Early Cardiovascular and Metabolic Benefits of rhGH Therapy in Adult Patients with Severe Growth Hormone Deficiency: Impact on Oxidative Stress Parameters
Abstract
1. Introduction
2. Results
2.1. Biochemical Analysis
2.1.1. IGF-1 and Ca Measurements
2.1.2. Endothelin-1, Asymmetric Dimethylarginine, and Oxidative Stress
2.1.3. Nitric Oxide and Lipid Profile
2.2. DXA and Body Composition
2.3. Correlations
3. Discussion
4. Materials and Methods
4.1. Studied Population
4.2. Biochemical Measurement
4.3. Statistical Analysis
4.4. Dual-Energy X-Ray Absorptiometry and Body Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Aguiar-Oliveira, M.H.; Bartke, A. Growth Hormone Deficiency: Health and Longevity. Endocr. Rev. 2019, 40, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, F.; Kelestimur, F. Classical and non-classical causes of GH deficiency in adults. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Gan, H.W.; Ibba, A.; Patti, G.; Dattani, M.; Loche, S.; Maghnie, M.; Salvatori, R. Advances in differential diagnosis and management of growth hormone deficiency in children. Nat. Rev. Endocrinol. 2021, 17, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Collett-Solberg, P.F.; Ambler, G.; Backeljauw, P.F.; Bidlingmaier, M.; Biller, B.M.; Boguszewski, M.C.; Cheung, P.T.; Choong, C.S.Y.; Cohen, L.E.; Cohen, P.; et al. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm. Res. Paediatr. 2019, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Laffan, E. Congenital Growth Hormone Deficiency—A Review with a Focus on Neuroimaging. Eur. Endocrinol. 2013, 9, 136–140. [Google Scholar] [CrossRef]
- Vázquez-Borrego, M.C.; del Rio-Moreno, M.; Kineman, R.D. Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism. Cells 2021, 10, 2532. [Google Scholar] [CrossRef]
- Wang, C.; Huang, H.; Zhao, C.; Zhao, J.; Xiong, R.; Jin, R.; Bai, Y. The impact of pegylated recombinant human growth hormone replacement therapy on glucose and lipid metabolism in children with growth hormone deficiency. Ann. Palliat. Med. 2021, 10, 1809–1814. [Google Scholar] [CrossRef]
- Kubo, T.; Furujo, M.; Takahashi, K.; Hyodo, Y.; Tsuchiya, H.; Hattori, M.; Fujinaga, S.; Urayama, K. Effects of Growth Hormone Treatment on Lipid Profiles. Indian J. Pediatr. 2017, 85, 261–265. [Google Scholar] [CrossRef]
- Møller, N.; Jørgensen, J.O.L. Effects of Growth Hormone on Glucose, Lipid, and Protein Metabolism in Human Subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Colao, A.; di Somma, C.; Pivonello, R.; Cuocolo, A.; Spinelli, L.; Bonaduce, D.; Salvatore, M.; Lombardi, G. The Cardiovascular Risk of Adult GH Deficiency (GHD) Improved after GH Replacement and Worsened in Untreated GHD: A 12-Month Prospective Study. J. Clin. Endocrinol. Metab. 2002, 87, 1088–1093. [Google Scholar] [CrossRef]
- Suzuki, K.; Yanagi, K.; Shimizu, M.; Wakamatsu, S.; Niitani, T.; Hosonuma, S.; Sagara, M.; Aso, Y. Effect of growth hormone replacement therapy on plasma diacron-reactive oxygen metabolites and endothelial function in Japanese patients: The GREAT clinical study. Endocr. J. 2018, 65, 101–111. [Google Scholar] [CrossRef]
