Genomic Size Is Critical to Guarantee the Genomic Stability of Non-Replicative HSV1 Vectors
Abstract
1. Introduction
2. Results
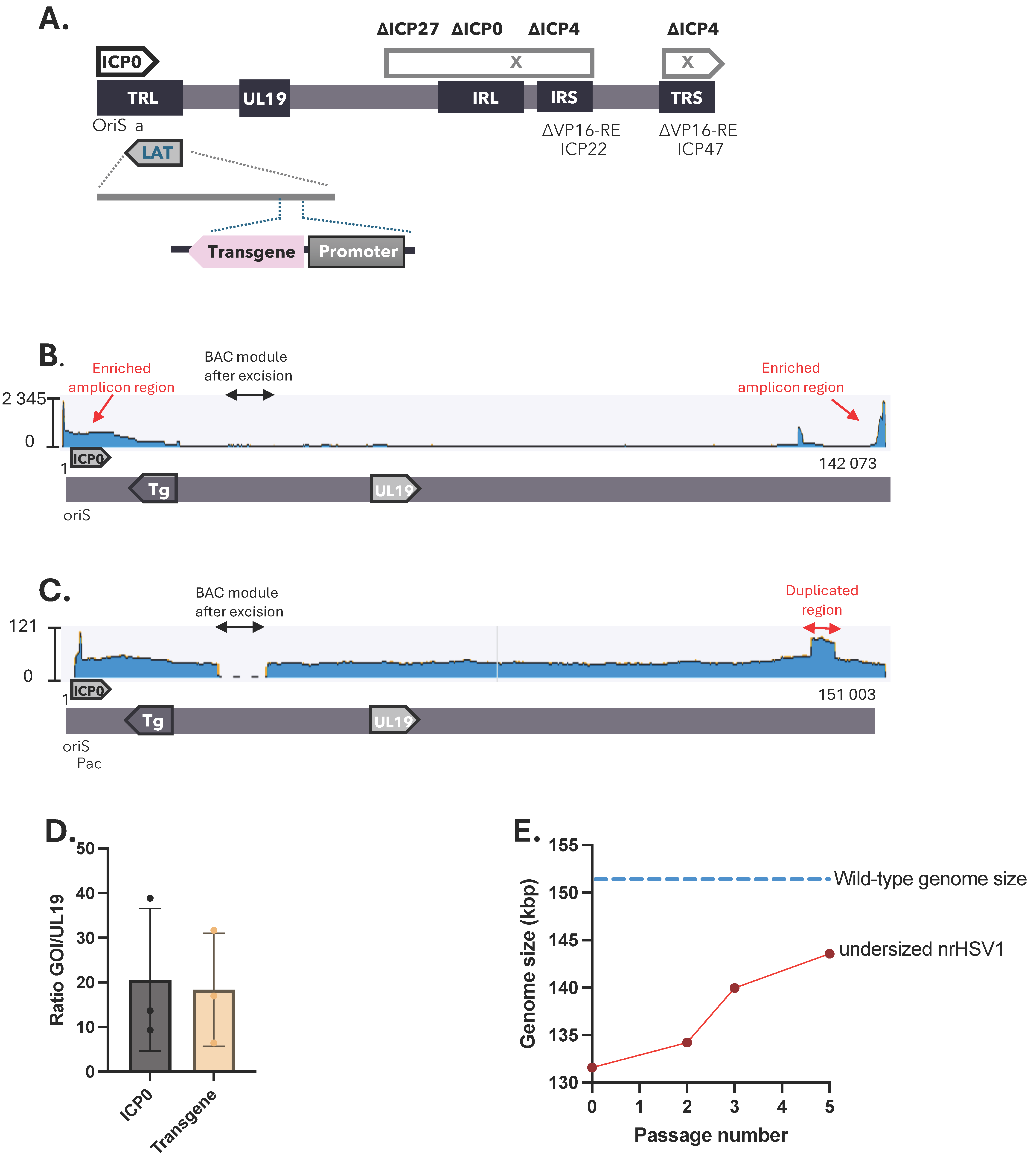
2.1. Genomic Stability of Non-Replicative HSV-1 Vector Depends on the Net Genome Size
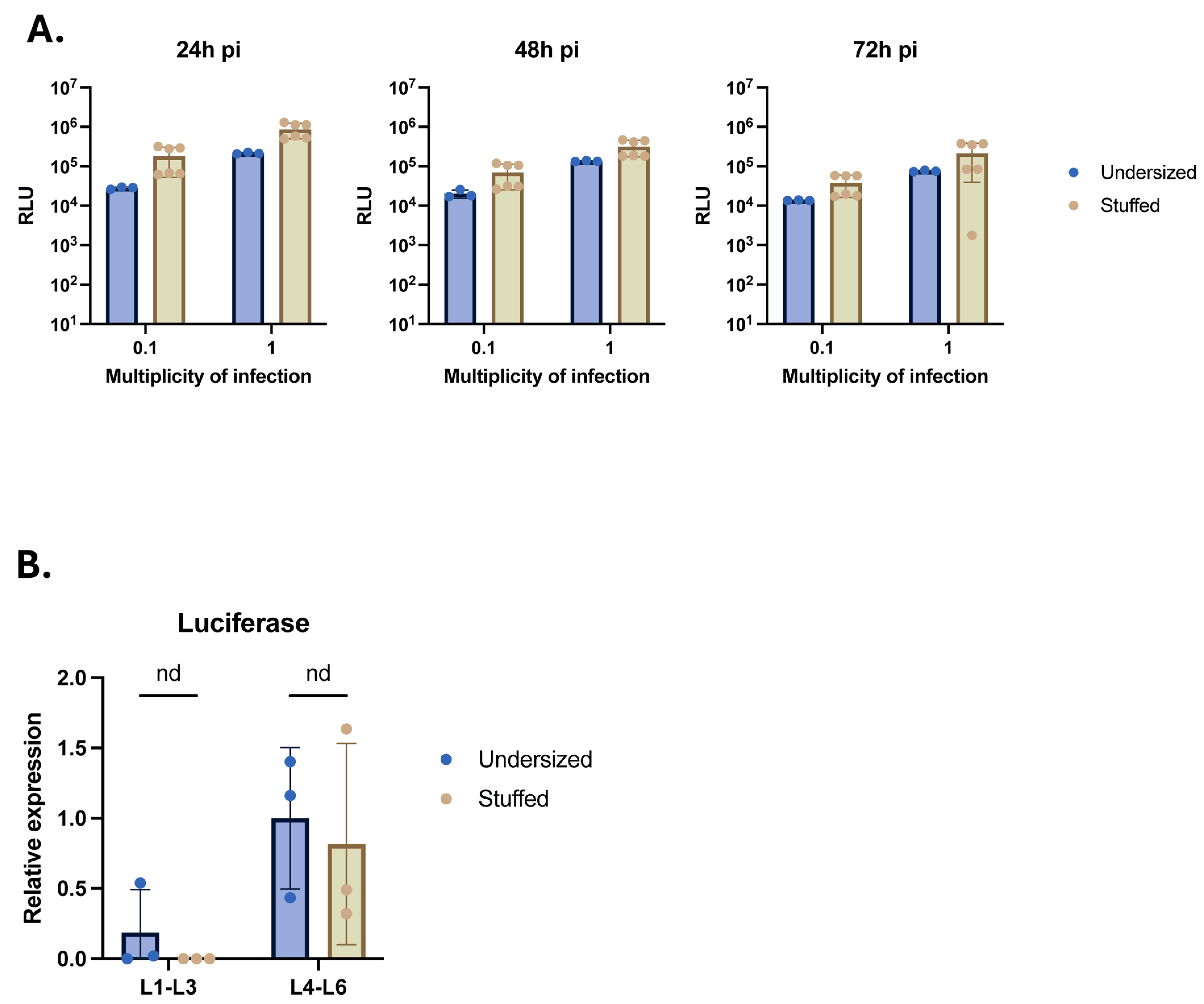
2.2. Stuffed nrHSV-1 Vectors Have Higher Productivity
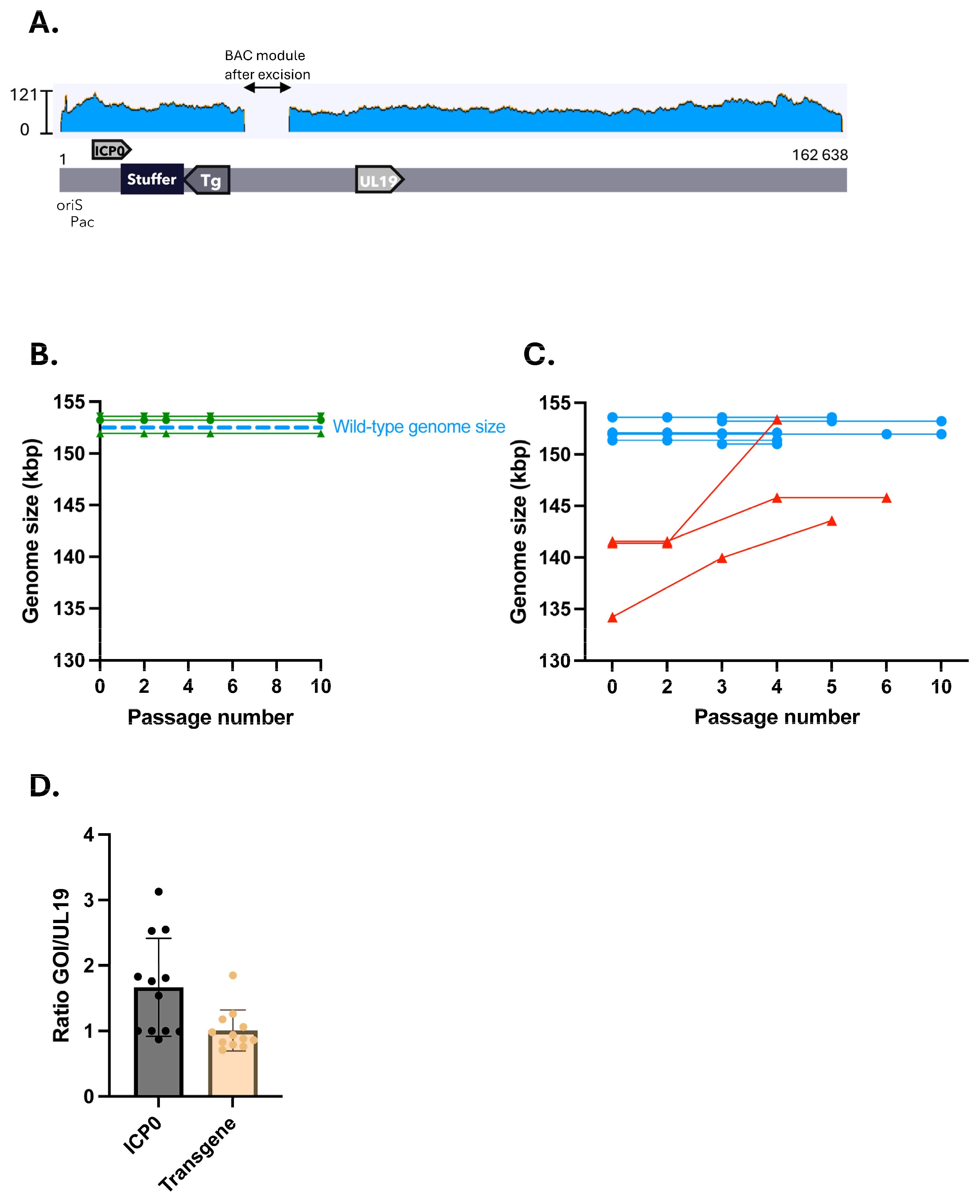
2.3. Stuffed nrHSV-1 Vectors Display Genomic Stability over Time
2.4. Insertion of the Stuffer DNA in nrHSV1 Vector Genome Does Not Impact Transgene Expression
3. Discussion
3.1. Genomic Instability of Small Non-Replicative HSV-1 Vectors
3.2. Restoration of nrHSV1 Genomic Stability by Inserting a Stuffer DNA
3.3. Stuffer DNA Does Not Compromise Transgene Expression
4. Materials and Methods
4.1. Cell Culture
4.2. Non-Replicative HSV-1 BAC Engineering
4.3. Viral Amplification
4.4. Plaque Forming Unit (PFU)
4.5. Sequencing
4.6. Viral Gene Copy
4.7. Productivity Assay
4.8. Toxicity
4.9. In Vitro Luciferase Transgene Activity
4.10. In Vivo nrHSV1 Injection and Tissue Collection
4.11. Transgene Expression Quantification by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saha, B.; Wong, C.M.; Parks, R.J. The Adenovirus Genome Contributes to the Structural Stability of the Virion. Viruses 2014, 6, 3563–3583. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Colosi, P. Effect of Genome Size on AAV Vector Packaging. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef]
- Smith, A.C.; Poulin, K.L.; Parks, R.J. DNA Genome Size Affects the Stability of the Adenovirus Virion. J. Virol. 2009, 83, 2025–2028. [Google Scholar] [CrossRef]
- Dong, B.; Nakai, H.; Xiao, W. Characterization of Genome Integrity for Oversized Recombinant AAV Vector. Mol. Ther. 2010, 18, 87–92. [Google Scholar] [CrossRef]
