Pentagalloylglucose Inhibits Melanogenesis via Suppression of MITF Signaling Pathway
Abstract
1. Introduction
2. Results
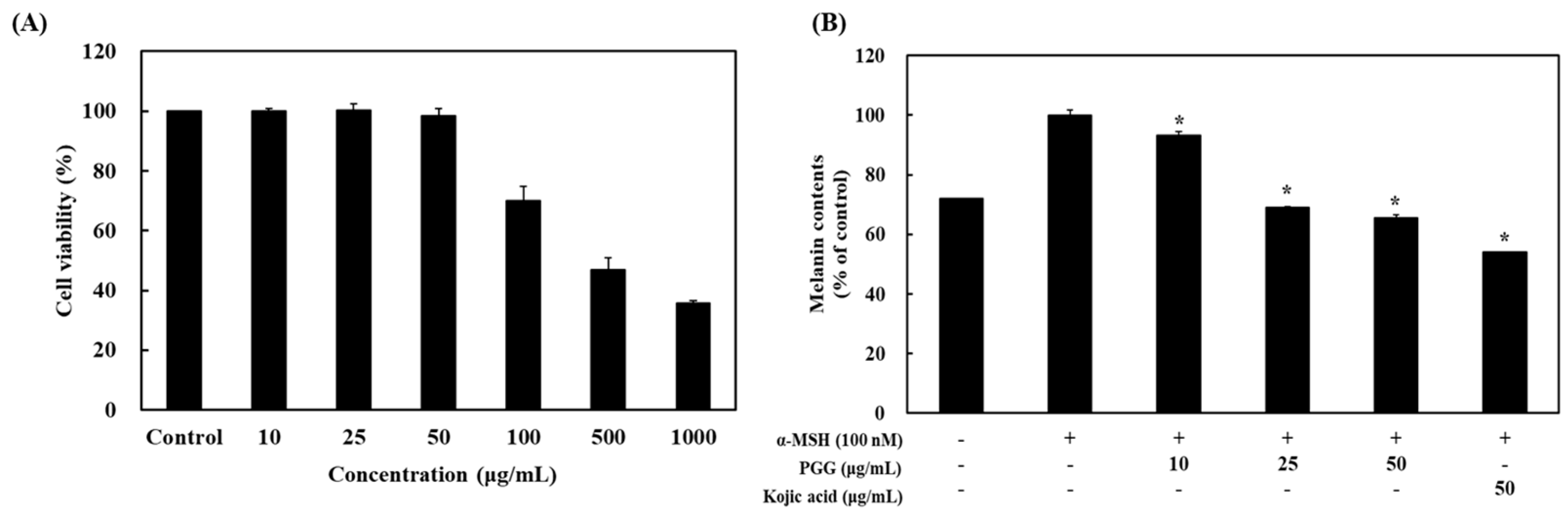
2.1. Effects of PGG on Cell Viability and Melanin Content in B16F10 Cells
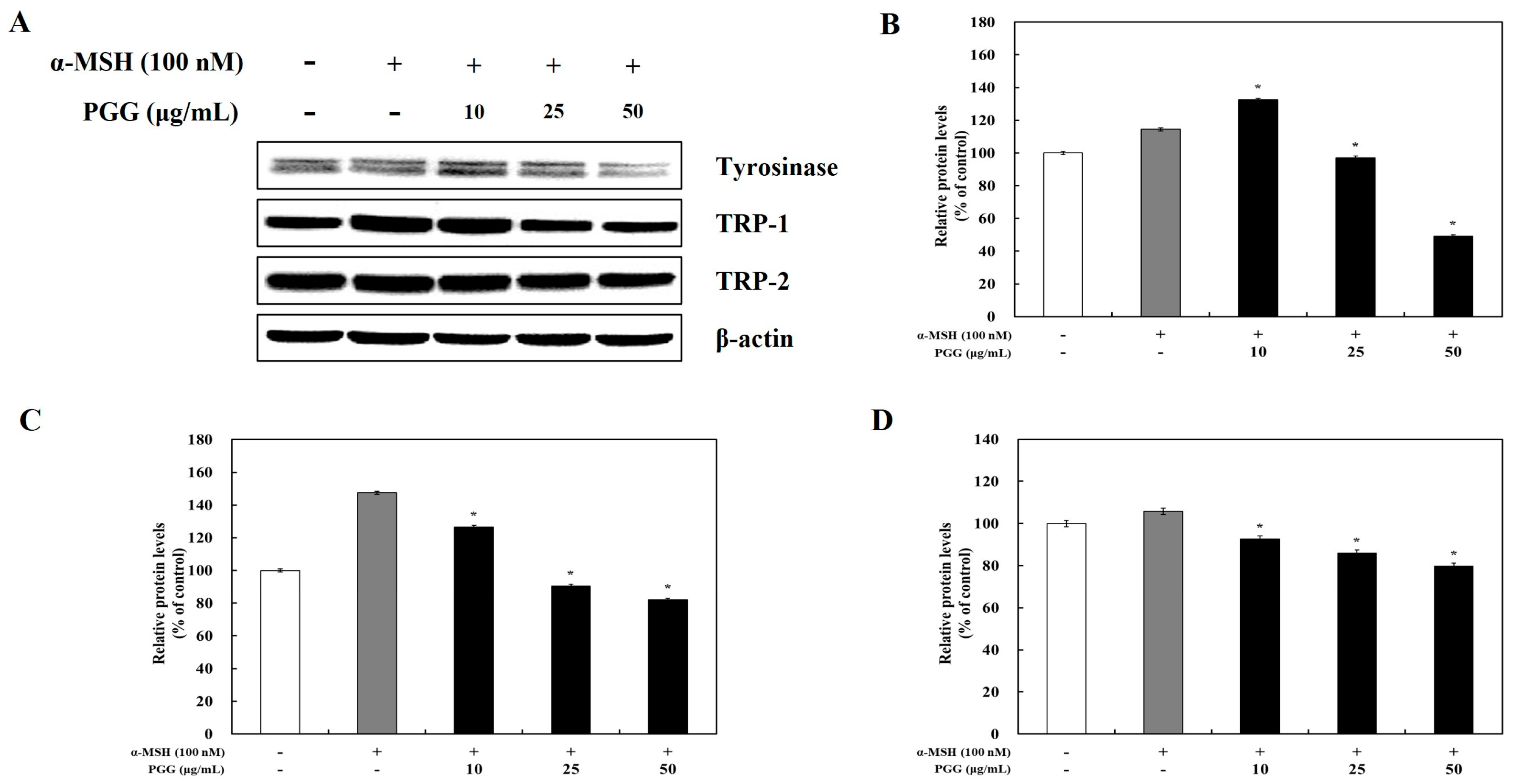
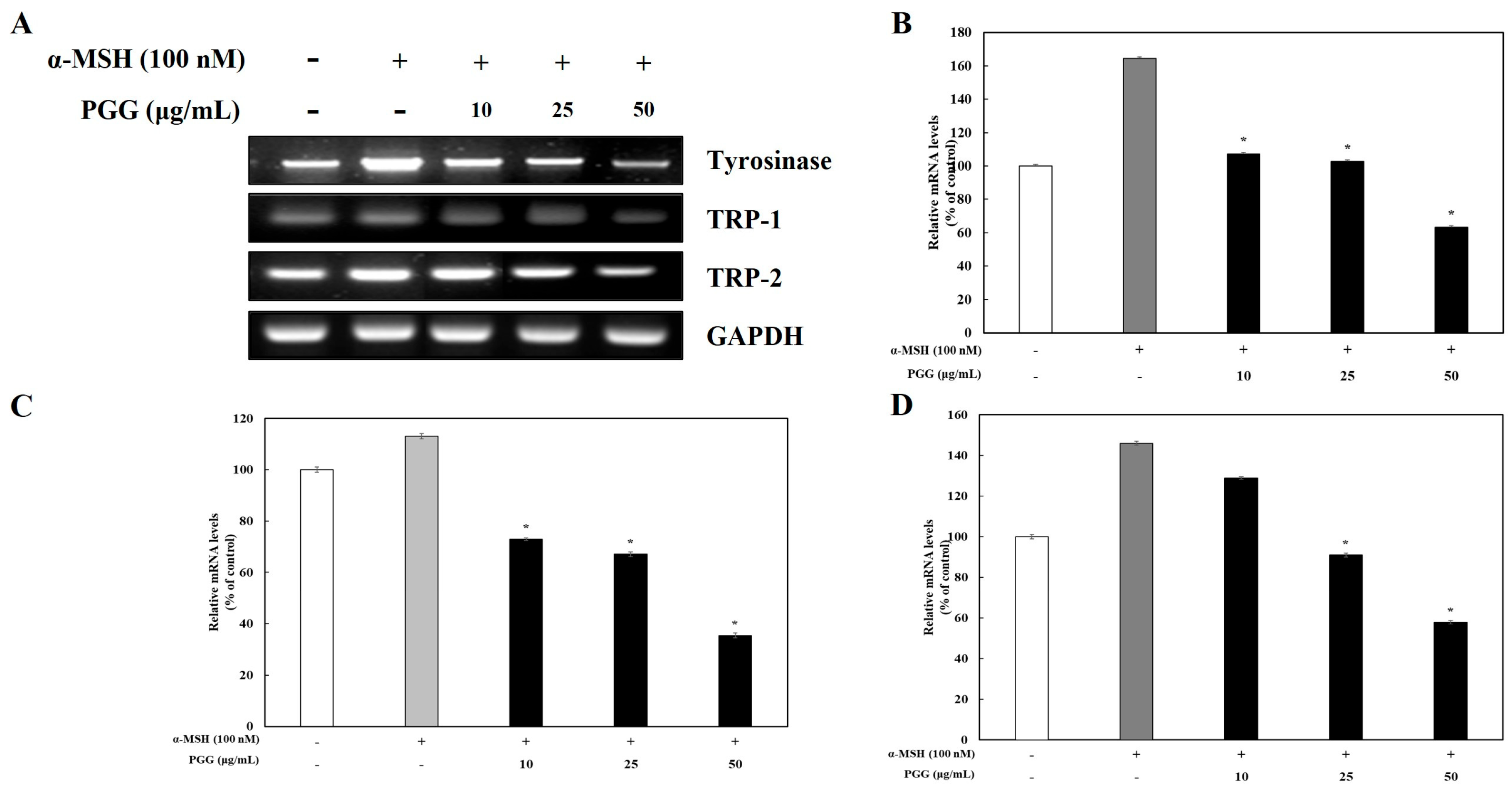
2.2. Effects of PGG on Melanogenic Enzyme Expression in B16F10 Cells
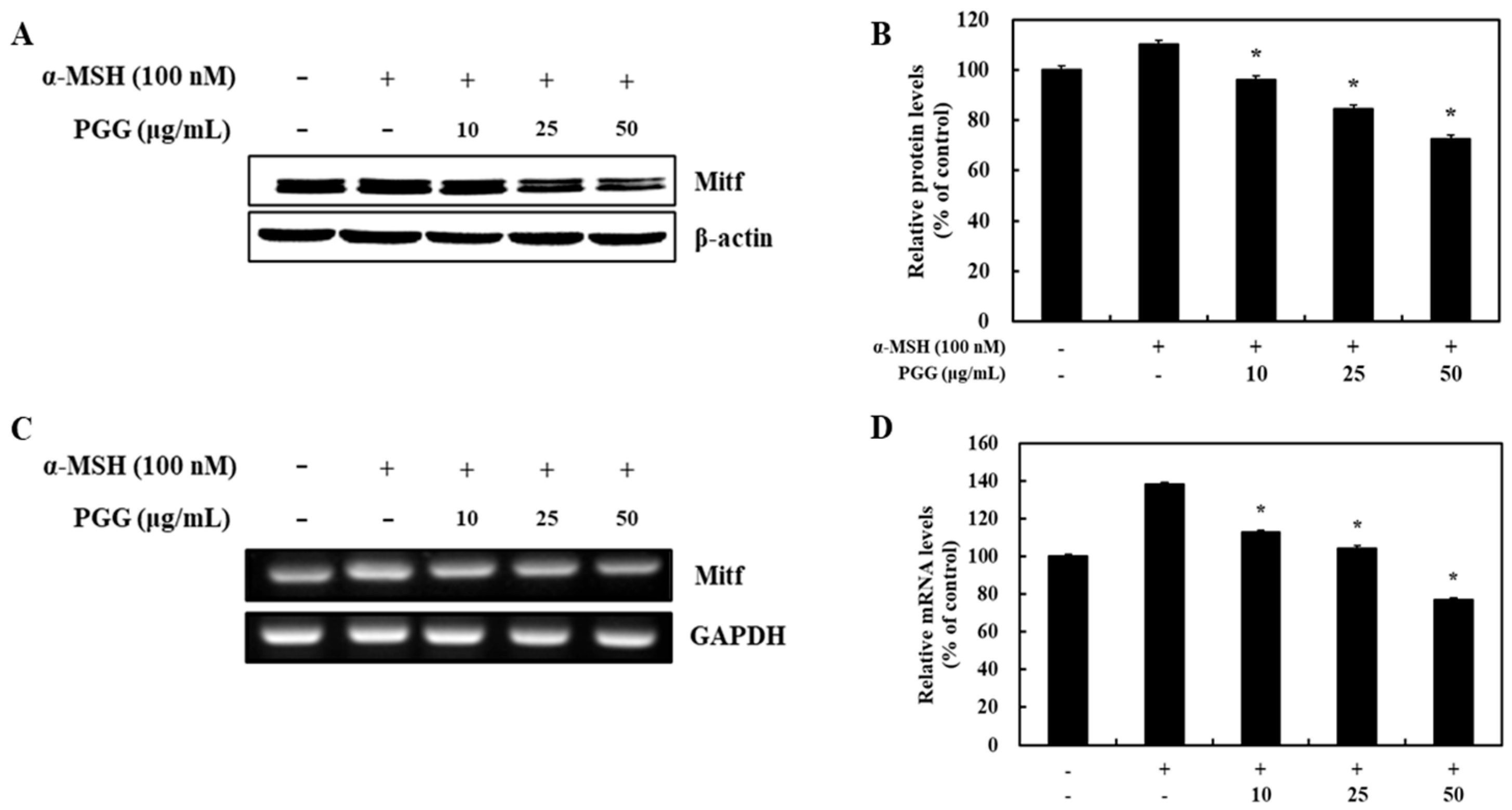
2.3. Effects of PGG on the Protein and mRNA Expression of MITF in B16F10 Cells
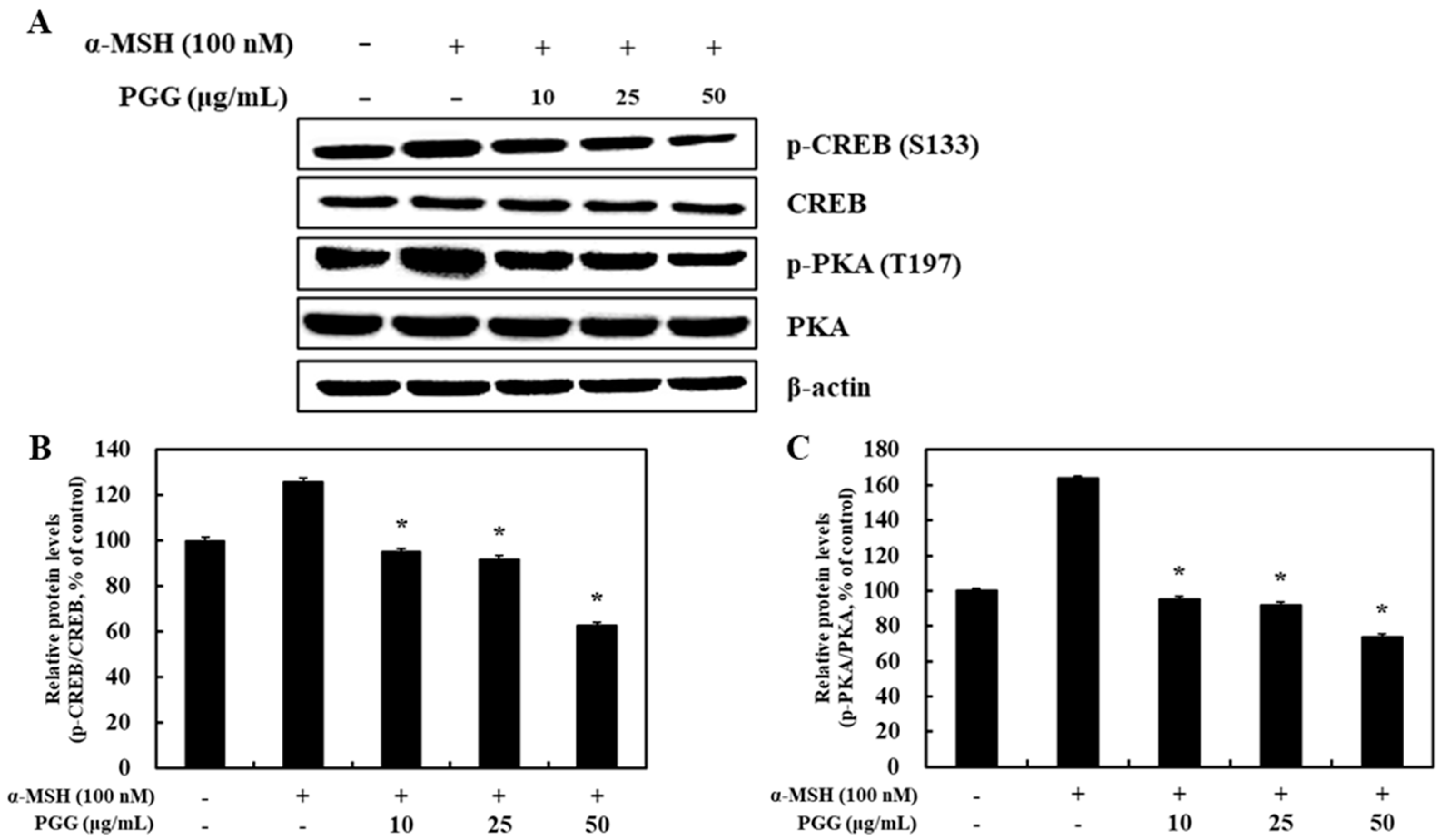
2.4. Effect of PGG on the Phosphorylation of PKA/CREB in B16F10 Cells
2.5. Effects of PGG on the MAPK Signaling Pathway in B16F10 Cells
3. Discussion
4. Materials and Methods
4.1. Chemical
4.2. Cell Culture
4.3. Cell Viability
4.4. Measurement of Antioxidant Ability
4.5. Measurement of Melanin Content
4.6. RT-PCR
4.7. Western Blotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gam, D.H.; Hong, J.W.; Kim, J.H.; Kim, J.W. Skin-whitening and anti-wrinkle effects of bioactive compounds isolated from peanut shell using ultrasound-assisted extraction. Molecules 2021, 26, 1231. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. The inhibitory effect of silk sericin against ultraviolet-induced melanogenesis and its potential use in cosmeceutics as an anti-hyperpigmentation compound. Photochem. Photobiol. Sci. 2019, 18, 2497–2508. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Chen, J.; Yang, J.; Chen, S.; Jameson, J.; Swope, V.B.; Cheng, T.; Kadakia, M.; Abdel-Malek, Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol. Cancer Res. 2012, 10, 778–786. [Google Scholar] [CrossRef]