- Evans, L.; Davies, J.; Anderson, R.; Ellis, G.; Jackson, S.; Lewis, M.; Frenneaux, M.; Rees, A.; Scanlon, M. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur. J. Endocrinol. 2000, 142, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, C.K.; Zhang, C.-L.; Huang, Y. Interplay Between Oxidative Stress, Cyclooxygenases, and Prostanoids in Cardiovascular Diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. (Landmark Ed.) 2022, 27, 105. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Wen, H.J.; Liu, G.F.; Xiao, L.Z.; Wu, Y.G. Involvement of endothelial nitric oxide synthase pathway in IGF-1 protects endothelial progenitor cells against injury from oxidized LDLs. Mol. Med. Rep. 2019, 19, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Obradovic, M.; Zafirovic, S.; Soskic, S.; Stanimirovic, J.; Trpkovic, A.; Jevremovic, D.; Isenovic, E.R. Effects of IGF-1 on the Cardiovascular System. Curr. Pharm. Des. 2019, 25, 3715–3725. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Shang, R.; Chen, Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide Biol. Chem. 2018, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.H.; Dora, K.A.; Lemmey, H.A.; Lin, J.; Alden, J.; Wallis, L.; Donovan, L.; Shorthose, O.; Leiper, F.C.; Leiper, J.; et al. Asymmetric Dimethylarginine Enables Depolarizing Spikes and Vasospasm in Mesenteric and Coronary Resistance Arteries. Hypertension 2024, 81, 764–775. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Barrea, L.; Colao, A. The Complex Relationship between Obesity and the Somatotropic Axis: The Long and Winding Road. Growth Horm. IGF Res. 2014, 24, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peng, J.; Wang, N.; Wu, Z.; Zhang, Y.; Cui, H.; Zang, D.; Lu, F.; Ma, X.; Yang, J. Comparison of seven surrogate insulin resistance indexes for prediction of incident coronary heart disease risk: A 10-year prospective cohort study. Front. Endocrinol. 2024, 15, 1290226. [Google Scholar] [CrossRef]
- Liu, W.; Weng, S.; Chen, Y.; Cao, C.; Peng, D. Age-adjusted visceral adiposity index (VAI) is superior to VAI for predicting mortality among US adults: An analysis of the NHANES 2011–2014. Aging Clin. Exp. Res. 2024, 35, 1367–1376. [Google Scholar] [CrossRef]
- Qin, Z.; Jiang, L.; Sun, J.; Geng, J.; Chen, S.; Yang, Q.; Su, B.; Liao, R. Higher visceral adiposity index is associated with increased likelihood of abdominal aortic calcification. Clinics 2022, 77, 100114. [Google Scholar] [CrossRef]
- Hansen, T.B.; Brixen, K.; Vahl, N.; Jørgensen, J.O.; Christiansen, J.S.; Mosekilde, L.; Hagen, C. Effects of 12 months of growth hormone (GH) treatment on calciotropic hormones, calcium homeostasis, and bone metabolism in adults with acquired GH deficiency: A double blind, randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 1996, 81, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Endo, T.; Tsuboi, S.; Fujio, S. Prevalence and risk of complications in untreated patients with adult growth hormone deficiency. Pituitary 2025, 28, 32. [Google Scholar] [CrossRef]
- Colao, A. Cardiovascular Effects of Growth Hormone Treatment: Potential Risks and Benefits. Horm. Res. 2004, 62 (Suppl. S3), 42–50. [Google Scholar] [CrossRef]
- Ahmid, M.; Perry, C.G.; Ahmed, S.F.; Shaikh, M.G. Growth hormone deficiency during young adulthood and the benefits of growth hormone replacement. Endocr. Connect. 2016, 5, R1–R11. [Google Scholar] [CrossRef] [PubMed]
