HERV Dysregulation in a Case of Myalgic Encephalomyelitis and Multiple Sclerosis Responsive to Rituximab
Abstract
1. Introduction
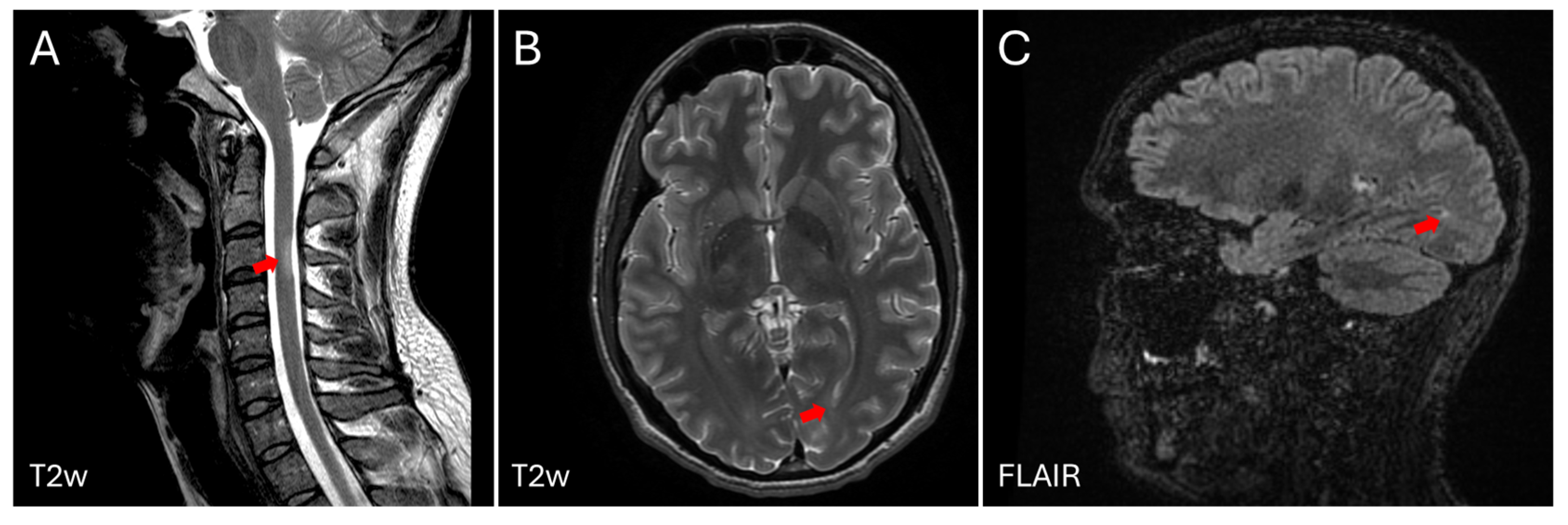
2. Case Description
- Physically, he was unable to leave the house (only for medical appointments, with significant subsequent deterioration, resulting in an increase in symptoms and increased fatigue requiring continuous rest). He was bedridden most of the day. He was able to perform personal self-care: grooming, dressing, eating at the table, and showering (he had to adapt his bathroom settings and did not do so daily).
- Cognitively, his computer activity ranged from 10 min to a maximum of one hour a day on days when he felt better.
- He suffered from a weekly migraine that could last up to three days.
- He experienced daily symptoms of moderate to severe intensity: dizziness upon standing, feeling immediately tired and unsteady, concentration problems, memory problems, palpitations, and muscle pain.
- Severe photosensitivity that impaired his vision.
- Physically, he could leave the house 2–3 times a week and take gentle walks for an hour. He could help around the house with simple tasks such as cooking or cleaning, although he could not fully perform these tasks.
- Cognitively, he could use the computer daily, without the deterioration that used to be triggered, but never for more than an hour at a time.
- He reported a migraine once a month.
- The rest of the symptoms persisted, but at a mild to moderate level. He tolerated standing for longer. For muscle pain, he is receiving treatment from a home physiotherapist, and it has almost completely resolved this issue.