- Yoon, Y.; Bae, S.; Kim, T.J.; An, S.; Lee, J.H. Nodakenin inhibits melanogenesis via the ERK/MSK1 signaling pathway. Pharmazie 2023, 78, 6–12. [Google Scholar] [CrossRef]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol. Cell Biochem. 2011, 354, 241–246. [Google Scholar] [CrossRef]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment. Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef]
- Watabe, H.; Soma, Y.; Ito, M.; Kawa, T.; Mizoguchi, M. All-trans Retinoic Acid Induces Differentiation and Apoptosis of Murine Melanocyte Precursors with Induction of the Microphthalmia-Associated Transcription Factor. J. Investig. Dermatol. 2022, 118, 35–42. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Kahn, V. Effect of kojic acid on the oxidation of DL-DOPA, norepinephrine, and dopamine by mushroom tyrosinase. Pigment. Cell Res. 1995, 8, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Evaluation of effect of kojic acid by a novel flow cytometric method in human epidermal melanocyte/keratinocyte co-culture System. Kor. J. Aesthet. Cosmetol. 2014, 12, 891–897. [Google Scholar]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Kim, B.H.; Choi, M.S.; Lee, H.G.; Lee, S.H.; Noh, K.H.; Kwon, S.; Jeong, A.J.; Lee, H.; Yi, E.H.; Park, J.Y.; et al. Photoprotective potential of penta-O-Galloyl-β-D-Glucose by targeting NF-κB and MAPK signaling in UVB radiation-induced human dermal fibroblasts and mouse skin. Mol. Cells 2015, 38, 982–990. [Google Scholar] [CrossRef]
