Impact of Genetic Testing Using Gene Panels, Exomes, and Genome Sequencing in Romanian Children with Epilepsy
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Genetic Findings
2.3. Impact of Genetic Diagnosis on Clinical Management
2.4. Clinical Predictors of a Genetic Diagnosis
3. Discussion
4. Material and Methods
4.1. Cohort Information
4.2. Genetic Testing
4.3. Data Analysis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- McTague, A.; Howell, K.B.; Cross, J.H.; Kurian, M.A.; Scheffer, I.E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016, 15, 304–316. [Google Scholar] [CrossRef]
- Smith, L.; Malinowski, J.; Ceulemans, S.; Peck, K.; Walton, N.; Sheidley, B.R.; Lippa, N. Genetic testing and counseling for the unexplained epilepsies: An evidence-based practice guideline of the National Society of Genetic Counselors. J. Genet. Couns. 2023, 32, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.; Albury, C.L.; Maksemous, N.; Benton, M.C.; Sutherland, H.G.; Smith, R.A.; Haupt, L.M.; Griffiths, L.R. Next Generation Sequencing Methods for Diagnosis of Epilepsy Syndromes. Front. Genet. 2018, 9, 20. [Google Scholar] [CrossRef]
- Lesca, G.; Baumgartner, T.; Monin, P.; De Dominicis, A.; Kunz, W.S.; Specchio, N. Genetic causes of rare and common epilepsies: What should the epileptologist know? Eur. J. Med. Genet. 2022, 65, 104570. [Google Scholar] [CrossRef] [PubMed]
- Thakran, S.; Guin, D.; Singh, P.; Singh, P.; Kukal, S.; Rawat, C.; Yadav, S.; Kushwaha, S.S.; Srivastava, A.K.; Hasija, Y.; et al. Genetic Landscape of Common Epilepsies: Advancing towards Precision in Treatment. Int. J. Mol. Sci. 2020, 21, 7784. [Google Scholar] [CrossRef] [PubMed]
- Haviland, I.; Daniels, C.I.; Greene, C.A.; Drew, J.; Love-Nichols, J.A.; Swanson, L.C.; Smith, L.; Nie, D.A.; Benke, T.; Sheidley, B.R.; et al. Genetic Diagnosis Impacts Medical Management for Pediatric Epilepsies. Pediatr. Neurol. 2023, 138, 71–80. [Google Scholar] [CrossRef]
- Sheidley, B.R.; Malinowski, J.; Bergner, A.L.; Bier, L.; Gloss, D.S.; Mu, W.; Mulhern, M.M.; Partack, E.J.; Poduri, A. Genetic testing for the epilepsies: A systematic review. Epilepsia 2022, 63, 375–387. [Google Scholar] [CrossRef]
- Stefanski, A.; Calle-López, Y.; Leu, C.; Pérez-Palma, E.; Pestana-Knight, E.; Lal, D. Clinical sequencing yield in epilepsy, autism spectrum disorder, and intellectual disability: A systematic review and meta-analysis. Epilepsia 2021, 62, 143–151. [Google Scholar] [CrossRef]
- Riza, A.L.; Streață, I.; Roza, E.; Budișteanu, M.; Iliescu, C.; Burloiu, C.; Dobrescu, M.A.; Dorobanțu, S.; Dragoș, A.; Grigorescu, A.; et al. Phenotypic and Genotypic Spectrum of Early-Onset Developmental and Epileptic Encephalopathies-Data from a Romanian Cohort. Genes 2022, 13, 1253. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Nabbout, R.; Scheffer, I.E.; Alsaadi, T.; Bogacz, A.; French, J.A.; Hirsch, E.; Jain, S.; Kaneko, S.; Riney, K.; et al. Methodology for classification and definition of epilepsy syndromes with list of syndromes: Report of the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Gene [Internet]. 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 15 January 2025).