- Binder, G.; Donner, J.; Becker, B.; Bauer, J.L.; Schweizer, R. Changes in body composition in male adolescents with childhood-onset GH deficiency during transition. Clin. Endocrinol. 2019, 91, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Bitti, S.R.; Franco, M.; Albertelli, M.; Gatto, F.; Vera, L.; Ferone, D.; Boschetti, M. GH Replacement in the Elderly: Is It Worth It? Front. Endocrinol. 2021, 12, 680579. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Martins, S.R.; Toledo, S.L.O.; da Silva, A.J.; Mendes, F.S.; de Oliveira, M.M.; Ferreira, L.G.R.; Dusse, L.M.S.; Carvalho, M.d.G.; Rios, D.R.A.; Alpoim, P.N.; et al. Endothelial dysfunction biomarkers in sickle cell disease: Is there a role for ADMA and PAI-1? Ann. Hematol. 2021, 101, 273–280. [Google Scholar] [CrossRef]
- Sonkar, S.K.; Verma, J.; Sonkar, G.K.; Gupta, A.; Singh, A.; Vishwakarma, P.; Bhosale, V. Assessing the Role of Asymmetric Dimethylarginine in Endothelial Dysfunction: Insights into Cardiovascular Risk Factors. Cureus 2025, 17, e77565. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, F.; Li, J.; Chen, L.; Mao, Y.-F.; Li, Q.-B.; Nie, C.-Y.; Lin, C.; Xiao, J. IGF-1 inhibits inflammation and accelerates angiogenesis via Ras/PI3K/IKK/NF-κB signaling pathways to promote wound healing. Eur. J. Pharm. Sci. 2024, 200, 106847. [Google Scholar] [CrossRef]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.-Y.; Delafontaine, P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metab. 2010, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Sawada, S.; Satoh, M.; Asai, Y.; Kodama, S.; Sato, T.; Tomiyama, S.; Seike, J.; Takahashi, K.; Kaneko, K.; et al. Insulin-like growth factor-1 levels are associated with high comorbidity of metabolic disorders in obese subjects; a Japanese single-center, retrospective-study. Sci. Rep. 2022, 12, 20130. [Google Scholar] [CrossRef]
- Hjelholt, A.; Høgild, M.; Bak, A.M.; Arlien-Søborg, M.C.; Baek, A.; Jessen, N.; Richelsen, B.; Pedersen, S.B.; Moller, N.; Jorgensen, J.O.L. Growth Hormone and Obesity. Endocrinol. Metab. Clin. N. Am. 2020, 49, 239–250. [Google Scholar] [CrossRef]
- Verhelst, J.; Abs, R.; Vandeweghe, M.; Mockel, J.; Legros, J.; Copinschi, G.; Mahler, C.; Velkeniers, B.; Vanhaelst, L.; Van Aelst, A.; et al. Two years of replacement therapy in adults with growth hormone deficiency. Clin. Endocrinol. 1997, 47, 485–494. [Google Scholar] [CrossRef]
- McCallum, R.W.; Sainsbury, C.A.R.; Spiers, A.; Dominiczak, A.F.; Petrie, J.R.; Sattar, N.; Connell, J.M.C. Growth hormone replacement reduces C-reactive protein and large-artery stiffness but does not alter endothelial function in patients with adult growth hormone deficiency. Clin. Endocrinol. 2005, 62, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Sakai, S.; Ieda, M. Endothelin-1 alters BMP signaling to promote proliferation of pulmonary artery smooth muscle cells. Can. J. Physiol. Pharmacol. 2022, 100, 1018–1027. [Google Scholar] [CrossRef]
- Stauffer, B.L.; Westby, C.M.; A DeSouza, C. Endothelin-1, aging and hypertension. Curr. Opin. Cardiol. 2008, 23, 350–355. [Google Scholar] [CrossRef]
- Sam, F.; Colucci, W.S. Endothelin-1 in heart failure: Does it play a role? Cardiologia 1998, 43, 889–892. [Google Scholar] [PubMed]