- Ibreljic, N.; Draper, B.E.; Lawton, C.W. Recombinant AAV Genome Size Effect on Viral Vector Production, Purification, and Thermostability. Mol. Ther. Methods Clin. Dev. 2024, 32, 101188. [Google Scholar] [CrossRef]
- Fisher, K.J.; Choi, H.; Burda, J.; Chen, S.-J.; Wilson, J.M. Recombinant Adenovirus Deleted of All Viral Genes for Gene Therapy of Cystic Fibrosis. Virology 1996, 217, 11–22. [Google Scholar] [CrossRef]
- Parks, R.J.; Graham, F.L. A Helper-Dependent System for Adenovirus Vector Production Helps Define a Lower Limit for Efficient DNA Packaging. J. Virol. 1997, 71, 3293–3298. [Google Scholar] [CrossRef]
- Li, S.; Roy, P.; Travesset, A.; Zandi, R. Why Large Icosahedral Viruses Need Scaffolding Proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10971–10976. [Google Scholar] [CrossRef]
- Gao, K.; Li, M.; Zhong, L.; Su, Q.; Li, J.; Li, S.; He, R.; Zhang, Y.; Hendricks, G.; Wang, J.; et al. Empty Virions in AAV8 Vector Preparations Reduce Transduction Efficiency and May Cause Total Viral Particle Dose-Limiting Side-Effects. Mol. Ther. Methods Clin. Dev. 2014, 1, 20139. [Google Scholar] [CrossRef] [PubMed]
- Krisky, D.M.; Marconi, P.C.; Oligino, T.; Rouse, R.J.D.; Fink, D.J.; Glorioso, J.C. Rapid Method for Construction of Recombinant HSV Gene Transfer Vectors. Gene Ther. 1997, 4, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Akkaraju, G.R.; Huard, J.; Hoffman, E.P.; Goins, W.F.; Pruchnc, R.; Watkins, S.C.; Cohen, J.B.; Glorioso, J.C. Herpes Simplex Virus Vector-Mediated Dystrophin Gene Transfer and Expression in MDX Mouse Skeletal Muscle. J. Gene Med. 1999, 1, 280–289. [Google Scholar] [CrossRef]