- Shipe, M.E.; Deppen, S.A.; Farjah, F.; Grogan, E.L. Developing prediction models for clinical use using logistic regression: An overview. J. Thorac. Dis. 2019, 11, S574–S584. [Google Scholar] [CrossRef] [PubMed]
- Habela, C.W.; Schatz, K.; Kelley, S.A. Genetic Testing in Epilepsy: Improving Outcomes and Informing Gaps in Research. Epilepsy Curr. 2024. [Google Scholar] [CrossRef]
- Howell, K.B.; Eggers, S.; Dalziel, K.; Riseley, J.; Mandelstam, S.; Myers, C.T.; McMahon, J.M.; Schneider, A.; Carvill, G.L.; Mefford, H.C.; et al. A population-based cost-effectiveness study of early genetic testing in severe epilepsies of infancy. Epilepsia 2018, 59, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.E.; Schofield, D.; Shrestha, R.; Kandula, T.; Macintosh, R.; Lawson, J.A.; Andrews, I.; Sampaio, H.; Johnson, A.M.; Farrar, M.A.; et al. Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: Evidence of clinical utility and cost effectiveness. Mol. Genet. Genom. Med. 2018, 6, 186–199. [Google Scholar] [CrossRef]
- Oates, S.; Tang, S.; Rosch, R.; Lear, R.; Hughes, E.F.; Williams, R.E.; Larsen, L.H.G.; Hao, Q.; Dahl, H.A.; Møller, R.S.; et al. Incorporating epilepsy genetics into clinical practice: A 360°evaluation. NPJ Genom. Med. 2018, 3, 13. [Google Scholar] [CrossRef]
- McKnight, D.; Morales, A.; Hatchell, K.E.; Bristow, S.L.; Bonkowsky, J.L.; Perry, M.S.; Berg, A.T.; Borlot, F.; Esplin, E.D.; Moretz, C.; et al. Genetic Testing to Inform Epilepsy Treatment Management from an International Study of Clinical Practice. JAMA Neurol. 2022, 79, 1267–1276. [Google Scholar] [CrossRef]
- Benson, K.A.; White, M.; Allen, N.M.; Byrne, S.; Carton, R.; Comerford, E.; Costello, D.; Doherty, C.; Dunleavey, B.; El-Naggar, H.; et al. A comparison of genomic diagnostics in adults and children with epilepsy and comorbid intellectual disability. Eur. J. Hum. Genet. 2020, 28, 1066–1077. [Google Scholar] [CrossRef]
- Rochtus, A.; Olson, H.E.; Smith, L.; Keith, L.G.; El Achkar, C.; Taylor, A.; Mahida, S.; Park, M.; Kelly, M.; Shain, C.; et al. Genetic diagnoses in epilepsy: The impact of dynamic exome analysis in a pediatric cohort. Epilepsia 2020, 61, 249–258. [Google Scholar] [CrossRef]
- Horák, O.; Burešová, M.; Kolář, S.; Španělová, K.; Jeřábková, B.; Gaillyová, R.; Česká, K.; Réblová, K.; Šoukalová, J.; Zídková, J.; et al. Next-generation sequencing in children with epilepsy: The importance of precise genotype-phenotype correlation. Epilepsy Behav. 2022, 128, 108564. [Google Scholar] [CrossRef]
- Blazekovic, A.; Gotovac Jercic, K.; Meglaj, S.; Duranovic, V.; Prpic, I.; Lozic, B.; Malenica, M.; Markovic, S.; Lujic, L.; Petelin Gadze, Z.; et al. Genetics of Pediatric Epilepsy: Next-Generation Sequencing in Clinical Practice. Genes 2022, 13, 1466. [Google Scholar] [CrossRef]
- Symonds, J.D.; McTague, A. Epilepsy and developmental disorders: Next generation sequencing in the clinic. Eur. J. Paediatr. Neurol. 2020, 24, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Costain, G.; Cordeiro, D.; Matviychuk, D.; Mercimek-Andrews, S. Clinical Application of Targeted Next-Generation Sequencing Panels and Whole Exome Sequencing in Childhood Epilepsy. Neuroscience 2019, 418, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.; Liang, X.Y.; Wang, J.; Gao, L.D.; Liao, H.J.; He, Y.H.; Yi, Y.H.; He, N.; Liao, W.P. Epilepsy-associated genes: An update. Seizure 2024, 116, 4–13. [Google Scholar] [CrossRef]
