Astragalus Extract Mixture HT042 Reverses Cyclophosphamide-Induced Immunosuppression Through Dual Modulation of Innate and Adaptive Immunity
Abstract
:1. Introduction
2. Results
2.1. HPLC Chromatograms of HT042
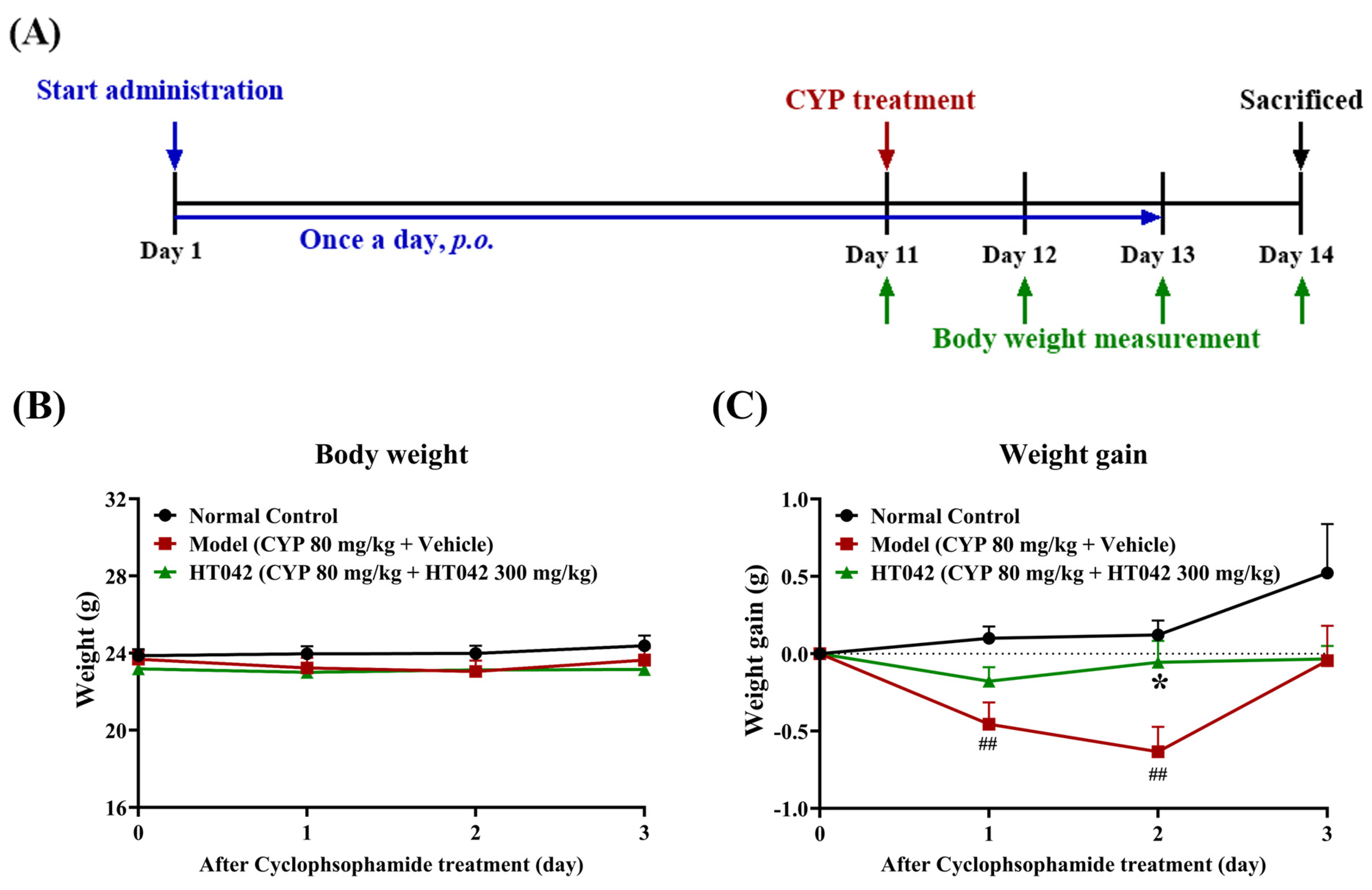
2.2. Effects of HT042 on the Body Weight of Mice with CYP-Induced Immunosuppression
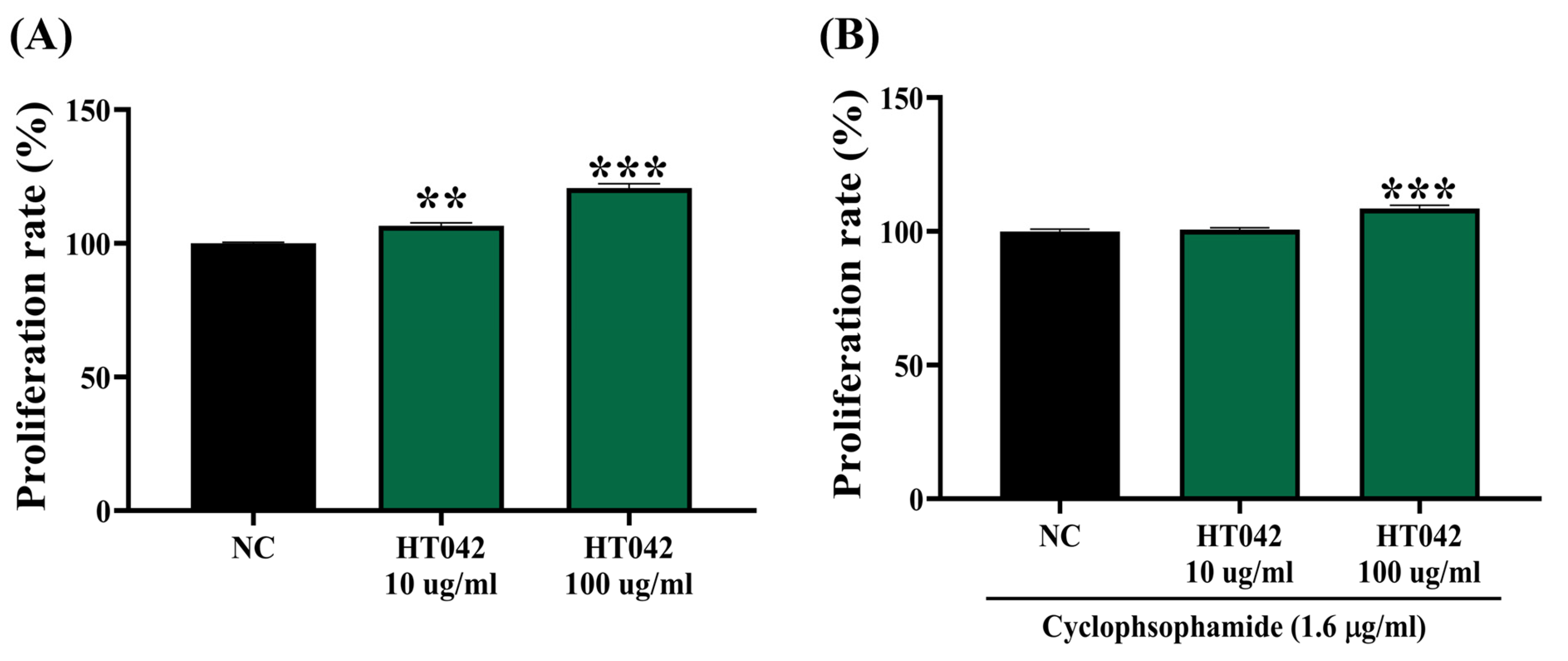
2.3. Effects of HT042 on the Proliferation of Primary Splenocytes
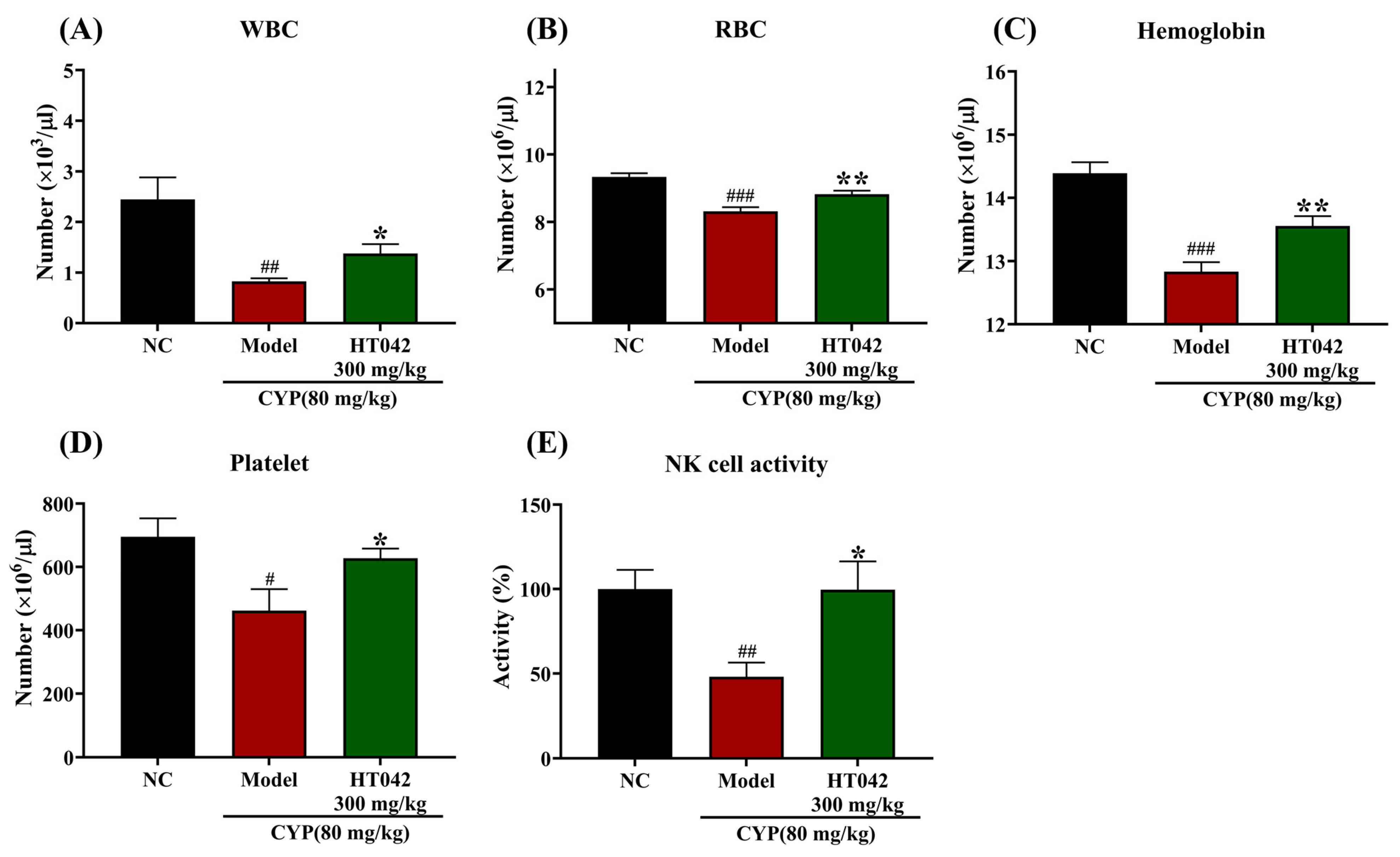
2.4. Effects of HT042 on the Hematocytes of Mice with CYP-Induced Immunosuppression
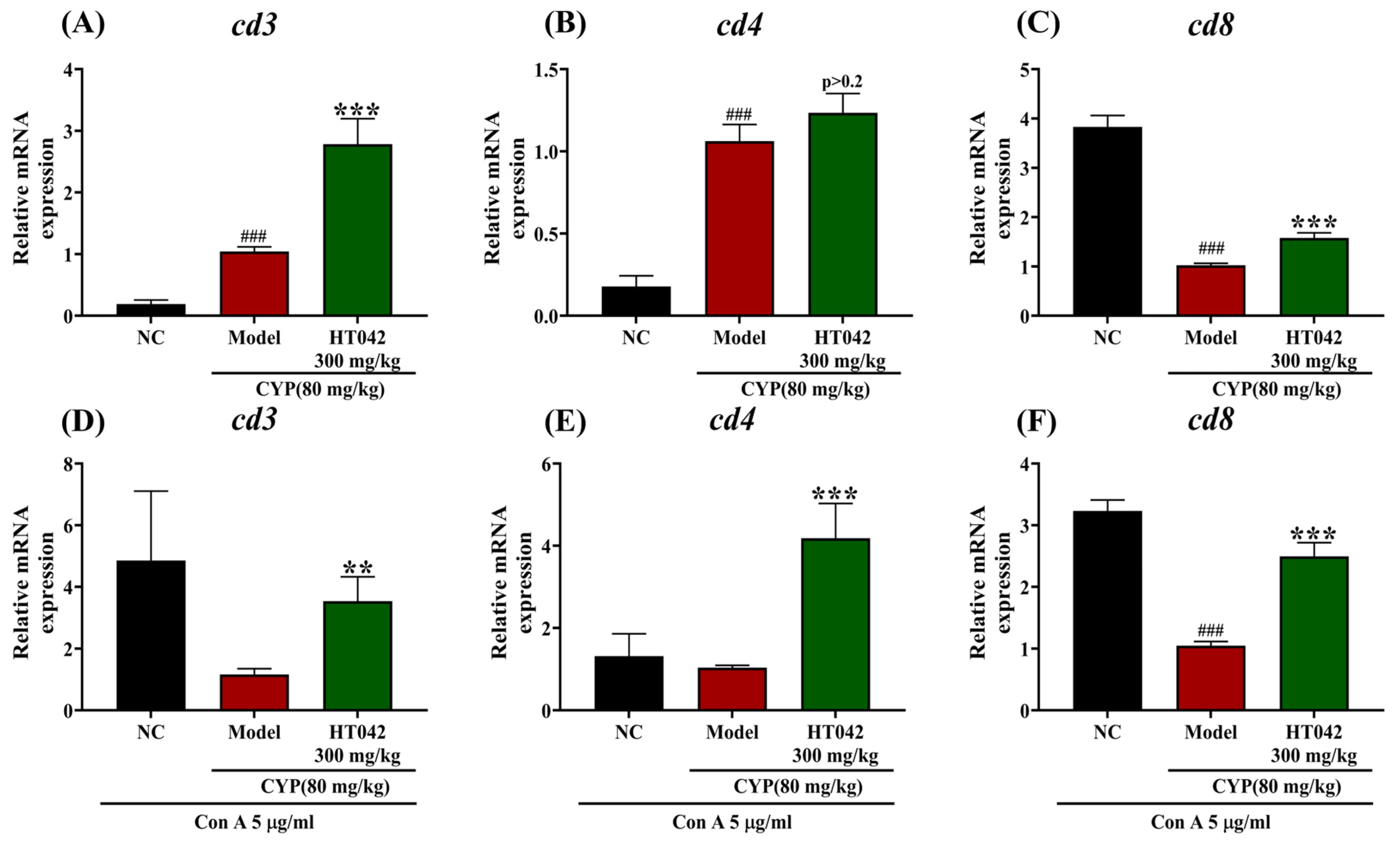
2.5. Effects of HT042 on the T-Cell-Related Gene of Mice with CYP-Induced Immunosuppression
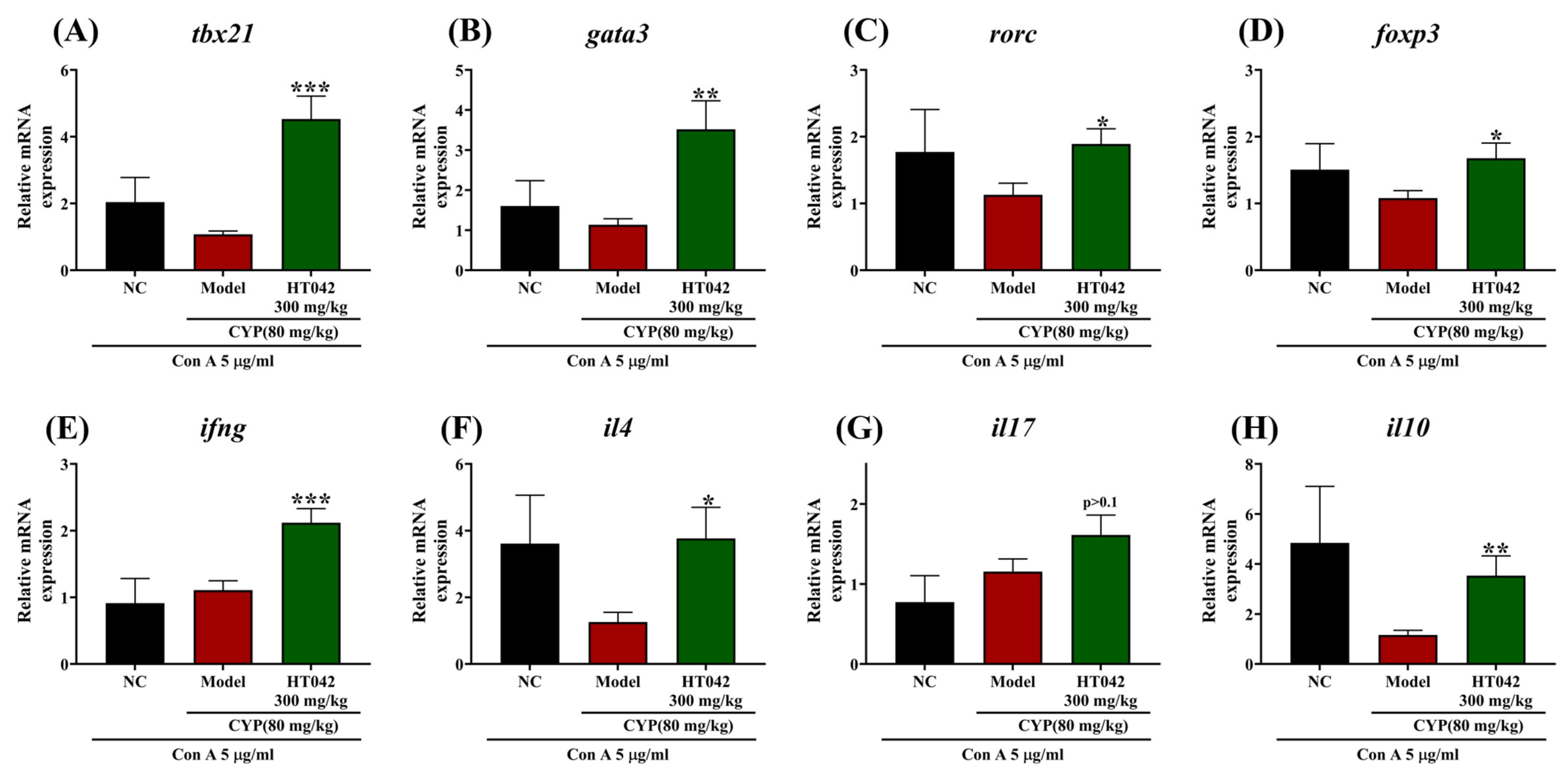
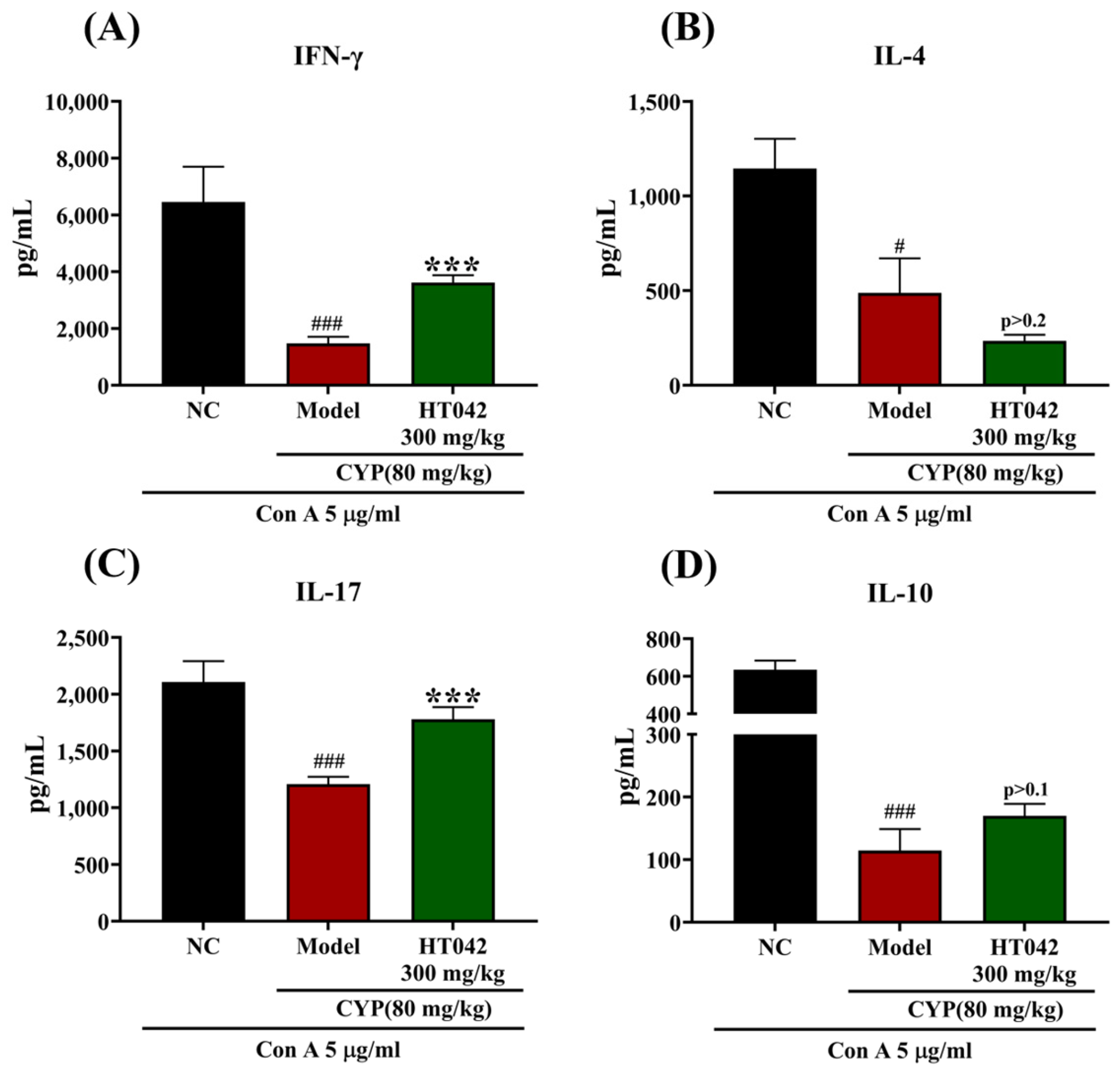
2.6. Effects of HT042 on CD4+ Th Subsets of Mice with CYP-Induced Immunosuppression
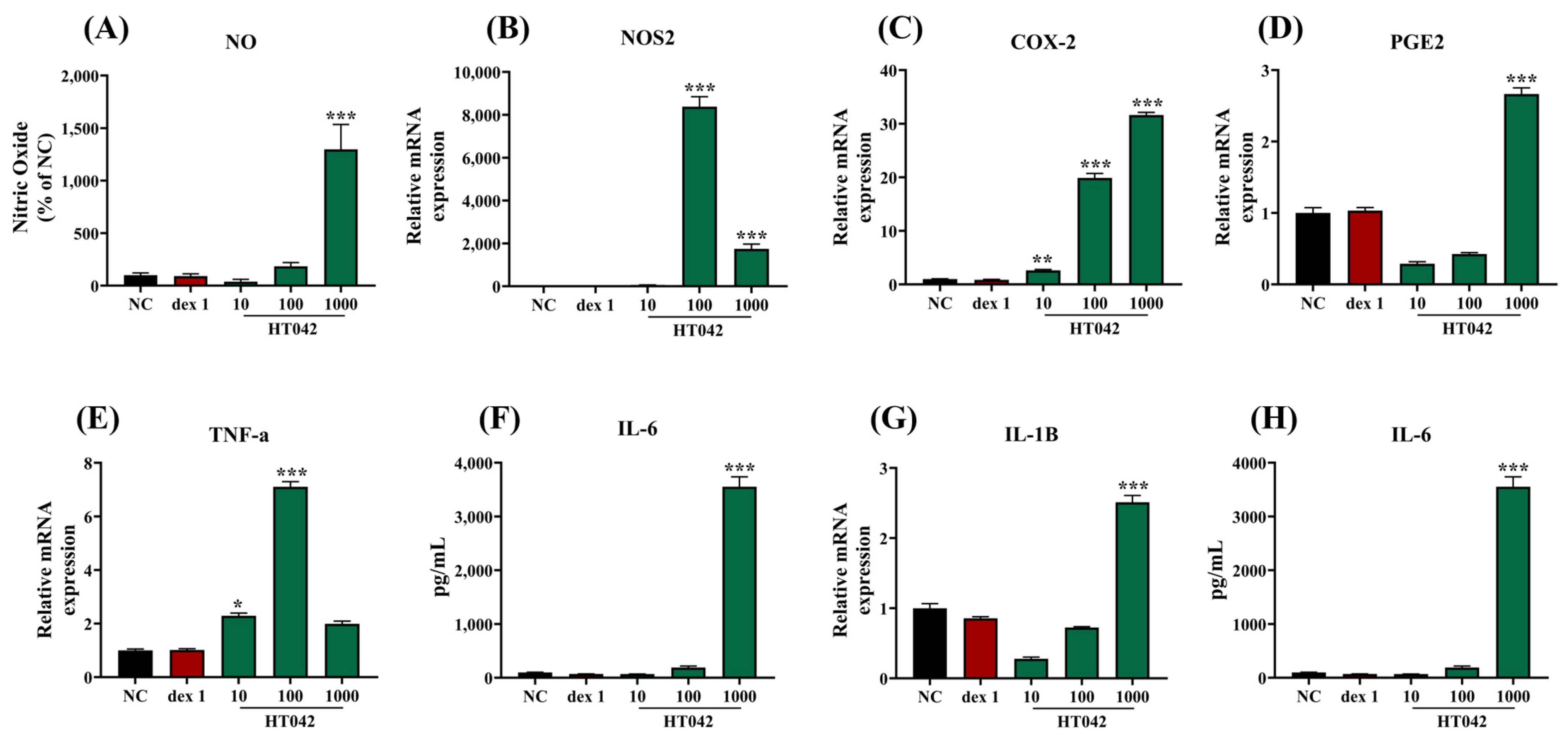
2.7. Effects of HT042 on NO and Cytokine Production in RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and HPLC Analysis
4.2. Animals
4.3. Development of the Immune-Suppressed Mouse Model
4.4. Hematological Analysis and NK Cell Activity Measurement
4.5. Primary Splenocyte Proliferation Analysis
4.6. Primary Splenocyte Culture Under Concanavalin A Treatment
4.7. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.8. Cytokine Measurement Using ELISA
4.9. Cell Culture and NO Assay of RAW 264.7 Macrophages
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NK | Natural killer |
| GH | Growth hormone |
| IGF-1 | Insulin-like growth factor-1 |
