Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
2.2. Key References Table
3. Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction
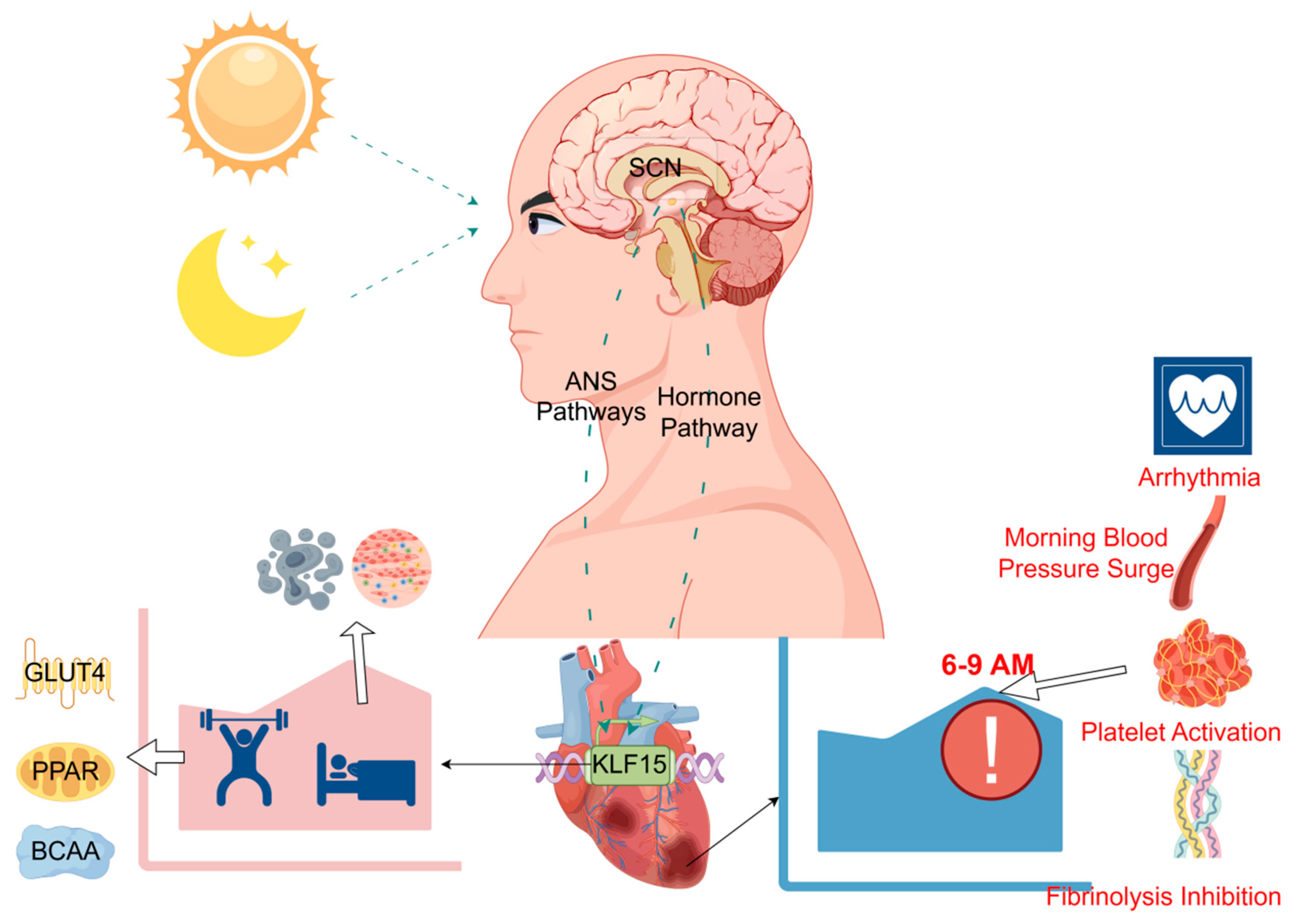
3.1. Myocardial Infarction Is Characterized by a Significant Circadian Rhythm
3.2. Effect of Circadian Rhythm Disturbances on Myocardial Infarction
3.3. KLF15 Gene: A Central Regulator of Cardiac Circadian Rhythm
3.4. The Role of KLF15 in the Pathologic Process of Myocardial Infarction
3.4.1. Modulation of the Inflammatory Response
3.4.2. Inhibition of Apoptosis
3.4.3. Regulation of Energy Metabolism
Glucose Metabolism and Cardiac Energy Supply
Homeostatic Maintenance of Lipid Metabolism
Circadian Rhythmicity of Branched-Chain Amino Acid Metabolism
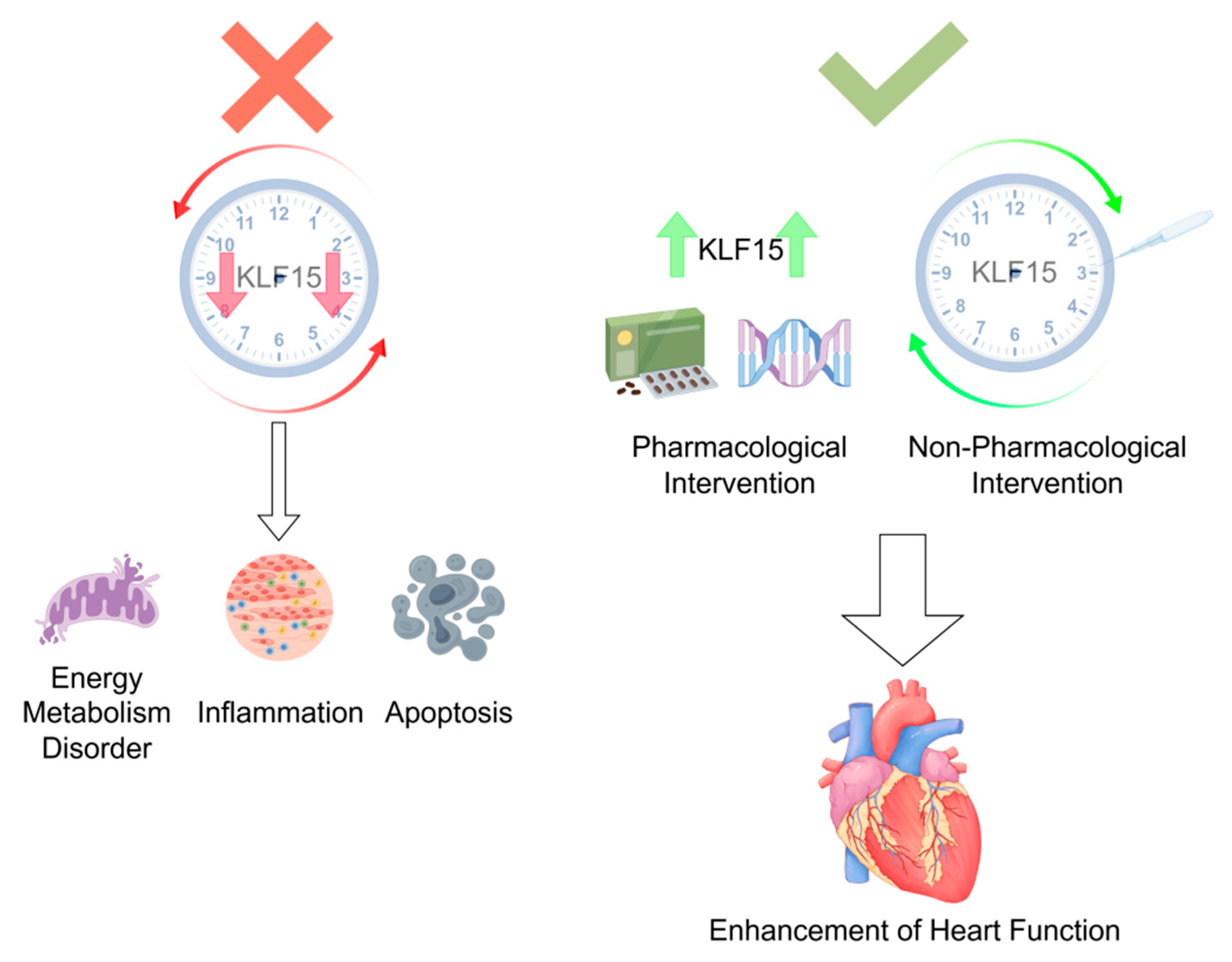
4. Discussion
5. Limitations and Future Research Directions
- (1)
- Restoring KLF15 Oscillation: Reprogramming downstream gene networks by reinstating KLF15’s circadian rhythm to facilitate myocardial repair.
- (2)
- Harnessing KLF15’s Multifunctional Effects: Leveraging its anti-inflammatory, antioxidant, and metabolic regulatory properties to mitigate inflammation, reduce cardiomyocyte death, limit infarct expansion, and prevent further cardiac dysfunction.
- (3)
- Timed Intervention Strategies: Prolonging KLF15 expression during its peak or trough phases to induce compensatory upregulation and prevent infarction.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; He, X.; Wang, Z.; Hu, S. Executive Summary of the China Cardiovascular Health and Disease Report 2023 (Epidemiology and Interventional Diagnosis/Treatment Status of Cardiovascular Diseases). Chin. J. Intervent. Cardiol. 2024, 32, 541–550. [Google Scholar]