- Oliver, K.L.; Scheffer, I.E.; Bennett, M.F.; Grinton, B.E.; Bahlo, M.; Berkovic, S.F. Genes4Epilepsy: An epilepsy gene resource. Epilepsia 2023, 64, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Poduri, A.; Lowenstein, D. Epilepsy genetics--past, present, and future. Curr Opin. Genet. Dev. 2011, 21, 325–332. [Google Scholar] [CrossRef]
- Olson, H.; Shen, Y.; Avallone, J.; Sheidley, B.R.; Pinsky, R.; Bergin, A.M.; Berry, G.T.; Duffy, F.H.; Eksioglu, Y.; Harris, D.J.; et al. Copy number variation plays an important role in clinical epilepsy. Ann. Neurol. 2014, 75, 943–958. [Google Scholar] [CrossRef]
- Pérez-Palma, E.; Helbig, I.; Klein, K.M.; Anttila, V.; Horn, H.; Reinthaler, E.M.; Gormley, P.; Ganna, A.; Byrnes, A.; Pernhorst, K.; et al. Heterogeneous contribution of microdeletions in the development of common generalised and focal epilepsies. J. Med. Genet. 2017, 54, 598–606. [Google Scholar] [CrossRef]
- Montanucci, L.; Lewis-Smith, D.; Collins, R.L.; Niestroj, L.M.; Parthasarathy, S.; Xian, J.; Ganesan, S.; Macnee, M.; Brünger, T.; Thomas, R.H.; et al. Genome-wide identification and phenotypic characterization of seizure-associated copy number variations in 741,075 individuals. Nat. Commun. 2023, 14, 4392. [Google Scholar] [CrossRef]
- Bayat, A.; Bayat, M.; Rubboli, G.; Møller, R.S. Epilepsy Syndromes in the First Year of Life and Usefulness of Genetic Testing for Precision Therapy. Genes 2021, 12, 1051. [Google Scholar] [CrossRef]
- Millichap, J.J.; Park, K.L.; Tsuchida, T.; Ben-Zeev, B.; Carmant, L.; Flamini, R.; Joshi, N.; Levisohn, P.M.; Marsh, E.; Nangia, S.; et al. KCNQ2 encephalopathy: Features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol. Genet. 2016, 2, e96. [Google Scholar] [CrossRef]
- Helbig, I.; Ellis, C. A Personalized medicine in genetic epilepsies—Possibilities, challenges, and new frontiers. Neuropharmacology 2020, 172, 107970. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Toffa, D.H.; Lefèbvre, M.; Tétreault, M.; Cossette, P.; Samarut, É.; Nguyen, D.K. Usage of Genetic Panels in an Adult Epilepsy Clinic. Can. J. Neurol. Sci. 2023, 50, 411–417. [Google Scholar] [CrossRef] [PubMed]
- McKnight, D.; Bristow, S.L.; Truty, R.M.; Morales, A.; Stetler, M.; Westbrook, M.J.; Robinson, K.; Riethmaier, D.; Borlot, F.; Kellogg, M.; et al. Multigene Panel Testing in a Large Cohort of Adults with Epilepsy: Diagnostic Yield and Clinically Actionable Genetic Findings. Neurol. Genet. 2021, 8, e650. [Google Scholar] [CrossRef]
- Iglesias, A.; Anyane-Yeboa, K.; Wynn, J.; Wilson, A.; Truitt Cho, M.; Guzman, E.; Sisson, R.; Egan, C.; Chung, W.K. The usefulness of whole-exome sequencing in routine clinical practice. Genet. Med. 2014, 16, 922–931. [Google Scholar] [CrossRef]
- Valencia, C.A.; Husami, A.; Holle, J.; Johnson, J.A.; Qian, Y.; Mathur, A.; Wei, C.; Indugula, S.R.; Zou, F.; Meng, H.; et al. Clinical Impact and Cost-Effectiveness of Whole Exome Sequencing as a Diagnostic Tool: A Pediatric Center’s Experience. Front. Pediatr. 2015, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Wusik, K.; Neilson, D.; Zhang, X.; Valencia, C.A.; Collins, K. Comparison of medical management and genetic counseling options pre- and post-whole exome sequencing for patients with positive and negative results. J. Genet. Couns. 2019, 28, 182–193. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Adesina, A.M.; Jones, J.; Scaglia, F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015, 116, 4–12. [Google Scholar] [CrossRef]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Gosnell, E.S.; Gupta, N.; Jansen, A.C.; Jóźwiak, S.; et al. International Tuberous Sclerosis Complex Consensus Group Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Kuchenbuch, M.; D’Onofrio, G.; Chemaly, N.; Barcia, G.; Teng, T.; Nabbout, R. Add-on cannabidiol significantly decreases seizures in 3 patients with SYNGAP1 developmental and epileptic encephalopathy. Epilepsia Open 2020, 5, 496–500. [Google Scholar] [CrossRef]
- Li, X.; Gao, K.; Li, Y.; Zhang, Y.; Zhang, H.; Jiang, Y. Effective treatment of NR2F1-related epilepsy with perampanel. Acta Epileptol. 2024, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, K.M.; Tümer, Z.; Weckhuysen, S.; Barakat, T.S.; Bayat, A. Solving the unsolved genetic epilepsies—Current and future perspectives. Epilepsia 2023, 64, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Vetter, T.R. Logistic Regression in Medical Research. Anesth. Analg. 2021, 132, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Luiza Benevides, M.; de Moraes, H.T.; Granados, D.M.M.; Bonadia, L.C.; Sauma, L.; Augusta Montenegro, M.; Guerreiro, M.M.; Lopes-Cendes, Í.; Carolina Coan, A. Predictors of genetic diagnosis in individuals with developmental and epileptic encephalopathies. Epilepsy Behav. 2024, 155, 109762. [Google Scholar] [CrossRef]
- Wong, N.R.; Klomhaus, A.; Adams, D.J.; Schneider, B.N.; Mehta, S.; DiStefano, C.; Wilson, R.B.; Martinez-Agosto, J.A.; Jeste, S.S.; Besterman, A.D. Clinical factors associated with genetic diagnosis in suspected neurogenetic disorders in a tertiary care clinic. Genet. Med. 2024, 27, 101252. [Google Scholar] [CrossRef]
- Wirrell, E.; Tinuper, P.; Perucca, E.; Moshé, S.L. Introduction to the epilepsy syndrome papers. Epilepsia 2022, 63, 1330–1332. [Google Scholar] [CrossRef]
- Jobst, B.C. Consensus Over Individualism: Validation of the ILAE Definition for Drug Resistant Epilepsy. Epilepsy Curr. 2015, 15, 172–173. [Google Scholar] [CrossRef]
- Wylie, T.; Sandhu, D.S.; Murr, N.I. Status Epilepticus [Internet]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel 2018. Available online: https://office.microsoft.com/excel (accessed on 21 January 2025).
- R Core Team. _R: A Language and Environment for Statistical Computing_ [Internet]. Vienna, Austria: R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 21 January 2025).
- RStudio Team. RStudio: Integrated Development for R [Internet]. Boston, MA, USA. 2020. Available online: http://www.rstudio.com (accessed on 21 January 2025).
- Gross, J.; Ligges, U. _nortest: Tests for Normality_ [Internet]. 2015. Available online: https://CRAN.R-project.org/package=nortest (accessed on 21 January 2025).
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 3, 7–10. [Google Scholar]
- Lele, S.R.; Keim, J.L.; Solymos, P. _ResourceSelection: Resource Selection (Probability) Functions for Use-Availability Data_ [Internet]. 2023. Available online: https://CRAN.R-project.org/package=ResourceSelection (accessed on 21 January 2025).
- Lüdecke, D. sjPlot:Data Visualization for Statistics in Social Science[R package] Version 2.8.15. 2024. Available online: https://CRAN.R-project.org/package=sjPlot (accessed on 21 January 2025).
- Canty, A.; Ripley, B. boot: Bootstrap R (S-Plus) Functions [R package]. Version 1.3-28. 2024. Available online: https://CRAN.R-project.org/package=boot (accessed on 21 January 2025).