| CYP | Cyclophosphamide |
| WBC | White blood cell |
| RBC | Red blood cell |
| IFN | Interferon |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| TNF | Tumor necrosis factor |
| NOS2 | Nitric oxide synthase2 |
| COX-2 | Cyclooxygenase 2 |
| PGE2 | Prostaglandin E2 |
| IL | Interleukin. |
| CD | Cluster of differentiation |
| NO | Nitric oxide |
| Con A | Concanavalin A |
| Th | T helper |
References
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Morey, J.N.; Boggero, I.A.; Scott, A.B.; Segerstrom, S.C. Current directions in stress and human immune function. Curr. Opin. Psychol. 2015, 5, 13–17. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- O’Connor, J.C.; McCusker, R.H.; Strle, K.; Johnson, R.W.; Dantzer, R.; Kelley, K.W. Regulation of IGF-I function by proinflammatory cytokines: At the interface of immunology and endocrinology. Cell. Immunol. 2008, 252, 91–110. [Google Scholar] [CrossRef]
- Ali, S.A.; Singh, G.; Datusalia, A.K. Potential therapeutic applications of phytoconstituents as immunomodulators: Pre-clinical and clinical evidences. Phytother. Res. 2021, 35, 3702–3731. [Google Scholar] [CrossRef]
- Mizuno, M.; Minato, K.-I. Anti-inflammatory and immunomodulatory properties of polysaccharides in mushrooms. Curr. Opin. Biotechnol. 2024, 86, 103076. [Google Scholar] [CrossRef]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Braga, F.C.; Buenz, E.J.; Campana, P.R.