- Qin, L.; Liu, X.; Li, Y. Correlation of serum BNP and ET-1 levels with cardiac pump function and ventricular remodeling in patients with heart failure. Cell. Mol. Biol. 2020, 66, 125–131. [Google Scholar] [CrossRef]
- Kaminski, H.J.; Andrade, F.H. Nitric oxide: Biologic effects on muscle and role in muscle diseases. Neuromuscul. Disord. 2001, 11, 517–524. [Google Scholar] [CrossRef]
- Alonso, D.; Radomski, M.W. The Nitric Oxide-Endothelin-1 Connection. Hear. Fail. Rev. 2003, 8, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Seccia, T.M.; Nussdorfer, G.G. Reciprocal regulation of endothelin-1 and nitric oxide: Relevance in the physiology and pathology of the cardiovascular system. Int. Rev. Cytol. 2001, 209, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Vierhapper, H. Effect of endothelin-1 in man—Impact on basal and stimulated concentrations of luteinizing hormone, follicle-stimulating hormone, thyrotropin, growth hormone, corticotropin, and prolactin with and without pretreatment with nifedipine. Metabolism 1996, 45, 658–661. [Google Scholar] [CrossRef]
- Bouras, G.; Deftereos, S.; Tousoulis, D.; Giannopoulos, G.; Chatzis, G.; Tsounis, D.; Cleman, M.W.; Stefanadis, C. Asymmetric Dimethylarginine (ADMA): A Promising Biomarker for Cardiovascular Disease? Curr. Top. Med. Chem. 2013, 13, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leiper, J. Cardiovascular Biology of the Asymmetric Dimethylarginine: Dimethylarginine Dimethylaminohydrolase Pathway. Arter. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef]
- Bekyarova, G.Y.; Vankova, D.G.; Madjova, V.H.; Bekyarov, N.A.; Salim, A.S.; Ivanova, D.G.; Stoeva, S.M.; Gerova, D.I.; Kiselova-Kaneva, Y.D. Association between Nfr2, HO-1, NF-kB Expression, Plasma ADMA, and Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 17067. [Google Scholar] [CrossRef]
- Gajecki, D.; Gawryś, J.; Wiśniewski, J.; Fortuna, P.; Szahidewicz-Krupska, E.; Doroszko, A. A Cross-Talk between the Erythrocyte L-Arginine/ADMA/Nitric Oxide Metabolic Pathway and the Endothelial Function in Subjects with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 2306. [Google Scholar] [CrossRef]
- Improda, N.; Moracas, C.; Raso, G.M.; Valente, V.; Crisci, G.; Lorello, P.; Di Mase, R.; Salerno, M.; Capalbo, D. Vascular Function and Intima-Media Thickness in Children and Adolescents with Growth Hormone Deficiency: Results from a Prospective Case-Control Study. Horm. Res. Paediatr. 2024, 97, 140–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Shao, R.; Chen, R.; Zheng, Q.; Yao, M.; Li, K.; Cao, Y.; Jiang, L. Oxidative stress disrupts vascular microenvironmental homeostasis affecting the development of atherosclerosis. Cell Biol. Int. 2024, 48, 1781–1801. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Oxidative Stress and Cardiovascular Aging: Interaction Between NRF-2 and ADMA. Curr. Cardiol. Rev. 2017, 13, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Bruno, C.; Vergani, E.; Guidi, F.; Angelini, F.; Meucci, E.; Silvestrini, A. Evaluation of oxidative stress effects on different macromolecules in adult growth hormone deficiency. PLoS ONE 2020, 15, e0236357. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Di Segni, C.; Bruno, C.; Olivieri, G.; Guidi, F.; Silvestrini, A.; Meucci, E.; Orlando, P.; Silvestri, S.; Tiano, L.; et al. Oxidative stress in adult growth hormone deficiency: Different plasma antioxidant patterns in comparison with metabolic syndrome. Endocrine 2018, 59, 130–136. [Google Scholar] [CrossRef]