- Coukos, G.; Makrigiannakis, A.; Kang, E.H.; Caparelli, D.; Benjamin, I.; Kaiser, L.R.; Rubin, S.C.; Albelda, S.M.; Molnar-Kimber, K.L. Use of Carrier Cells to Deliver a Replication-Selective Herpes Simplex Virus-1 Mutant for the Intraperitoneal Therapy of Epithelial Ovarian Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 1523–1537. [Google Scholar]
- Goss, J.R.; Mata, M.; Goins, W.F.; Wu, H.H.; Glorioso, J.C.; Fink, D.J. Antinociceptive Effect of a Genomic Herpes Simplex Virus-Based Vector Expressing Human Proenkephalin in Rat Dorsal Root Ganglion. Gene Ther. 2001, 8, 551–556. [Google Scholar] [CrossRef]
- Goss, J.R.; Goins, W.F.; Lacomis, D.; Mata, M.; Glorioso, J.C.; Fink, D.J. Herpes Simplex-Mediated Gene Transfer of Nerve Growth Factor Protects against Peripheral Neuropathy in Streptozotocin-Induced Diabetes in the Mouse. Diabetes 2002, 51, 2227–2232. [Google Scholar] [CrossRef]
- Fradette, J.; Wolfe, D.; Goins, W.F.; Huang, S.; Flanigan, R.M.; Glorioso, J.C. HSV Vector-Mediated Transduction and GDNF Secretion from Adipose Cells. Gene Ther. 2005, 12, 48–58. [Google Scholar] [CrossRef]
- Gurevich, I.; Agarwal, P.; Zhang, P.; Dolorito, J.A.; Oliver, S.; Liu, H.; Reitze, N.; Sarma, N.; Bagci, I.S.; Sridhar, K.; et al. In Vivo Topical Gene Therapy for Recessive Dystrophic Epidermolysis Bullosa: A Phase 1 and 2 Trial. Nat. Med. 2022, 28, 780. [Google Scholar] [CrossRef]
- Manservigi, R.; Argnani, R.; Marconi, P. HSV Recombinant Vectors for Gene Therapy. Open Virol. J. 2010, 4, 123. [Google Scholar] [CrossRef]
- Garber, D.A.; Beverley, S.M.; Coen, D.M. Demonstration of Circularization of Herpes Simplex Virus DNA Following Infection Using Pulsed Field Gel Electrophoresis. Virology 1993, 197, 459–462. [Google Scholar] [CrossRef]