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; et al. Future directions for the discovery of natural product-derived immunomodulating drugs: An IUPHAR positional review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhang, Y.; Li, J.; Lai, J. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Jia, A.; Zhang, Y.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2021, 268, 113586. [Google Scholar] [CrossRef]
- Lau, K.-M.; Yue, G.G.-L.; Chan, Y.-Y.; Kwok, H.-F.; Gao, S.; Wong, C.-W.; Lau, C.B.-S. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin. Med. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Dobie, R.; Altowati, M.A.; Werther, G.A.; Farquharson, C.; Ahmed, S.F. Growth and the Growth Hormone-Insulin Like Growth Factor 1 Axis in Children with Chronic Inflammation: Current Evidence, Gaps in Knowledge, and Future Directions. Endocr. Rev. 2016, 37, 62–110. [Google Scholar] [CrossRef]
- Ni, F.; Sun, R.; Fu, B.; Wang, F.; Guo, C.; Tian, Z.; Wei, H. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 2013, 4, 1479. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- Napolitano, L.A.; Schmidt, D.; Gotway, M.B.; Ameli, N.; Filbert, E.L.; Ng, M.M.; Clor, J.L.; Epling, L.; Sinclair, E.; Baum, P.D.; et al. Growth hormone enhances thymic function in HIV-1–infected adults. J. Clin. Investig. 2008, 118, 1085–1098. [Google Scholar] [CrossRef]
- Baek, C.Y.; Lee, J.; Lee, D.; Kim, H. Astragalus Extract Mixture HT042 Alleviates Dexamethasone-Induced Bone Growth Retardation in Rat Metatarsal Bones. Nutrients 2024, 16, 2333. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Swan, D.; Gurney, M.; Krawczyk, J.; Ryan, A.E.; O’Dwyer, M. Beyond DNA Damage: Exploring the Immunomodulatory Effects of Cyclophosphamide in Multiple Myeloma. HemaSphere 2020, 4, e350. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, T.; Liu, C.; Shan, X.; Xu, Z.; Hong, H.; Lin, H.; Chen, J.; Sun, H. Cyclophosphamide induced physiological and biochemical changes in mice with an emphasis on sensitivity analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111889. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wusiman, A.; Liu, Z.; Gu, P.; Ni, H.; Zhang, Y.; Hu, Y.; Liu, J.; Wu, Y.; Wang, D. pH-responsive Astragalus polysaccharides-loaded poly(lactic-co-glycolic acid) nanoparticles and their in vitro immunogenicity. Int. J. Biol. Macromol. 2019, 125, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Zhao, G.D.; Xiong, W.; Linghu, K.G.; Ma, Q.S.; Cheang, W.S.; Yu, H.; Wang, Y. Correction to: Immunomodulatory effects of a new whole ingredients extract from Astragalus: A combined evaluation on chemistry and pharmacology. Chin. Med. 2021, 16, 38. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef]
- Shen, M.L.; Zhai, S.K.; Chen, H.L.; Luo, Y.D.; Tu, G.R.; Ou, D.W. Immunomopharmacological effects of polysaccharides from Acanthopanax senticosus on experimental animals. Int. J. Immunopharmacol. 1991, 13, 549–554. [Google Scholar] [CrossRef]