- Hao, C.-N.; Geng, Y.-J.; Li, F.; Yang, T.; Su, D.-F.; Duan, J.-L.; Li, Y. Insulin-like growth factor-1 receptor activation prevents hydrogen peroxide-induced oxidative stress, mitochondrial dysfunction and apoptosis. Apoptosis 2011, 16, 1118–1127. [Google Scholar] [CrossRef]
- Pankratova, M.S.; Baizhumanov, A.A.; Yusipovich, A.I.; Faassen, M.; Shiryaeva, T.Y.; Peterkova, V.A.; Kovalenko, S.S.; Kazakova, T.A.; Maksimov, G.V. Imbalance in the blood antioxidant system in growth hormone-deficient children before and after 1 year of recombinant growth hormone therapy. PeerJ 2015, 3, e1055. [Google Scholar] [CrossRef]
- Mohn, A.; Di Marzio, D.; Giannini, C.; Capanna, R.; Marcovecchio, M.; Chiarelli, F. Alterations in the oxidant-antioxidant status in prepubertal children with growth hormone deficiency: Effect of growth hormone replacement therapy. Clin. Endocrinol. 2005, 63, 537–542. [Google Scholar] [CrossRef]
- Jayachandran, I.; Sundararajan, S.; Venkatesan, S.; Paadukaana, S.; Balasubramanyam, M.; Mohan, V.; Manickam, N. Asymmetric dimethylarginine (ADMA) accelerates renal cell fibrosis under high glucose condition through NOX4/ROS/ERK signaling pathway. Sci. Rep. 2020, 10, 16005. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Targeting on Asymmetric Dimethylarginine-Related Nitric Oxide-Reactive Oxygen Species Imbalance to Reprogram the Development of Hypertension. Int. J. Mol. Sci. 2016, 17, 2020. [Google Scholar] [CrossRef]
- Fadaei, R.; Davies, S.S. Oxidative modification of HDL by lipid aldehydes impacts HDL function. Arch. Biochem. Biophys. 2022, 730, 109397. [Google Scholar] [CrossRef]
- Mahrooz, A. Pharmacological Interactions of Paraoxonase 1 (PON1): A HDL-Bound Antiatherogenic Enzyme. Curr. Clin. Pharmacol. 2016, 11, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef] [PubMed]
- Soumyarani, V.S.; Jayakumari, N. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes–macrophages by enhanced production of ROS, TNF-α, MMP-9, and MMP-2. Mol. Cell. Biochem. 2012, 366, 277–285. [Google Scholar] [CrossRef]
- Karabacak, M.; Uysal, B.A.; Turkdogan, A.K. Alteration in serum oxidative stress balance in patients with different circulating high-density lipoprotein cholesterol levels. Rev. Port. De Cardiol. 2022, 41, 833–839. [Google Scholar] [CrossRef]
- Huang, D.; Cui, L.; Guo, P.; Xue, X.; Wu, Q.; Hussain, H.I.; Wang, X.; Yuan, Z. Nitric oxide mediates apoptosis and mitochondrial dysfunction and plays a role in growth hormone deficiency by nivalenol in GH3 cells. Sci. Rep. 2017, 7, 17079. [Google Scholar] [CrossRef]