- Shaikh, Q.U.A.; Yang, M.; Memon, K.H.; Lateef, M.; Na, D.; Wan, S.; Eric, D.; Zhang, L.; Jiang, T. 1,2,3,4,6-Pentakis[-O-(3,4,5-trihydroxybenzoyl)]-α, β-D-glucopyranose (PGG) analogs: Design, synthesis, anti-tumor and anti-oxidant activities. Carbohydr. Res. 2016, 430, 72–81. [Google Scholar] [CrossRef]
- Chen, X.; Daniels, N.A.; Cottrill, D.; Cao, Y.; Wang, X.; Li, Y.; Shriwas, P.; Qian, Y.; Archer, M.W.; Whitticar, N.B.; et al. Natural compound α-pgg and its synthetic derivative 6cl-tgq alter insulin secretion: Evidence for diminishing glucose uptake as a mechanism. Diabetes. Metab. Syndr. Obes. 2021, 14, 759–772. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis:The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Kant, R.; Yen, C.H.; Lu, C.K.; Lin, Y.C.; Li, J.H.; Chen, Y.M.A. Identification of 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranoside as a glycine N-methyltransferase enhancer by high-throughput screening of natural products inhibits hepatocellular carcinoma. Int. J. Mol. Sci. 2016, 17, 669. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lii, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Deiab, S.; Mazzio, E.; Eyunni, S.; McTier, O.; Mateeva, N.; Elshami, F.; Soliman, K.F.A. 1,2,3,4,6-Penta-O-galloylglucose within Galla Chinensis inhibits human LDH-A and attenuates cell proliferation in MDA-MB-231 breast cancer cells. Evidence-Based Complement. Altern. Med. 2015, 2015, 276946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Hao, W.; Zhao, M.; Peng, S. In vitro inhibition of fatty acid synthase by 1,2,3,4,6-penta-O-galloyl- β-d-glucose plays a vital role in anti-tumour activity. Biochem. Biophys. Res. Commun. 2014, 445, 346–351. [Google Scholar] [CrossRef]
