; Qin, J.; Xu, P.; Mao, C.; Chen, L.-N.; Zhang, H.; et al. Molecular insights into ligand recognition and activation of chemokine receptors CCR2 and CCR3. Cell Discov. 2022, 8, 44. [Google Scholar] [CrossRef]
- Isaikina, P.; Tsai, C.-J.; Dietz, N.; Pamula, F.; Grahl, A.; Goldie, K.N.; Guixà-González, R.; Branco, C.; Paolini-Bertrand, M.; Calo, N.; et al. Structural basis of the activation of the CC chemokine receptor 5 by a chemokine agonist. Sci. Adv. 2021, 7, eabg8685. [Google Scholar] [CrossRef] [PubMed]
- Wasilko, D.J.; Johnson, Z.L.; Ammirati, M.; Che, Y.; Griffor, M.C.; Han, S.; Wu, H. Structural basis for chemokine receptor CCR6 activation by the endogenous protein ligand CCL20. Nat. Commun. 2020, 11, 3031. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, X.; Wu, L.; Wang, L.; Wu, Y.; Xu, Z.; Xu, F. Unveiling the structural mechanisms of nonpeptide ligand recognition and activation in human chemokine receptor CCR8. Sci. Adv. 2024, 10, eadj7500. [Google Scholar] [CrossRef]
- Oswald, C.; Rappas, M.; Kean, J.; Doré, A.S.; Errey, J.C.; Bennett, K.; Deflorian, F.; Christopher, J.A.; Jazayeri, A.; Mason, J.S.; et al. Intracellular allosteric antagonism of the CCR9 receptor. Nature 2016, 540, 462–465. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Godwin, C.D.; Laszlo, G.S.; Fiorenza, S.; Garling, E.E.; Phi, T.-D.; Bates, O.M.; Correnti, C.E.; Hoffstrom, B.G.; Lunn, M.C.; Humbert, O.; et al. Targeting the membrane-proximal C2-set domain of CD33 for improved CD33-directed immunotherapy. Leukemia 2021, 35, 2496–2507. [Google Scholar] [CrossRef]
- Lim, S.Y.T.; Cole, F.M.; Laszlo, G.S.; Lunn-Halbert, M.C.; Huo, J.; Li, J.; Kehret, A.R.; Walter, R.B. Targeting the Membrane-Proximal domain of CD33 to maximize the efficacy of natural killer Cell-Based immunotherapies. Blood 2024, 144 (Suppl. S1), 3436. [Google Scholar] [CrossRef]
- Shim, H. One target, different effects: A comparison of distinct therapeutic antibodies against the same targets. Exp. Mol. Med. 2011, 43, 539. [Google Scholar] [CrossRef] [PubMed]
- Craigen, J.L.; Mackus, W.J.M.; Engleberts, P.; Miller, S.R.; Speller, S.; Chamberlain, L.C.; Davis, B.G.; McHugh, S.M.; Bullmore, E.; Cox, C.J.; et al. Ofatumumab, a human MAB targeting a Membrane-Proximal Small-Loop epitope on CD20, induces potent NK Cell-Mediated ADCC. Blood 2009, 114, 1725. [Google Scholar] [CrossRef]
- Freeman, R.; Shahid, S.; Khan, A.G.; Mathew, S.C.; Souness, S.; Burns, E.R.; Um, J.S.; Tanaka, K.; Cai, W.; Yoo, S.; et al. Developing a membrane-proximal CD33-targeting CAR T cell. J. Immunother. Cancer 2024, 12, e009013. [Google Scholar] [CrossRef]
- Cunningham, O.; Scott, M.; Zhou, Z.S.; Finlay, W.J.J. Polyreactivity and polyspecificity in therapeutic antibody development: Risk factors for failure in preclinical and clinical development campaigns. mAbs 2021, 13, e1999195. [Google Scholar] [CrossRef]