- Photosensitivity persists, and he still requires sunglasses, but it does not impair his vision.
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ME/CFS | Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome |
| MS | Multiple Sclerosis |
| MG | Myasthenia gravis |
| HERV | Human endogenous retrovirus |
| HERV-W ENV | Human Endogenous Retrovirus type W (tryptophan), Envelope protein |
| MSRV | Multiple Sclerosis Retrovirus |
| PBMC | Peripheral Blood Mononuclear Cells |
| EBV | Epstein–Barr Virus |
| DMT | Disease-modifying therapy |
| AUC | Area under the curve |
| AchR | Acetylcholine receptor |
| aAb | Autoantibody |
References
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An international classification of diseases for the twenty-first century. BMC Med. Inform. Decis. Mak. 2021, 21 (Suppl. S6), 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carruthers, B.M.; Van De Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International consensus criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Zwibel, H.L.; Smrtka, J. Improving quality of life in multiple sclerosis: An unmet need. Am. J. Manag. Care 2011, 17 (Suppl. S5), S139–S145. [Google Scholar]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Degenhardt, A.; Rice, G.P.; Muraro, P.A.; Daumer, M.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study 10, relapses and long-term disability. Brain 2010, 133 Pt. 7, 1914–1929. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129 Pt 3, 606–616. [Google Scholar] [CrossRef]
- Koch, M.; Kingwell, E.; Rieckmann, P.; Tremlett, H.; UBC MS Clinic Neurologists. The natural history of secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1039–1043. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Sánchez-Fito, T.; Ovejero, T.; Nathanson, L.; Oltra, E. Impact of Polypharmacy on Candidate Biomarker miRNomes for the Diagnosis of Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Striking Back on Treatments. Pharmaceutics 2019, 11, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. J. 2018, 24, 96–120. [Google Scholar] [CrossRef]
- Rae-Grant, A.; Day, G.S.; Marrie, R.A.; Rabinstein, A.; Cree, B.A.; Gronseth, G.S.; Haboubi, M.; Halper, J.; Hosey, J.P.; Jones, D.E.; et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018, 90, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Williams, O.; Willis, M.; Hrastelj, J.; Rimmer, A.; Joseph, F.; Tomassini, V.; Wardle, M.; Pickersgill, T.; Robertson, N.; et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019, 76, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.L.; Coles, A.; Horakova, D.; Havrdova, E.; Izquierdo, G.; Prat, A.; Girard, M.; Duquette, P.; Trojano, M.; Lugaresi, A.; et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019, 321, 175–187. [Google Scholar] [CrossRef]
- He, A.; Merkel, B.; Brown, J.W.L.; Ryerson, L.Z.; Kister, I.; Malpas, C.B.; Sharmin, S.; Horakova, D.; Havrdova, E.K.; Spelman, T.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Buron, M.D.; Chalmer, T.A.; Sellebjerg, F.; Barzinji, I.; Bech, D.; Christensen, J.R.; Christensen, M.K.; Hansen, V.; Illes, Z.; Jensen, H.B.; et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: A nationwide cohort study. Neurology 2020, 95, e1041–e1051. [Google Scholar] [CrossRef]
- Spelman, T.; Magyari, M.; Piehl, F.; Svenningsson, A.; Rasmussen, P.V.; Kant, M.; Sellebjerg, F.; Joensen, H.; Hillert, J.; Lycke, J. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: Data from 2 different national strategies. JAMA Neurol. 2021, 78, 1197–1204. [Google Scholar] [CrossRef]
- Uher, T.; Krasensky, J.; Malpas, C.; Bergsland, N.; Dwyer, M.G.; Havrdova, E.K.; Vaneckova, M.; Horakova, D.; Zivadinov, R.; Kalincik, T. Evolution of brain volume loss rates in early stages of multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e979. [Google Scholar] [CrossRef]
- Hanninen, K.; Viitala, M.; Atula, S.; Laakso, S.M.; Kuusisto, H.; Soilu-Hanninen, M. Initial treatment strategy and clinical outcomes in Finnish MS patients: A propensity-matched study. J. Neurol. 2022, 269, 913–922. [Google Scholar] [CrossRef]
- Carlson, A.K.; Amin, M.; Cohen, J.A. Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability. Drugs 2024, 84, 285–304. [Google Scholar] [CrossRef]
- Pescovitz, M.D. Rituximab, an anti-cd20 monoclonal antibody: History and mechanism of action. Am. J. Transplant. 2006, 6 Pt 1, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Arunkumar, A.; Kingdon, C.; Lacerda, E.; Nacul, L. Prevalence of and risk factors for severe cognitive and sleep symptoms in ME/CFS and MS. BMC Neurol. 2017, 17, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braley, T.J.; Chervin, R.D. Fatigue in multiple sclerosis: Mechanisms, evaluation, and treatment. Sleep 2010, 33, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cleveland Clinic. Multiple Sclerosis and Fatigue [Internet]. 2023. Available online: https://my.clevelandclinic.org/departments/neurological/depts/multiple-sclerosis/ms-approaches/ms-and-fatigue (accessed on 18 April 2025).