- Kwon, T.R.; Lee, M.H.; Seong, S.J.; Sohn, E.J.; Lee, D.; Shin, E.A.; Lee, H.J.; Lee, E.O.; Jung, J.H.; Kim, J.H.; et al. Penta-O-galloyl-beta-D-glucose enhances antitumor activity of imatinib and suppresses the growth of K562 cells in mice. African J. Pharm. Pharmacol. 2013, 7, 552–559. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, X.; Wang, D. Lysosomes contribute to radioresistance in cancer. Cancer Lett. 2018, 439, 39–46. [Google Scholar] [CrossRef]
- Ni, J.; Bucci, J.; Malouf, D.; Knox, M.; Graham, P.; Li, Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019, 9, 869. [Google Scholar] [CrossRef]
- Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules 2023, 28, 4856. [Google Scholar] [CrossRef]
- Kantapan, J.; Dechsupa, N.; Tippanya, D.; Nobnop, W.; Chitapanarux, I. Gallotannin from bouea macrophylla seed extract suppresses cancer stem-like cells and radiosensitizes head and neck cancer. Int. J. Mol. Sci. 2021, 22, 9253. [Google Scholar] [CrossRef]
- Fujimaki, T.; Sato, C.; Yamamoto, R.; Watanabe, S.; Fujita, H.; Kikuno, H.; Sue, M.; Matsushima, Y. Isolation of phenolic acids and tannin acids from Mangifera indica L. kernels as inhibitors of lipid accumulation in 3T3-L1 cells. Biosci. Biotechnol. Biochem. 2022, 86, 665–671. [Google Scholar] [CrossRef]