- Efstathiou, S.; Minson, A.C.; Field, H.J.; Anderson, J.R.; Wildy, P. Detection of Herpes Simplex Virus-Specific DNA Sequences in Latently Infected Mice and in Humans. J. Virol. 1986, 57, 446–455. [Google Scholar] [CrossRef]
- Krisky, D.M.; Wolfe, D.; Goins, W.F.; Marconi, P.C.; Ramakrishnan, R.; Mata, M.; Rouse, R.J.D.; Fink, D.J.; Glorioso, J.C. Deletion of Multiple Immediate–Early Genes from Herpes Simplex Virus Reduces Cytotoxicity and Permits Long-Term Gene Expression in Neurons. Gene Ther. 1998, 5, 1593–1603. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Marino, P.; Verlengia, G.; Uchida, H.; Goins, W.F.; Yokota, S.; Geller, D.A.; Yoshida, O.; Mester, J.; Cohen, J.B.; et al. Herpes Simplex Viral-Vector Design for Efficient Transduction of Nonneuronal Cells without Cytotoxicity. Proc. Natl. Acad. Sci. USA 2015, 112, E1632–E1641. [Google Scholar] [CrossRef]
- Ventosa, M.; Ortiz-Temprano, A.; Khalique, H.; Lim, F. Synergistic Effects of Deleting Multiple Nonessential Elements in Nonreplicative HSV-1 BAC Genomic Vectors Play a Critical Role in Their Viability. Gene Ther. 2017, 24, 433–440. [Google Scholar] [CrossRef]
- Le Hars, M.; Joussain, C.; Jégu, T.; Epstein, A.L. Non-Replicative Herpes Simplex Virus Genomic and Amplicon Vectors for Gene Therapy—An Update. Gene Ther. 2024, 1–11. [Google Scholar] [CrossRef]
- Cuchet, D.; Ferrera, R.; Lomonte, P.; Epstein, A.L. Characterization of Antiproliferative and Cytotoxic Properties of the HSV-1 Immediate-Early ICPo Protein. J. Gene Med. 2005, 7, 1187–1199. [Google Scholar] [CrossRef]
- Ecob-Prince, M.S.; Preston, C.M.; Rixon, F.J.; Hassan, K.; Kennedy, P.G. Neurons Containing Latency-Associated Transcripts Are Numerous and Widespread in Dorsal Root Ganglia Following Footpad Inoculation of Mice with Herpes Simplex Virus Type 1 Mutant In 1814. J. Gen. Virol. 1993, 74 Pt 6, 985–994. [Google Scholar] [CrossRef]