- Cleveland Clinic. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) [Internet]. 2023. Available online: https://my.clevelandclinic.org/health/diseases/17720-myalgic-encephalomyelitis-chronic-fatigue-syndrome-me-cfs (accessed on 18 April 2025).
- Hedström, A.K. Risk factors for multiple sclerosis in the context of Epstein-Barr virus infection. Front. Immunol. 2023, 14, 1212676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernard, C.; Bertrand, J.-B.; Stefas, I.; Veas, F.; Lang, A.B.; Popa, I.; Sanhadji, K.; Mancuso, R.; Saresella, M.; Clerici, M.; et al. HERV-W envelope is significantly expressed in Multiple Sclerosis and causes neuroinflammation in animal models with specific antibody inhibition. Retrovirology 2009, 6 (Suppl. S2), P70. [Google Scholar] [CrossRef][Green Version]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Karen, G.-O.; Eva, M.-M.; Lubov, N.; Elisa, O. HERV activation segregates ME/CFS from fibromyalgia while defining a novel nosologic entity. eLife 2025, 14, RP104441. [Google Scholar] [CrossRef]
- Yang, T.T.; Wang, L.; Deng, X.Y.; Yu, G. Pharmacological treatments for fatigue in patients with multiple sclerosis: A sys-tematic review and meta-analysis. J. Neurol. Sci. 2017, 380, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Orenga, K.; Oltra, E. Human Endogenous Retrovirus as Therapeutic Targets in Neurologic Disease. Pharmaceuticals 2021, 14, 495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perron, H.; Lazarini, F.; Ruprecht, K.; Péchoux-Longin, C.; Seilhean, D.; Sazdovitch, V.; Créange, A.; Battail-Poirot, N.; Sibaï, G.; Santoro, L.; et al. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: Physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J. Neurovirol. 2005, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Perron, H.; Oltra, E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front. Immunol. 2022, 13, 1020064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becker, J.; Pérot, P.; Cheynet, V.; Oriol, G.; Mugnier, N.; Mommert, M.; Tabone, O.; Textoris, J.; Veyrieras, J.B.; Mallet, F. A comprehensive hybridization model allows whole HERV transcriptome profiling using high density microarray. BMC Genom. 2017, 18, 286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolde, R.; Lizee, A.; Metsalu, T. Pheatmap: Pretty Heatmaps GitHub. 2018. Available online: https://github.com/raivokolde/pheatmap (accessed on 18 January 2025).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses R Package 2019. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 18 January 2025).