- Kim, J.H.; Ha, W.R.; Park, J.H.; Lee, G.; Choi, G.; Lee, S.H.; Kim, Y.S. Influence of herbal combinations on the extraction efficiencies of chemical compounds from Cinnamomum cassia, Paeonia lactiflora, and Glycyrrhiza uralensis, the herbal components of Gyeji-tang, evaluated by HPLC method. J. Pharm. Biomed. Anal. 2016, 129, 50–59. [Google Scholar] [CrossRef]
- Thody, A.J.; Graham, A. Does α-MSH have a role in regulating skin pigmentation in humans? Pigm. Cell Res. 1988, 11, 265–274. [Google Scholar] [CrossRef]
- Kamilijiang, M.; Zang, D.; Abudukelimu, N.; Aidarhan, N.; Liu, G.; Aisa, H.A. Anti-Melanogenesis Effect of Polysaccharide from Saussurea involucrata on Forskolin-Induced Melanogenesis in B16F10 Melanoma Cells. Nutrients 2022, 14, 5044. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, H.; Hwang-Bo, J.; Kim, K.M.; Kwon, J.E.; Lee, S.R.; Hwang, S.H.; Kang, S.C.; Lee, Y. Anti-Melanogenic Effects of Cnidium monnieri Extract via p38 Signaling-Mediated Proteasomal Degradation of Tyrosinase. Plants 2024, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, N.; Lee, J.; Lee, J.N.; An, S.; Bae, S. Anti-Melanogenic Effects of Lilium lancifolium Root Extract via Downregulation of PKA/CREB and MAPK/CREB Signaling Pathways in B16F10 Cells. Plants 2023, 12, 3666. [Google Scholar] [CrossRef]
- Busca, R.; Abbe, P.; Mantoux, F.; Aberdam, E.; Peyssonnaux, C.; Eychene, A.; Ortonne, J.P.; Ballotti, R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000, 19, 2900–2910. [Google Scholar] [CrossRef]