| Variables | Variable Categories | Total n = 140 (100%) | Diagnostic n = 40 | Non-Diagnostic n = 100 | p-Value |
|---|---|---|---|---|---|
| Test performed | Multigene Panel | 116 (82.9%) | 32 (80.0%) | 85 (85.0%) | 0.639 |
| WES | 30 (21.4%) | 6 (15.0%) | 24 (24.0%) | 0.345 | |
| WGS | 3 (2.1%) | 2 (5.0%) | 1 (1.0%) | 1.000 | |
| Gender | Female | 70 (50.0%) | 26 (65.0%) | 44 (44.0%) | 0.040 |
| Age at seizure onset | Median = 2.5 IQR = 0.8–4.0 | Median = 1.0 IQR = 0.3–2.5 | Median = 3.0 IQR = 1.3–6.0 | 0.009 | |
| <2 years | 60 (42.9%) | 27 (67.5%) | 33 (33.0%) | 0.000 | |
| Age at genetic testing | Median = 5.0 IQR = 3.0–9.0 | Median = 3.5 IQR = 1.8–6.0 | Median = 6.0 IQR = 3.0–10.5 | 0.002 | |
| <2 years | 21 (15.0%) | 12 (30.0%) | 9 (9.0%) | 0.004 | |
| Time from seizure onset to genetic testing | Median = 2.1 IQR = 0.0–5.9 | Median= −1.2 IQR = (−3.9)–(−0.2) | Median= −1.7 IQR = (−4.8)–(−0.1) | 0.837 | |
| Number of genes included in the panel | Median = 306.0 IQR = 192.0–320.0 | Median = 306.0 IQR = 188.5–320 | Median = 306.0 IQR = 274.5–320.0 | 0.405 | |
| Seizure type * | Focal | 50/129 (38.8%) | 12/35 (34.3%) | 38/94 (40.4%) | 0.665 |
| Generalized | 38/129 (29.5%) | 9/35 (25.7%) | 29/94 (30.9%) | 0.725 | |
| Mixed | 41/129 (31.8%) | 14/35 (40.0%) | 27/94 (28.7) | 0.312 | |
| Status epilepticus | Present | 10 (7.1%) | 2 (5.0%) | 8 (8.0%) | 1.000 |
| Frequency of seizures ** | less than one seizure per year | 46/112 (41.1%) | 9/30 (30.0%) | 37/82 (45.1%) | 0.221 |
| 1 seizure per year | 23/112 (20.5%) | 3/30 (10.0%) | 20/82 (24.4%) | 1.000 | |
| 2–3 seizures per year | 12/112 (10.7%) | 3/30 (10.0%) | 9/82 (11.0%) | 1.000 | |
| monthly seizures | 7/112 (6.3%) | 3/30 (10.0%) | 4/82 (4.9%) | 1.000 | |
| weekly seizures | 9/112 (8.0%) | 5/30 (16.7%) | 4/82 (4.9%) | 1.000 | |
| daily seizures | 15/112 (13.4%) | 7/30 (23.3%) | 8/82 (9.8%) | 0.120 | |
| EE/DEE | Present | 24 (17.1%) | 15 (37.5%) | 9 (9.0%) | 0.000 |
| Intellectual disability | Present | 74 (52.9%) | 33 (82.5%) | 41 (41.0%) | 0.014 |
| Developmental delay | Present | 74 (52.9%) | 32 (80.0%) | 42 (42.0%) | 0.000 |
| Speech delay | Present | 80 (57.1%) | 32 (80.0%) | 48 (48.0%) | 0.001 |