- Liu, X.; Guo, P.; Liu, A.; Wu, Q.; Xue, X.; Dai, M.; Hao, H.; Qu, W.; Xie, S.; Wang, X.; et al. Nitric oxide (NO)-mediated mitochondrial damage plays a critical role in T-2 toxin-induced apoptosis and growth hormone deficiency in rat anterior pituitary GH3 cells. Food Chem. Toxicol. 2017, 102, 11–23. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Björntorp, P.; Edén, S. Additive effects of growth hormone and testosterone on lipolysis in adipocytes of hypophysectomized rats. J. Endocrinol. 1995, 147, 147–152. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2019, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- de Castro Barbosa, T.; Salgueiro, R.; Serrano-Nascimento, C.; Amaral, F.; Cipolla-Neto, J.; Nunes, M. Molecular basis of growth hormone daily mRNA and protein synthesis in rats. Life Sci. 2018, 207, 36–41. [Google Scholar] [CrossRef]
- Chanson, P. The heart in growth hormone (GH) deficiency and the cardiovascular effects of GH. Ann. Endocrinol. 2021, 82, 210–213. [Google Scholar] [CrossRef]
- Belceanu, A.D.; Bîlha, Ş.C.; Leuştean, L.; Ungureanu, M.-C.; Preda, C. Changes in body composition, adipokines, ghrelin, and FGF23 in growth hormone-deficient children during rhGH therapy. Endokrynol. Pol. 2024, 75, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Postma, M.R.; van Beek, A.P.; Jönsson, P.J.; van Bunderen, C.C.; Drent, M.L.; Mattsson, A.F.; Camacho-Hubner, C. Improvements in Body Composition after 4 Years of Growth Hormone Treatment in Adult-Onset Hypopituitarism Compared to Age-Matched Controls. Neuroendocrinology 2019, 109, 131–140. [Google Scholar] [CrossRef]
- Shen, Y.-Y.; Ma, J.-N.; Ren, Z.-Y.; Liu, J.; Zhou, X.-Y.; Xie, X.-R.; Ren, W. Effects of 18 Months of Growth Hormone Replacement Therapy on Bone Mineral Density in Patients with Adult Growth Hormone Deficiency: A Retrospective Study. Int. J. Endocrinol. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.; van der Lely, A.J. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Garmes, H.M.; Castillo, A.R. Insulin signaling in the whole spectrum of GH deficiency. Arch. Endocrinol. Metab. 2019, 63, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Fowelin, J.; Attvall, S.; Lager, I.; Bengtsson, B. Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism 1993, 42, 1443–1447. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J.K.; Chen, C. Influence of insulin on growth hormone secretion, level and growth hormone signalling. Sheng Li Xue Bao 2017, 69, 541–556. [Google Scholar] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef]
- Dong, X.; Su, L.; Patti, M.-E. Growth Hormone and Counterregulation in the Pathogenesis of Diabetes. Curr. Diabetes Rep. 2022, 22, 511–524. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, M.-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Scarano, E.; Riccio, E.; Somma, T.; Arianna, R.; Romano, F.; Di Benedetto, E.; de Alteriis, G.; Colao, A.; Di Somma, C. Impact of Long-Term Growth Hormone Replacement Therapy on Metabolic and Cardiovascular Parameters in Adult Growth Hormone Deficiency: Comparison Between Adult and Elderly Patients. Front. Endocrinol. 2021, 12, 635983. [Google Scholar] [CrossRef] [PubMed]

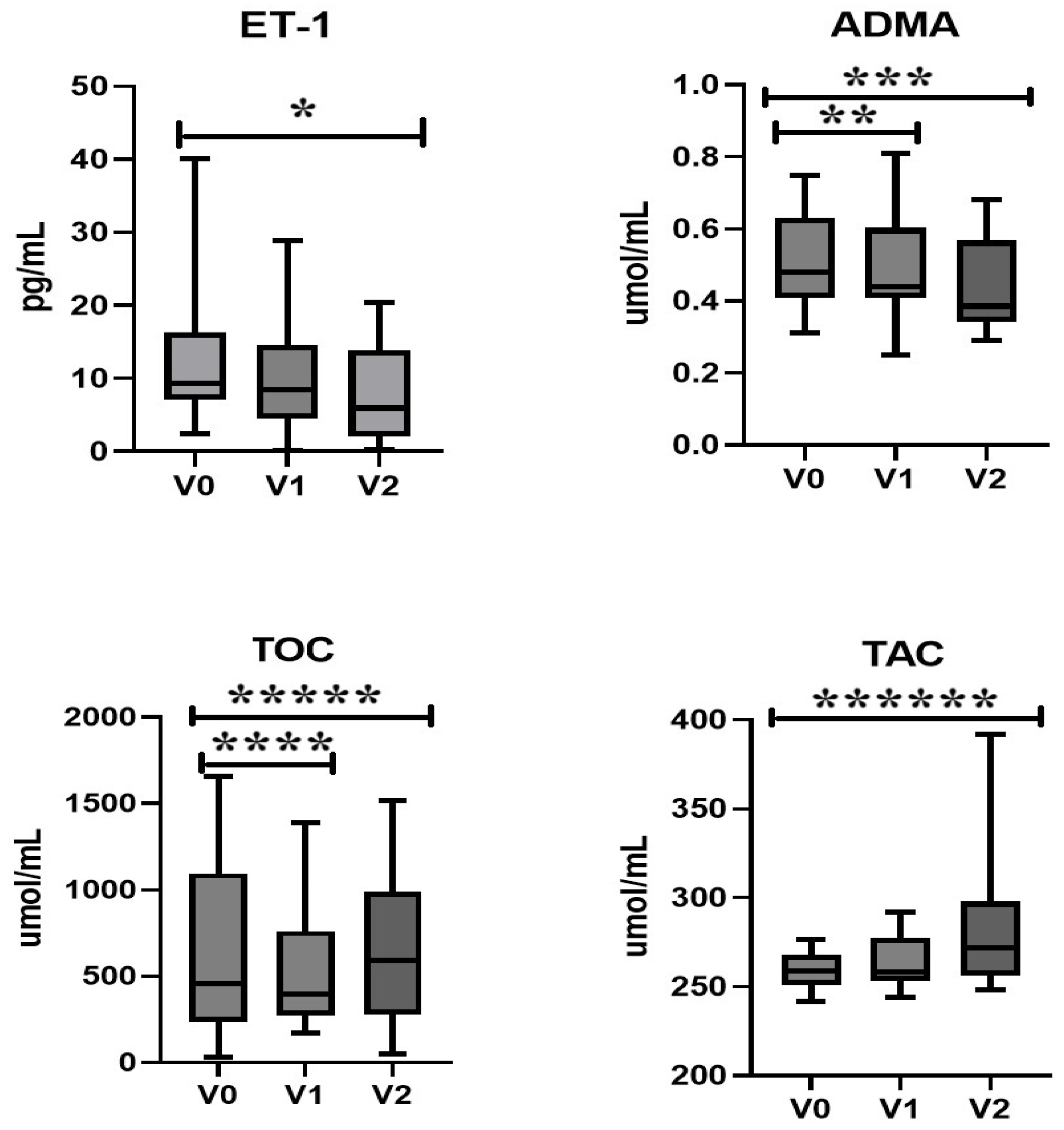
| Parameters | GHD Group (n = 15) | ||||
|---|---|---|---|---|---|
| V0 | V1 | V2 | p Value (V0 vs. V1) | p * Value (V0 vs. V2) | |
| IGF-1 (ng/mL) | 47.07 (8.57–138.8) | 122.8 (44.1–278.1) | 155.1 (36.04–265.1) | 0.0003 | 0.0001 |
| ET-1 (pg/mL) | 8.67 (0.18–40.09) | 8.4 (0.03–28.89) | 5.93 (0.18–20.44) | 0.24 | 0.007 |
| ADMA (umol/mL) | 0.5 (0.31–0.75) | 0.43 (0.25–0.67) | 0.38 (0.29–0.59) | 0.01 | 0.01 |