- Zhou, X.; Oh, J.H.; Karadeniz, F.; Yang, J.; Lee, H.; Seo, Y.; Kong, C.S. Anti-Melanogenesis Effect of Rosa rugosa on α-MSH-Induced B16F10 Cells via PKA/CREB Pathway Activation. Appl. Sci. 2023, 13, 184. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, D.C.; Noh, S.G.; Choi, H.; Choi, I.; Ryu, I.Y.; Chung, H.Y.; Moon, H.R. New Benzimidazothiazolone Derivatives as Tyrosinase Inhibitors with Potential Anti-Melanogenesis and Reactive Oxygen Species Scavenging Activities. Antioxidants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Kim, M.M. Recent Natural Products Involved in the Positive Modulation of Melanogenesis. J. Life Sci. 2018, 28, 745–752. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Orozco-Covarrubias, M.D.L.L. Postinflammatory hypopigmentation and hyperpigmentation. Semin. Cutan. Med. Sur. 1997, 16, 36–43. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 22, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Woolery-Lloyd, H.; Kammer, J.N. Treatment of Hyperpigmentation. Semin. Cutan. Med. Surg. 2011, 30, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 0877–10895. [Google Scholar] [CrossRef]
- Cillo, C.; Dick, J.E.; Hill, R.P. Generation of drug-resistant variants in metastatic B16 mouse melanoma cell lines. Cancer Res. 1987, 15, 2604–2608. [Google Scholar]
- Cho, M.; Kim, O.; Lee, Y.; Kang, L.J.; Nguyen, G.N.; Ishihara, A.; Kim, H.E. Feruloylserotonin inhibits hydrogen peroxide-induced melanogenesis and apoptosis in B16F10 and SK-Mel-2 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 973–979. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Hyang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, C.P.; Gowrisankar, Y.V.; Huang, P.J.; Chang, W.L.; Shrestha, S.; Hseu, Y.C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021, 185, 114454. [Google Scholar] [CrossRef]