| Autism spectrum disorder | Present | 28 (20.0%) | 13 (32.5%) | 15 (15.0%) | 0.035 |
| Developmental regression | Present | 11 (7.9%) | 4 (10.0%) | 7 (7.0%) | 1.000 |
| Drug-resistant epilepsy | Present | 14 (10.0%) | 6 (15.0%) | 8 (8.0%) | 0.340 |
| Facial dysmorphisms | Present | 17 (12.1%) | 10 (25.0%) | 7 (7.0%) | 0.008 |
| Congenital malformations | Yes | 9 (6.4%) | 4 (10.0%) | 5 (5.0%) | 1.000 |
| Ataxia | Yes | 7 (5.0%) | 4 (10.0%) | 3 (3.0%) | 1.000 |
| Ophthalmologic features | Yes | 15 (10.7%) | 7 (17.5%) | 8 (8.0%) | 0.180 |
| Muscle tone | Abnormal | 20 (14.3%) | 8 (20.0%) | 12 (12.0%) | 0.340 |
| Movement disorder | Present | 12 (8.6%) | 4 (10.0%) | 8 (8.0%) | 1.000 |
| Motor deficit | Present | 10 (7.1%) | 1 (2.5%) | 9 (9.0%) | 1.000 |
| Cerebral palsy | Present | 2 (1.4%) | 0 (0%) | 2 (2.0%) | 1.000 |
| Behavioral disorder | Present | 11 (7.9%) | 5 (12.5%) | 6 (6.0%) | 0.345 |
| Number of antiepileptic drugs at last visit *** | 0 | 12/119 (10.1%) | 1/31 (3.2%) | 11/88 (12.5%) | 1.000 |
| 1 | 55/119 (46.2%) | 9/31 (29.0%) | 46/88(52.3%) | 0.043 | |
| 2 | 34/119 (28.6%) | 13/31 (41.9%) | 21/88 (23.9%) | 0.092 | |
| 3 | 8/119 (6.7%) | 4/31 (12.9%) | 4/88 (4.5%) | 0.214 | |
| 4 | 10/119 (8.4%) | 4/31 (12.9%) | 6/88 (6.8%) | 1.000 | |
| EEG findings **** | Pathological | 107/117 (91.5%) | 30/30 (100.0%) | 77/87(88.5%) | 0.118 |
| Evolution ***** | Favorable | 53/94(56.4%) | 10/32 (31.3%) | 43/62 (69.4%) | 0.000 |
| Stationary | 37/94 (39.4%) | 20/32 (62.5%) | 17/62 (27.4%) | 0.002 | |
| Unfavorable | 4/94 (4.3%) | 2/32 (6.3%) | 2/62 (3.2%) | 1.000 | |
| Type of Genetic Testing Impact | Values from the 40 Children with a Genetic Diagnosis (%) | Causative Gene/Syndrome |
|---|---|---|
| Drug impact | n = 34 (85.0%) | CACNA1A, EIF2B5, KCNC1, KCNH5, KCNQ2, MECP2, MT-CYB, NEXMIF(KIAA2022), NR2F1, NRXN1, PPP2R5D, PRRT2, PURA, SCN1, SCN2A, SCN8A, SLC2A1, STXBP1, SYNGAP1, TSC1, TSC2, UBE3A, WWOX |
| Ketogenic diet | n = 7 (17.5%) | NR2F1, PPP2R5D, SCN2A, SLC2A1, STXBP1, SYNGAP |
| Clinical trials | n = 1 (2.5%) | EIF2B5 |
| Multidisciplinary dispensary | n = 18 (45.0%) | Phelan–McDermid syndrome, 16p12.2p11.2 deletion syndrome, EIF2B5, MECP2, MT-CYB, NEXMIF(KIAA2022), NFIA, NR2F1, NRXN1, P4HTM, PPP2R5D, PURA, RAI1, TSC1, TSC2, UBE3A |