- Haecker, S.E.; Stedman, H.H.; Balice-Gordon, R.J.; Smith, D.B.; Greelish, J.P.; Mitchell, M.A.; Wells, A.; Sweeney, H.L.; Wilson, J.M. In Vivo Expression of Full-Length Human Dystrophin from Adenoviral Vectors Deleted of All Viral Genes. Hum. Gene Ther. 1996, 7, 1907–1914. [Google Scholar] [CrossRef]
- Cui, X.; McGregor, A.; Schleiss, M.R.; McVoy, M.A. The Impact of Genome Length on Replication and Genome Stability of the Herpesvirus Guinea Pig Cytomegalovirus. Virology 2009, 386, 132–138. [Google Scholar] [CrossRef]
- Čičin-Šain, L.; Bubić, I.; Schnee, M.; Ruzsics, Z.; Mohr, C.; Jonjić, S.; Koszinowski, U.H. Targeted Deletion of Regions Rich in Immune-Evasive Genes from the Cytomegalovirus Genome as a Novel Vaccine Strategy. J. Virol. 2007, 81, 13825–13834. [Google Scholar] [CrossRef]
- Verlengia, G.; Miyagawa, Y.; Ingusci, S.; Cohen, J.B.; Simonato, M.; Glorioso, J.C. Engineered HSV Vector Achieves Safe Long-Term Transgene Expression in the Central Nervous System. Sci. Rep. 2017, 7, 1507. [Google Scholar] [CrossRef]
- Parks, R.J.; Bramson, J.L.; Wan, Y.; Addison, C.L.; Graham, F.L. Effects of Stuffer DNA on Transgene Expression from Helper-Dependent Adenovirus Vectors. J. Virol. 1999, 73, 8027–8034. [Google Scholar] [CrossRef]
- Ross, P.J.; Kennedy, M.A.; Parks, R.J. Host Cell Detection of Noncoding Stuffer DNA Contained in Helper-Dependent Adenovirus Vectors Leads to Epigenetic Repression of Transgene Expression. J. Virol. 2009, 83, 8409–8417. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kagawa, H.; Yamanashi, Y.; Sata, T.; Kawaguchi, Y. Construction of an Excisable Bacterial Artificial Chromosome Containing a Full-Length Infectious Clone of Herpes Simplex Virus Type 1: Viruses Reconstituted from the Clone Exhibit Wild-Type Properties In Vitro and In Vivo. J. Virol. 