- Pae, H.O.; Oh, G.S.; Jeong, S.O.; Jeong, G.S.; Lee, B.S.; Choi, B.M.; Lee, H.S.; Chung, H.T. 1,2,3,4,6-penta-O-galloyl-β-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase dependent manner in HepG2 cells. World, J. Gastroenterol. 2006, 12, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Lee, I.C. Antioxidant and Skin Whitening Effects of Jeju Mango Kernel Extracts (Mangifera indica L. var. Irwin). J. Korean Soc. Food Sci. Nutr. 2022, 51, 1266–1271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-W.; Lee, I.-C. Pentagalloylglucose Inhibits Melanogenesis via Suppression of MITF Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 4861. https://doi.org/10.3390/ijms26104861
Kang J-W, Lee I-C. Pentagalloylglucose Inhibits Melanogenesis via Suppression of MITF Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(10):4861. https://doi.org/10.3390/ijms26104861
Chicago/Turabian StyleKang, Jung-Wook, and In-Chul Lee. 2025. "Pentagalloylglucose Inhibits Melanogenesis via Suppression of MITF Signaling Pathway" International Journal of Molecular Sciences 26, no. 10: 4861. https://doi.org/10.3390/ijms26104861
APA StyleKang, J.-W., & Lee, I.-C. (2025). Pentagalloylglucose Inhibits Melanogenesis via Suppression of MITF Signaling Pathway. International Journal of Molecular Sciences, 26(10), 4861. https://doi.org/10.3390/ijms26104861