- Yoon, T.J.; Yoo, Y.C.; Lee, S.-W.; Shin, K.-S.; Choi, W.-H.; Hwang, S.-H.; Ha, E.S.; Jo, S.K.; Kim, S.-H.; Park, W.-M. Anti-metastatic activity of Acanthopanax senticosus extract and its possible immunological mechanism of action. J. Ethnopharmacol. 2004, 93, 247–253. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.H.; Lee, Y.H.; Song, J.; Kim, H. Astragalus Extract Mixture HT042 Increases Longitudinal Bone Growth Rate by Upregulating Circulatory IGF-1 in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 6935802. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Gao, J.; Yang, H.; Duan, Y.; Feng, Y.; He, X.; Gong, X.; Wang, H.; Wu, X.; et al. Astragaloside III Enhances Anti-Tumor Response of NK Cells by Elevating NKG2D and IFN-γ. Front. Pharmacol. 2019, 10, 898. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of various Eleutherococcus senticosus cortex on swimming time, natural killer activity and corticosterone level in forced swimming stressed mice. J. Ethnopharmacol. 2004, 95, 447–453. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Q.; Masubuchi, T.; Shi, X.; Li, H.; Xu, X.; Huang, M.; Meng, L.; He, X.; Zhu, H.; et al. Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy. Cell 2020, 182, 855–871.e23. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Love, P.E.; Hayes, S.M. ITAM-mediated Signaling by the T-Cell Antigen Receptor. Cold Spring Harb. Perspect. Biol. 2010, 2, a002485. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010, 20, 4–12. [Google Scholar] [CrossRef]
- Jasemi, S.V.; Khazaei, H.; Morovati, M.R.; Joshi, T.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Phytochemicals as treatment for allergic asthma: Therapeutic effects and mechanisms of action. Phytomedicine 2024, 122, 155149. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Jia, N.; Qiao, H.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Synthesis and evaluation of a novel water-soluble high Se-enriched Astragalus polysaccharide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1438–1448. [Google Scholar] [CrossRef]
- Yu, Z.M.; Huang, X.H.; Yan, C.Q.; Jin, G.A.O.; Liang, Z.S. Effect of Fuzheng Jiedu granule on immunological function level of immune-related cytokines in immune-suppressed mice. J. Integr. Agric. 2016, 15, 650–657. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.H.; Lee, S.H.; Cho, W.Y.; Kim, Y.S.; Kim, H. Effects of Astragalus Extract Mixture HT042 on Circulating IGF-1 Level and Growth Hormone Axis in Rats. Children 2021, 8, 975. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.H.; Song, J.; Jee, H.; Cha, S.H.; Chang, G.T. Effects of Astragalus Extract Mixture HT042 on Height Growth in Children with Mild Short Stature: A Multicenter Randomized Controlled Trial. Phytother. Res. 2018, 32, 49–57. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharma 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, D.; Min, B.; Bae, J.S.; Chang, G.T.; Kim, H. Safety evaluation of Astragalus extract mixture HT042 and its constituent herbs in Sprague–Dawley rats. Phytomedicine 2017, 32, 59–67. [Google Scholar] [CrossRef]
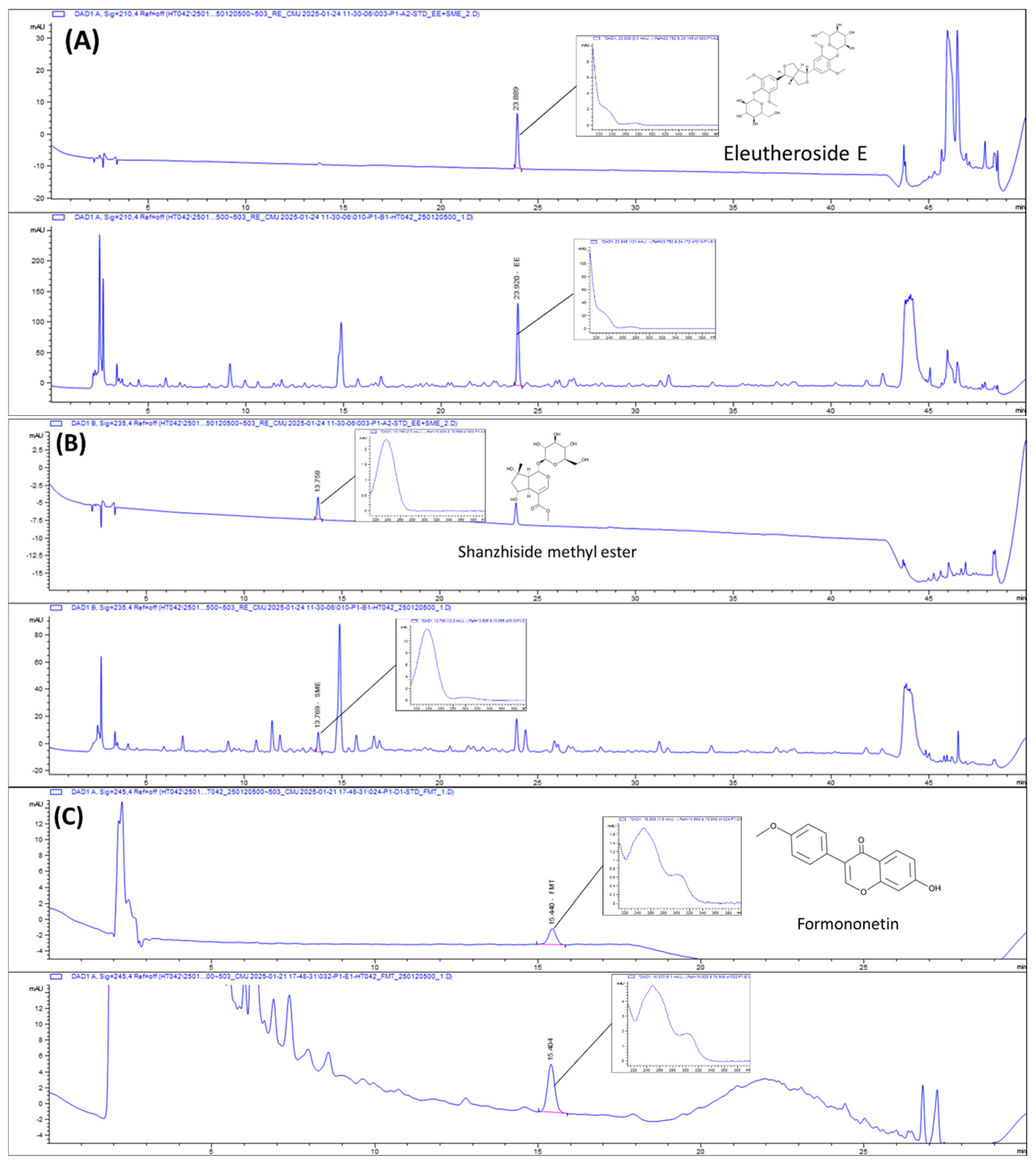
| Botanical Name | Ratio (%) | Estimated Dose (mg/kg) |
|---|---|---|
| Astragalus mongholicus Bunge | 26.5 | 79.5 |
| Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. | 31.2 | 93.6 |