| NO (umol/mL) | 23.54 (7.57–58.52) | 30.79 (5.63–55.94) | 31.11 (7.57–82.06) | 1.0 | 0.73 |
| TAC (umol/L) | 258.6 (241.8–276.7) | 258.3 (244.3–291.9) | 271.1 (248.4–392.1) | 0.22 | 0.02 |
| TOC (umol/L) | 457.3 (32.12–1655) | 394.4 (171.9–1391) | 589.5 (47.24–1514) | 0.02 | 0.04 |
| Cholesterol (mg/dL) | 201 (114–302) | 188 (87–296) | 199 (114–295) | 0.28 | 0.69 |
| LDL (mg/dL) | 126 (65–219) | 121.5 (48–173) | 131 (58–216) | 0.85 | 0.20 |
| HDL (mg/dL) | 43 (24–85) | 45 (26–76) | 50 (27–80) | 0.41 | 0.20 |
| TG (mg/dL) | 120 (51–684) | 125 (55–259) | 120.5 (45–326) | 0.91 | 0.67 |
| NT-pro-BNP (pg/mL) | 45.13 (10–2025) | 35.71 (10–1546) | 27.16 (10–1325) | 0.38 | 0.23 |
| Ca (mmol/L) | 2.31 (2.03–2.8) | 2.32 (2.02–2.44) | 2,37 (2.17–3.02) | 0.18 | 0.01 |
| Glucose (mg/dL) | 89 (80–180) | 90 (75–172) | 86 (75–147) | 0.19 | 0.15 |
| VAI | 4.31 (1.56–11.77) | 4.23 (1.58–7.03) | 3.58 (1.34–9.05) | 0.59 | 0.69 |
| Parameters | GHD Group (n = 15) | ||||
|---|---|---|---|---|---|
| V0 | V1 | V2 | p Value (V0 vs. V1) | p * Value (V0 vs. V2) | |
| Total mass (kg) | 78.6 (39.6–167.3) | 85.8 (67.6–122.3) | 78.0 (62.3–156) | 0.72 | 0.51 |
| Tissue fat % | 37.5 (27.4–50.4) | 36.3 (30.6–48.8) | 38.4 (26.7–48.7) | 0.006 | 0.04 |
| Tissue mass (g) | 76,222 (37,887–163,689) | 79,671 (65,189–119,236) | 73,551 (15,232–113,051) | 0.19 | 0.11 |
| Fat tissue (g) | 28,434 (13,891–82,462) | 30,931 (19,970–52,232) | 29,937 (16,939–67,385) | 0.23 | 0.08 |
| Lean mass (g) | 48,646 (23,996–81,228) | 52,144 (36,852–73,131) | 45,550 (36,530–84,977) | 0.08 | 0.49 |
| BMC (g) | 2547 (1261–3778) | 2637 (2149–3829) | 2568 (1770–3650) | 0.77 | 0.42 |
| L1-L4 BMD (g/cm2) | 1.09 (0.8–1.6) | 1.08 (0.9–1.6) | 1.1 (0.9–1.5) | 1.0 | 0.73 |
| L1-L4 T score | −1.1 (−3.4–3.2) | −1.0 (−2.1–2.8) | −0.3 (−2.0–2.4) | 0.56 | 0.17 |
| L1-L4 Z score | −1.1 (−3.7–3.0) | −0.7 (−2.3–2.7) | −0.9 (−2.3–2.0) | 0.20 | 0.73 |
| Femoral neck BMD | 0.95 (0.7–1.4) | 0.97 (0.78–1,4) | 0.96 (0.77–1.5) | 0.30 | 0.29 |
| Femoral neck T score | −0.8 (−2.1–2.3) | −0.7 (−1.9–2.0) | −0.6 (−1.9–2.5) | 0.58 | 0.79 |
| Femoral neck Z score | −0.9 (−2.2–2.0) | −1.1 (−2.2–1.6) | −1.0 (−2.0–2.3) | 0.59 | 0.72 |
| Parameters | V0 | V1 | V2 |
|---|---|---|---|
| IGF-1 vs. TOC | p < 0.006; R = −0.73 | NS | p < 0.01; R = −0.69 |
| IGF-1 vs. TAC | p < 0.001; R = 0.83 | NS | p < 0.01; R = 0.69 |
| IGF -1 vs. ADMA | NS | NS | p < 0.01; R = −0.65 |
| IGF-1 vs. NO | NS | NS | p < 0.03; R = −0.67 |
| IGF-1 vs. NT-pro-BNP | p < 0.02; R = −0.62 | NS | NS |
| TOC vs. NT-pro-BNP | NS | p < 0.04; R = 0.56 | NS |
| TOC vs. Lean mass | p < 0.035; R = −0.52 | NS | NS |
| TOC vs. HDL | p < 0.04; R = 0.49 | NS | NS |
| TAC vs. HDL | NS | NS | p < 0.01; R = −0.72 |
| NO vs. Fat tissue % | p < 0.04; R = 0.51 | NS | NS |
| NO vs. TG | NS | NS | p < 0.004; R = 0.67 |
| NT-pro-BNP vs. cholesterol | NS | p < 0.01; R = −0.70 | NS |
| NT-pro-BNP vs. LDL | NS | p < 0.001; R = −0.84 | NS |
| ET-1 vs. Total mass | p < 0.05; R = −0.53 | NS | NS |
| ET-1 vs. Tissue mass | p < 0.05; R = −0.51 | NS | NS |
| ET-1 vs. Lean mass | p < 0.05; R = −0.55 | NS | p < 0.001; R = −0.81 |
| ET-1 vs. BMC | p < 0.01; R = −0.51 | NS | p < 0.0001; R = −0.84 |
| ET-1 vs. BMD | p < 0.01; R = −0.61 | NS | NS |
| ET-1 vs. Ca | p < 0.03; R = −0.65 | NS | NS |