| Variable | OR/Adjusted OR | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Univariate Logistic Regression Models | |||
| Age Onset seizures | 0.673 | 0.528–0.818 | 0.004 |
| EE/DEE | 6.066 | 2.419–16.047 | 0.036 |
| ID | 6.783 | 2.875–18.065 | 0.024 |
| DD | 5.523 | 2.408–13.988 | 0.018 |
| ASD | 2.728 | 1.147–6.478 | 0.022 |
| Status epilepticus | 0.605 | 0.088–2.553 | 0.537 |
| Facial dysmorphism | 4.428 | 1.567–13.187 | 0.005 |
| Number of genes in the panel | 1.001 | 0.998–1.002 | 0.856 |
| Multivariate Logistic Regression Model | |||
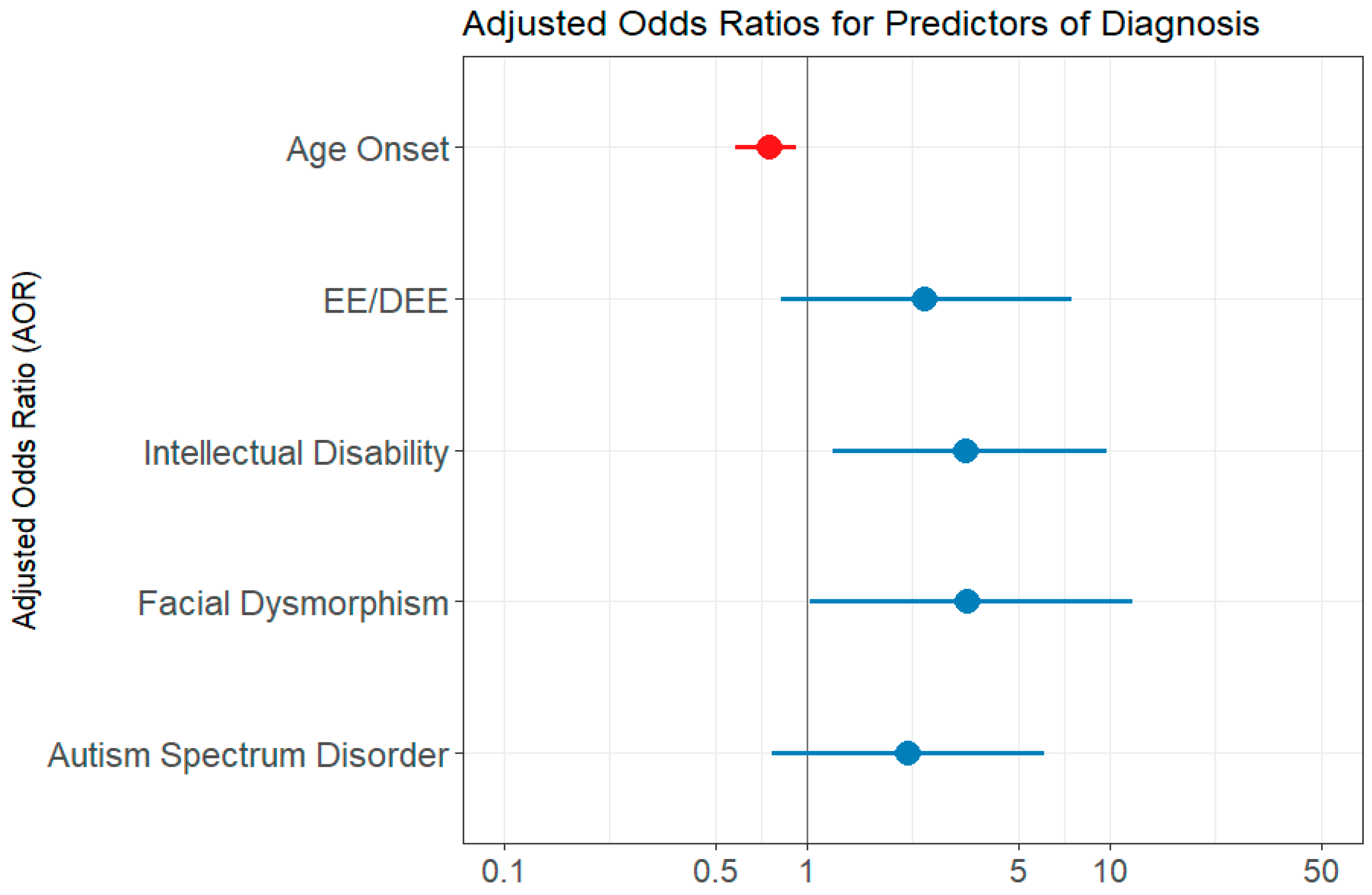
| Age onset | 0.752 | 0.580–0.920 | 0.015 |
| EE/DEE | 2.438 | 0.824–7.502 | 0.111 |
| Intellectual disability | 3.331 | 1.223–9.759 | 0.021 |
| Facial Dysmorphism | 3.377 | 1.026–11.959 | 0.049 |
| ASD | 2.156 | 0.772–6.078 | 0.141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabau, I.M.; Bacos-Cosma, I.S.; Streata, I.; Dragulescu, B.; Puiu, M.; Chirita-Emandi, A. Impact of Genetic Testing Using Gene Panels, Exomes, and Genome Sequencing in Romanian Children with Epilepsy. Int. J. Mol. Sci. 2025, 26, 4843. https://doi.org/10.3390/ijms26104843
Sabau IM, Bacos-Cosma IS, Streata I, Dragulescu B, Puiu M, Chirita-Emandi A. Impact of Genetic Testing Using Gene Panels, Exomes, and Genome Sequencing in Romanian Children with Epilepsy. International Journal of Molecular Sciences. 2025; 26(10):4843. https://doi.org/10.3390/ijms26104843
Chicago/Turabian StyleSabau, Iulia Maria, Iuliu Stefan Bacos-Cosma, Ioana Streata, Bogdan Dragulescu, Maria Puiu, and Adela Chirita-Emandi. 2025. "Impact of Genetic Testing Using Gene Panels, Exomes, and Genome Sequencing in Romanian Children with Epilepsy" International Journal of Molecular Sciences 26, no. 10: 4843. https://doi.org/10.3390/ijms26104843
APA StyleSabau, I. M., Bacos-Cosma, I. S., Streata, I., Dragulescu, B., Puiu, M., & Chirita-Emandi, A. (2025). Impact of Genetic Testing Using Gene Panels, Exomes, and Genome Sequencing in Romanian Children with Epilepsy. International Journal of Molecular Sciences, 26(10), 4843. https://doi.org/10.3390/ijms26104843