- Bhasin, J.M.; Ting, A.H. Goldmine integrates information placing genomic ranges into meaningful biological contexts. Nucleic Acids Res. 2016, 44, 5550–5556. [Google Scholar] [CrossRef]
- Machtoub, D.; Fares, C.; Sinan, H.; Al Hariri, M.; Nehme, R.; Chami, J.; Joukhdar, R.; Tcheroyan, R.; Adib, S.; Khoury, S.J. Factors affecting fatigue progression in multiple sclerosis patients. Sci. Rep. 2024, 14, 31682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridley, B.; Minozzi, S.; Gonzalez-Lorenzo, M.; Del Giovane, C.; Piggott, T.; Filippini, G.; Peryer, G.; Foschi, M.; Tramacere, I.; Baldin, E.; et al. Immunomodulators and immunosuppressants for progressive multiple sclerosis: A network meta-analysis. Cochrane Database Syst. Rev. 2024, 9, CD015443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fluge, Ø.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Kristoffersen, E.K.; Sørland, K.; Bruland, O.; Dahl, O.; et al. B-Lymphocyte Depletion in Myalgic Encephalopathy/Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fluge, Ø.; Rekeland, I.G.; Lien, K.; Thürmer, H.; Borchgrevink, P.C.; Schäfer, C.; Sørland, K.; Aßmus, J.; Ktoridou-Valen, I.; Herder, I.; et al. B-Lymphocyte Depletion in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2019, 170, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.; Tzartos, S.J. Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Front. Neurol. 2020, 11, 596981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilhus, N.E.; Andersen, H.; Andersen, L.K.; Boldingh, M.; Laakso, S.; Leopoldsdottir, M.O.; Madsen, S.; Piehl, F.; Popperud, T.H.; Punga, A.R.; et al. Generalized myasthenia gravis with acetylcholine receptor antibodies: A guidance for treatment. Eur. J. Neurol. 2024, 31, e16229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meisel, A.; Baggi, F.; Behin, A.; Evoli, A.; Kostera-Pruszczyk, A.; Mantegazza, R.; Morales, R.J.; Punga, A.R.; Sacconi, S.; Schroeter, M.; et al. Role of autoantibody levels as biomarkers in the management of patients with myasthenia gravis: A systematic review and expert appraisal. Eur. J. Neurol. 2023, 30, 266–282. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; van der Pol, S.; Nijland, P.; Amor, S.; Perron, H. Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Burgelin, I.; Perron, H.; Curtin, F.; Lang, A.B.; Faucard, R. MSRV envelope protein is a potent, endogenous and pathogenic agonist of human toll-like receptor 4: Relevance of GNbAC1 in multiple sclerosis treatment. J. Neuroimmunol. 2016, 291, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gamer, J.; Van Booven, D.J.; Zarnowski, O.; Arango, S.; Elias, M.; Kurian, A.; Joseph, A.; Perez, M.; Collado, F.; Klimas, N.; et al. Sex-Dependent Transcriptional Changes in Response to Stress in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Project. Int. J. Mol. Sci. 2023, 24, 10255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Germain, A.; Giloteaux, L.; Moore, G.E.; Levine, S.M.; Chia, J.K.; Keller, B.A.; Stevens, J.; Franconi, C.J.; Mao, X.; Shungu, D.C.; et al. Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2022, 7, e157621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheema, A.K.; Sarria, L.; Bekheit, M.; Collado, F.; Almenar-Pérez, E.; Martín-Martínez, E.; Alegre, J.; Castro-Marrero, J.; Fletcher, M.A.; Klimas, N.G.; et al. Unravelling myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Gender-specific changes in the microRNA expression profiling in ME/CFS. J. Cell Mol. Med. 2020, 24, 5865–5877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macchietto, M.G.; Langlois, R.A.; Shen, S.S. Virus-induced transposable element expression up-regulation in human and mouse host cells. Life Sci. Alliance 2020, 3, e201900536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armangue, T.; Spatola, M.; Vlagea, A.; Mattozzi, S.; Cárceles-Cordon, M.; Martinez-Heras, E.; Llufriu, S.; Muchart, J.; Erro, M.E.; Abraira, L.; et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018, 17, 760–772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iversen, R.; Sollid, L.M. Dissecting autoimmune encephalitis through the lens of intrathecal B cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2401337121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Pablos, M.; Paiva, B.; Zabaleta, A. Epstein-Barr virus-acquired immunodeficiency in myalgic encephalomyelitis-Is it present in long COVID? J. Transl. Med. 2023, 21, 633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasimir, F.; Toomey, D.; Liu, Z.; Kaiping, A.C.; Ariza, M.E.; Prusty, B.K. Tissue specific signature of HHV-6 infection in ME/CFS. Front. Mol. Biosci. 2022, 9, 1044964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O'Neal, A.J.; Hanson, M.R. The Enterovirus Theory of Disease Etiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Critical Review. Front. Med. 2021, 8, 688486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, G.; Maes, M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013, 11, 205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflamm. 2017, 14, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Carbonell, L.; Iranzo, A. Sleep Disturbances in Autoimmune Neurological Diseases. Curr. Neurol. Neurosci. Rep. 2023, 23, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Orenga, K.; Martín-Martínez, E.; Oltra, E. Over-Representation of Torque Teno Mini Virus 9 in a Subgroup of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Study. Pathogens 2024, 13, 751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
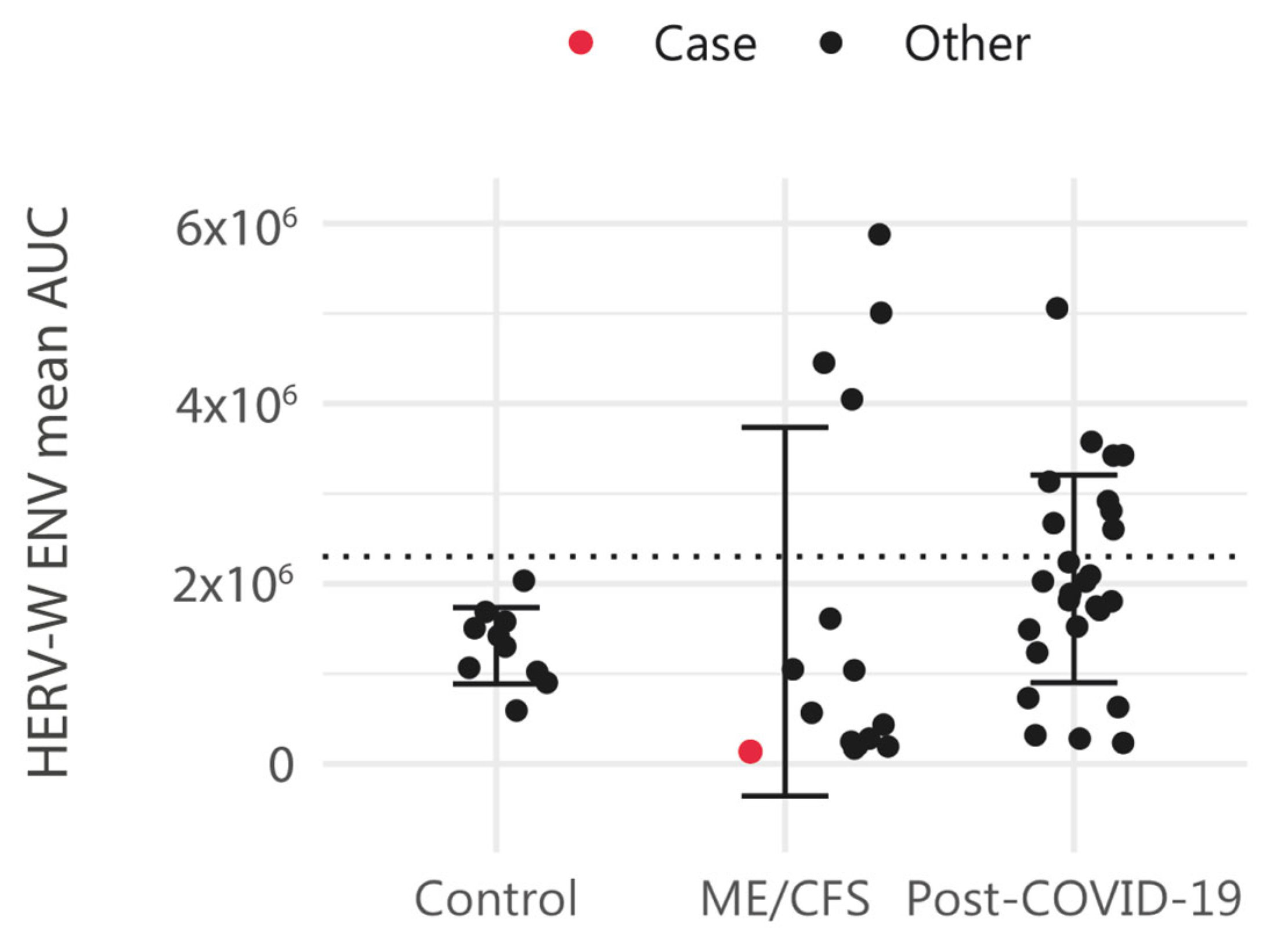

| HERV Element | Gene | Alias | Description | Distance (bp) | Subregion |
|---|---|---|---|---|---|
| ERVL_8q11.23 | RB1CC1 | RB1 Inducible Coiled-Coil 1 | The protein encoded by this gene interacts with signaling pathways to coordinately regulate cell growth, cell proliferation, apoptosis, autophagy, and cell migration. | 0 | intron |