| ADMA vs. Total mass (kg) | p < 0.02; R = 0.54 | NS | NS |
| ADMA vs. Tissue mass (g) | p < 0.02; R = 0.53 | NS | NS |
| ADMA vs. Lean mass (g) | p < 0.04; R = 0.49 | NS | NS |
| ADMA vs. Fat tissue (g) | p < 0.03; R = 0.52 | NS | NS |
| ADMA vs. BMI | NS | NS | p < 0.02; R = 0.64 |
| VAI vs. HDL | p = 0.002; R = −0.77 | NS | NS |
| VAI vs. TG | p = 0.01; R = 0.68 | NS | NS |
| Patients (n = 15) | Sex | Age (Years) | Treatment (Before rhGH) | Dose of rhGH | Etiology GHD | IGF-1 (ng/mL) Initially | BMI (kg/m2) Initially | CO-GHD in History |
|---|---|---|---|---|---|---|---|---|
| P1 | F | 41 | HCT, L, D, Es/Pg | 0.5 mg | CPGP | 68.6 | 30.9 | + |
| P2 | M | 25 | L,T | 0.5 mg | NFPM | 62.8 | 24.8 | + |
| P3 | M | 18 | T | 0.4 mg | CPH | 27.3 | 22.8 | + |
| P4 | F | 26 | HCT, L, Es/Pg | 0.6 mg | CPH | 40.1 | 29.0 | + |
| P5 | M | 19 | D, L, T, HCT | 0.3 mg | CPGP | 74.8 | 34.9 | + |
| P6 | F | 60 | HCT, L | 0.4 mg | ES | 15.11 | 24.3 | - |
| P7 | M | 20 | L, HCT, T | 0.3 mg | CPH | 91.8 | 28.1 | + |
| P8 | M | 23 | - | 0.3 mg | I | 138.8 | 25.9 | - |
| P9 | F | 38 | L, HCT, Es/Pg | 0.5 mg | NFPM | 47.07 | 24.9 | - |
| P10 | M | 18 | T | 0.2 mg | I | 120.2 | 20.4 | + |
| P11 | M | 28 | L, HCT, T, D | 0.3 mg | CPGP | 22.6 | 27.1 | + |
| P12 | M | 42 | L, T, D | 0.3 mg | CPGP | 63.0 | 54.1 | + |
| P13 | M | 36 | HCT, L, T | 0.5 mg | CPGP | 48.9 | 21.5 | + |
| P14 | M | 18 | L, HCT, D, T | 0.7 mg | CPGP | 54.4 | 24.4 | + |
| P15 | M | 25 | L, HCT, T | 0.5 mg | CPGP | 8.6 | 35.8 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kościuszko, M.; Buczyńska, A.; Hryniewicka, J.; Jankowska, D.; Adamska, A.; Siewko, K.; Jacewicz-Święcka, M.; Zaniuk, M.; Krętowski, A.J.; Popławska-Kita, A. Early Cardiovascular and Metabolic Benefits of rhGH Therapy in Adult Patients with Severe Growth Hormone Deficiency: Impact on Oxidative Stress Parameters. Int. J. Mol. Sci. 2025, 26, 5434. https://doi.org/10.3390/ijms26125434
Kościuszko M, Buczyńska A, Hryniewicka J, Jankowska D, Adamska A, Siewko K, Jacewicz-Święcka M, Zaniuk M, Krętowski AJ, Popławska-Kita A. Early Cardiovascular and Metabolic Benefits of rhGH Therapy in Adult Patients with Severe Growth Hormone Deficiency: Impact on Oxidative Stress Parameters. International Journal of Molecular Sciences. 2025; 26(12):5434. https://doi.org/10.3390/ijms26125434
Chicago/Turabian StyleKościuszko, Maria, Angelika Buczyńska, Justyna Hryniewicka, Dorota Jankowska, Agnieszka Adamska, Katarzyna Siewko, Małgorzata Jacewicz-Święcka, Marcin Zaniuk, Adam Jacek Krętowski, and Anna Popławska-Kita. 2025. "Early Cardiovascular and Metabolic Benefits of rhGH Therapy in Adult Patients with Severe Growth Hormone Deficiency: Impact on Oxidative Stress Parameters" International Journal of Molecular Sciences 26, no. 12: 5434. https://doi.org/10.3390/ijms26125434
APA StyleKościuszko, M., Buczyńska, A., Hryniewicka, J., Jankowska, D., Adamska, A., Siewko, K., Jacewicz-Święcka, M., Zaniuk, M., Krętowski, A. J., & Popławska-Kita, A. (2025). Early Cardiovascular and Metabolic Benefits of rhGH Therapy in Adult Patients with Severe Growth Hormone Deficiency: Impact on Oxidative Stress Parameters. International Journal of Molecular Sciences, 26(12), 5434. https://doi.org/10.3390/ijms26125434