2003, 77, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Tischer, B.K.; Smith, G.A.; Osterrieder, N. En Passant Mutagenesis: A Two Step Markerless Red Recombination System. In Vitro Mutagenesis Protocols: Third Edition; Braman, J., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 421–430. ISBN 978-1-60761-652-8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basset, J.; Seraffin, A.; Ratelade, J.; Dickx, Y.; Benedyk, T.; Sarek, G.; Jégu, T.; Epstein, A.L. Genomic Size Is Critical to Guarantee the Genomic Stability of Non-Replicative HSV1 Vectors. Int. J. Mol. Sci. 2025, 26, 4941. https://doi.org/10.3390/ijms26104941
Basset J, Seraffin A, Ratelade J, Dickx Y, Benedyk T, Sarek G, Jégu T, Epstein AL. Genomic Size Is Critical to Guarantee the Genomic Stability of Non-Replicative HSV1 Vectors. International Journal of Molecular Sciences. 2025; 26(10):4941. https://doi.org/10.3390/ijms26104941
Chicago/Turabian StyleBasset, Justine, Alexandra Seraffin, Julien Ratelade, Yohann Dickx, Tomasz Benedyk, Grzegorz Sarek, Teddy Jégu, and Alberto L. Epstein. 2025. "Genomic Size Is Critical to Guarantee the Genomic Stability of Non-Replicative HSV1 Vectors" International Journal of Molecular Sciences 26, no. 10: 4941. https://doi.org/10.3390/ijms26104941
APA StyleBasset, J., Seraffin, A., Ratelade, J., Dickx, Y., Benedyk, T., Sarek, G., Jégu, T., & Epstein, A. L. (2025). Genomic Size Is Critical to Guarantee the Genomic Stability of Non-Replicative HSV1 Vectors. International Journal of Molecular Sciences, 26(10), 4941. https://doi.org/10.3390/ijms26104941