| MST_5p14.1 | PURPL | P53 Upregulated Regulator Of P53 Levels | lncRNA | 0 | intron |
| MLT1_Xq25 | TENM1 | Teneurin Transmembrane Protein 1 | Involved in neural development, regulating the establishment of proper connectivity within the nervous system. | 0 | intron |
| MLT1_11q23.3 | PHLDB1 | Pleckstrin Homology Like Domain Family B Member 1 | Involved in regulation of embryonic development; regulation of epithelial to mesenchymal transition; and regulation of microtubule cytoskeleton organization. Located in several cellular components, including basal cortex; cytosol; and intercellular bridge. | 0 | exon |
| MLT1_11p15.2 | SOX6 | SRY-Box Transcription Factor 6 | The encoded protein is a transcriptional activator that is required for normal development of the central nervous system, chondrogenesis, and maintenance of cardiac and skeletal muscle cells. | 0 | intron |
| MLT1_4p16.3 | PCGF3 | Polycomb Group Ring Finger 3 | Component of a Polycomb group (PcG) multiprotein PRC1-like complex, a complex class required to maintain the transcriptionally repressive state of many genes, including Hox genes, throughout development | 0 | intron |
| MLT1_8q24.21 | CYRIB | CYFIP Related Rac1 Interactor B | Involved in several processes, including cellular response to molecule of bacterial origin; negative regulation of small GTPase-mediated signal transduction; and regulation of organelle organization. Located in mitochondrion. | 0 | intron |
| MLT1_9p21.1 | LINGO2 | Leucine Rich Repeat And Ig Domain Containing 2 | Predicted to act upstream of or within positive regulation of synapse assembly. Predicted to be located in membrane. Predicted to be active in several cellular components, including extracellular space; glutamatergic synapse; and synaptic membrane. | 0 | intron |
| MER4_1q31.2 | Lnc-BRINP3-7 | Novel transcript | Novel transcript | 0 | promoter |
| ERV9_2q32.3 | CAVIN2-AS1 | CAVIN2 And TMEFF2 Antisense RNA 1 | lncRNA | 0 | promoter |
| MER21_6q26 | QKI | QKI, KH Domain Containing RNA Binding | The encoded protein is involved in myelinization and oligodendrocyte differentiation. | 0 | intron |
| LTR84_15q24.3 | LOC105370906 | Novel transcript | lncRNA | 0 | intron |
| ERV9_21q21.3 | MAP3K7CL | MAP3K7 C-Terminal Like | Located in cytosol and nucleus. | 0 | promoter |
| MER21_22q12.1 | GRK3 | G Protein-Coupled Receptor Kinase 3 | Specifically phosphorylates the agonist-occupied form of the beta-adrenergic and closely related receptors. | 0 | intron |
| MST_2q12.3 | LINC01789 | Long Intergenic Non-Protein Coding RNA 1789 | lncRNA | 0 | intron |
| MLT1_6p11.2 | RAB23 | RAB23, Member RAS Oncogene Family | The encoded protein may play a role in central nervous system development by antagonizing sonic hedgehog signaling. | 0 | intron |