| Phlomis umbrosa (Turcz.) Kamelin & Makhm | 42.3 | 126.9 |
| Gene | Primer | Sequence |
|---|---|---|
| CD3 | Forward | GCTCCAGGATTTCTCGGAAGTC |
| Reverse | ATGGCTACTGCTGTCAGGTCCA | |
| CD4 | Forward | GTTCAGGACAGCGACTTCTGGA |
| Reverse | GAAGGAGAACTCCGCTGACTCT | |
| CD8α | Forward | ACTACCAAGCCAGTGCTGCGAA |
| Reverse | ATCACAGGCGAAGTCCAATCCG | |
| T-bet | Forward | CCACCTGTTGTGGTCCAAGTTC |
| Reverse | CCACAAACATCCTGTAATGGCTTG | |
| GATA3 | Forward | CCTCTGGAGGAGGAACGCTAAT |
| Reverse | GTTTCGGGTCTGGATGCCTTCT | |
| RORγt | Forward | CCGCTGAGAGGGCTTCAC |
| Reverse | TGCAGGAGTAGGCCACATTACA | |
| FoxP3 | Forward | CCTGGTTGTGAGAAGGTCTTCG |
| Reverse | TGCTCCAGAGACTGCACCACTT | |
| IFN-γ | Forward | CAGCAACAGCAAGGCGAAAAAGG |
| Reverse | TTTCCGCTTCCTGAGGCTGGAT | |
| IL-4 | Forward | ATCATCGGCATTTTGAACGAGGTC |
| Reverse | ACCTTGGAAGCCCTACAGACGA | |
| IL-17 | Forward | CAGACTACCTCAACCGTTCCAC |
| Reverse | TCCAGCTTTCCCTCCGCATTGA | |
| IL-10 | Forward | CGGGAAGACAATAACTGCACCC |
| Reverse | CGGTTAGCAGTATGTTGTCCAGC | |
| β-actin | Forward | TGCTGTCCCTGTATGCCTCTG |
| Reverse | TGATGTCACGCACGATTTCC |
| Gene | Primer | Sequence |
|---|---|---|
| GAPDH | Forward | CTTGTGACAAAGTGGACATTGTT |
| Reverse | TGACCAGCTTCCCATTCTC | |
| TNF-α | Forward | GAGAAGTTCCCAAATGGCCT |
| Reverse | AGCCACTCCAGCTGCTCCT | |
| COX-2 | Forward | ATCCATGTCAAAACCGTGGG |
| Reverse | TTGGGGTGGGCTTCAGCAG | |
| NOS2 | Forward | ACCAAGATGGCCTGGAGGAA |
| Reverse | CCGACCTGATGTTGCCATTG | |
| PGE2 | Forward | CTGGTAACGGAATTGGTGC |