- Musnier, A.; Bourquard, T.; Vallet, A.; Mathias, L.; Bruneau, G.; Ayoub, M.A.; Travert, O.; Corde, Y.; Gallay, N.; Boulo, T.; et al. A New in Silico Antibody Similarity Measure Both Identifies Large Sets of Epitope Binders with Distinct CDRs and Accurately Predicts Off-Target Reactivity. Int. J. Mol. Sci. 2022, 23, 9765. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
| Mogamulizumab /CCR4 (N2-C29) /VHH | Mogamulizumab /CCR4 (S14-S24) | Mogamulizumab /VHH | |
|---|---|---|---|
| Data Collection | |||
| X-ray Source | PLS 5C | PLS 5C | PLS 7A |
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 |
| Space Group | P1 | P1 | P3221 |
| a, b, c (Å) | 65.39, 71.19, 73.45 | 41.27, 67.44, 87.83 | 110.02, 110.02, 120.97 |
| α, β, γ (°) | 81.24, 61.41, 71.05 | 87.2, 82.7, 79.2 | 90.00, 90.00, 120.00 |
| Resolution (Å) | 2.01 (2.07–2.01) 1 | 1.63 (1.69–1.63) | 2.94 (3.00–2.94) |
| Rmeas (%) | 9.50 (57.70) | 5.40 (67.90) | 7.30 (65.5) |
| I/σI | 7.27 (1.62) | 11.28 (1.59) | 22.7 (2.31) |
| Completeness (%) | 93.6 (94.6) | 94.8 (93.5) | 99.8 (99.6) |
| Redundancy | 1.8 (1.9) | 2.0 (1.8) | 9.6 (9.7) |
| CC1/2 | 0.995 (0.792) | 0.999 (0.710) | 0.983 (0.853) |
| Refinement | |||
| Resolution (Å) | 2.01 | 1.63 | 2.94 |
| No. of Reflections | 70,477 | 109,223 | 17,972 |
| Rwork/Rfree (%) | 19.7/24.4 | 16.8/19.7 | 22.6/27.6 |
| No. of Atoms | |||
| Protein | 8481 | 6776 | 4254 |
| Water | 874 | 1187 | 0 |
| Average B-factor (Å2) | 37.0 | 27.0 | 71.0 |
| R.m.s. Deviation | |||
| Bond Lengths (Å) | 0.007 | 0.005 | 0.009 |
| Bond Angles (°) | 0.885 | 0.765 | 1.292 |
| Ramachandran | |||
| Favored (%) | 99.26 | 98.15 | 99.27 |
| Allowed (%) | 0.74 | 1.85 | 0.73 |
| Outlier (%) | 0.00 | 0.00 | 0.00 |
| PDB Code | 9ULM | 9ULL | 9ULN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.B.; Lee, H.T.; Gu, N.; Jang, Y.-J.; Park, U.B.; Jeong, T.J.; Lee, S.H.; Heo, Y.-S. Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma. Int. J. Mol. Sci. 2025, 26, 5500. https://doi.org/10.3390/ijms26125500
Choi SB, Lee HT, Gu N, Jang Y-J, Park UB, Jeong TJ, Lee SH, Heo Y-S. Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma. International Journal of Molecular Sciences. 2025; 26(12):5500. https://doi.org/10.3390/ijms26125500
Chicago/Turabian StyleChoi, Seung Beom, Hyun Tae Lee, Nahyeon Gu, Yu-Jeong Jang, Ui Beom Park, Tae Jun Jeong, Sang Hyung Lee, and Yong-Seok Heo. 2025. "Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma" International Journal of Molecular Sciences 26, no. 12: 5500. https://doi.org/10.3390/ijms26125500
APA StyleChoi, S. B., Lee, H. T., Gu, N., Jang, Y.-J., Park, U. B., Jeong, T. J., Lee, S. H., & Heo, Y.-S. (2025). Structural Insights into the ADCC Mechanism and Resistance of Mogamulizumab, a First-in-Class Anti-CCR4 Therapy for Cutaneous T Cell Lymphoma. International Journal of Molecular Sciences, 26(12), 5500. https://doi.org/10.3390/ijms26125500