| THE1_12q21.31 | MGAT4C | MGAT4 Family Member C | Predicted to enable acetylglucosaminyltransferase activity. Among its related pathways are Translation of Structural Proteins and Infectious disease. | 0 | 3' end |
| MST_22q11.21 | USP18 | Ubiquitin Specific Peptidase 18 | Among its related pathways are Toll Like Receptor 7/8 (TLR7/8) Cascade and Overview of interferons-mediated signaling pathway. | 0 | intron |
| MST_18q21.1 | MIR4527HG | MIR4527 Host Gene | lncRNA | 94 | promoter |
| MLT1_4q35.1 | FAM149A | amily With Sequence Similarity 149 Member A | An important paralog of this gene is FAM149B1. | 3119 | intergenic |
| MER61_11q12.1 | GLYAT | Glycine-N-Acyltransferase | Mitochondrial acyltransferase, which transfers an acyl group to the N-terminus of glycine and glutamine, although much less efficiently. | 7837 | intergenic |
| HERV32_Xq13.1 | COX6CP12 | Cytochrome C Oxidase Subunit 6C Pseudogene 12 | Pseudogene | 10,819 | intergenic |
| HERVL33_14q32.32 | ENSG00000289207 | Lnc-LBHD2-4 | lncRNA | 11,605 | intergenic |
| MER61_8p12 | MTND5P41 | MT-ND5 Pseudogene 41 | Pseudogene | 12,771 | intergenic |
| MLT1_6p12.1 | TINAG | Tubulointerstitial Nephritis Antigen | This gene encodes a glycoprotein that is restricted within the kidney to the basement membranes underlying the epithelium of Bowman's capsule and proximal and distal tubules. | 18,001 | intergenic |
| LTR48_12q14.2 | LDHAL6CP | Lactate Dehydrogenase A Like 6C | Pseudogene | 19,473 | intergenic |
| THE1_9q34.11 | Lnc-NTMT1-2 | Uncharacterized LOC105376292 | lncRNA | 30,032 | intergenic |
| HERV16_1p34.3 | LINC01343 | Long Intergenic Non-Protein Coding RNA 1343 | lncRNA | 39,532 | intergenic |
| MER4_18q23 | Lnc-PQLC1-5 | NONHSAG024230.2 | lncRNA | 71,187 | intergenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Martínez, E.; Gil-Perotin, S.; Giménez-Orenga, K.; Barea-Moya, L.; Oltra, E. HERV Dysregulation in a Case of Myalgic Encephalomyelitis and Multiple Sclerosis Responsive to Rituximab. Int. J. Mol. Sci. 2025, 26, 4885. https://doi.org/10.3390/ijms26104885
Martín-Martínez E, Gil-Perotin S, Giménez-Orenga K, Barea-Moya L, Oltra E. HERV Dysregulation in a Case of Myalgic Encephalomyelitis and Multiple Sclerosis Responsive to Rituximab. International Journal of Molecular Sciences. 2025; 26(10):4885. https://doi.org/10.3390/ijms26104885
Chicago/Turabian StyleMartín-Martínez, Eva, Sara Gil-Perotin, Karen Giménez-Orenga, Lucas Barea-Moya, and Elisa Oltra. 2025. "HERV Dysregulation in a Case of Myalgic Encephalomyelitis and Multiple Sclerosis Responsive to Rituximab" International Journal of Molecular Sciences 26, no. 10: 4885. https://doi.org/10.3390/ijms26104885
APA StyleMartín-Martínez, E., Gil-Perotin, S., Giménez-Orenga, K., Barea-Moya, L., & Oltra, E. (2025). HERV Dysregulation in a Case of Myalgic Encephalomyelitis and Multiple Sclerosis Responsive to Rituximab. International Journal of Molecular Sciences, 26(10), 4885. https://doi.org/10.3390/ijms26104885