| Reverse | TGGCCAGACTAAAGAAGGTC | |
| IL-6 | Forward | CACTTCACAAGTCGGAGGCT |
| Reverse | CAAGTGCATCATCGTTGTTC | |
| IL-1B | Forward | CCAGCTTCAAATCTCGCAGC |
| Reverse | GTGCTCATGTCCTCATCCTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Son, J.; Kim, M.; Baek, C.Y.; Kim, M.-Y.; Shin, A.; Lee, D.; Kim, H. Astragalus Extract Mixture HT042 Reverses Cyclophosphamide-Induced Immunosuppression Through Dual Modulation of Innate and Adaptive Immunity. Int. J. Mol. Sci. 2025, 26, 4850. https://doi.org/10.3390/ijms26104850
Kim S-Y, Son J, Kim M, Baek CY, Kim M-Y, Shin A, Lee D, Kim H. Astragalus Extract Mixture HT042 Reverses Cyclophosphamide-Induced Immunosuppression Through Dual Modulation of Innate and Adaptive Immunity. International Journal of Molecular Sciences. 2025; 26(10):4850. https://doi.org/10.3390/ijms26104850
Chicago/Turabian StyleKim, Se-Young, Joohee Son, Minju Kim, Chae Yun Baek, Mi-Yeon Kim, Ari Shin, Donghun Lee, and Hocheol Kim. 2025. "Astragalus Extract Mixture HT042 Reverses Cyclophosphamide-Induced Immunosuppression Through Dual Modulation of Innate and Adaptive Immunity" International Journal of Molecular Sciences 26, no. 10: 4850. https://doi.org/10.3390/ijms26104850
APA StyleKim, S.-Y., Son, J., Kim, M., Baek, C. Y., Kim, M.-Y., Shin, A., Lee, D., & Kim, H. (2025). Astragalus Extract Mixture HT042 Reverses Cyclophosphamide-Induced Immunosuppression Through Dual Modulation of Innate and Adaptive Immunity. International Journal of Molecular Sciences, 26(10), 4850. https://doi.org/10.3390/ijms26104850