- Xu, F.; Wang, G.; Ye, N.; Bian, W.; Yang, L.; Ma, C.; Zhao, D.; Liu, J.; Hao, Y.; Liu, J.; et al. Mild renal insufficiency and attributable risk of adverse In-hospital outcomes in patients with Acute Coronary Syndrome from the improving care for Cardiovascular Disease in China (CCC) project. BMC Nephrol. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D.; Jaffe, A.S.; Apple, F.S.; Galvani, M.; Katus, H.A.; Newby, L.K.; Ravkilde, J.; Chaitman, B.; et al. Universal definition of myocardial infarction. Circulation 2007, 116, 2634–2653. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, E.; Kirshenbaum, L.A. Autophagy, Clock Genes, and Cardiovascular Disease. Can. J. Cardiol. 2023, 39, 1772–1780. [Google Scholar] [CrossRef]
- Delisle, B.P.; Prabhat, A.; Burgess, D.E.; Stumpf, I.G.; McCarthy, J.J.; Procopio, S.B.; Zhang, X.; Esser, K.A.; Schroder, E.A. Circadian influences on sudden cardiac death and cardiac electrophysiology. J. Mol. Cell. Cardiol. 2025, 200, 93–112. [Google Scholar] [CrossRef]
- Hou, T.; Guo, Z.; Gong, M.C. Circadian variations of vasoconstriction and blood pressure in physiology and diabetes. Curr. Opin. Pharmacol. 2021, 57, 125–131. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Shi, G.; Xing, L.; Wang, X.; Gu, X.; Qu, Z.; Dong, Z.; Xiong, J.; Gao, X.; et al. The circadian clock influences heart performance. J. Biol. Rhythm. 2011, 26, 402–411. [Google Scholar] [CrossRef]
- Hurley, J.M.; Loros, J.J.; Dunlap, J.C. Circadian Oscillators: Around the Transcription-Translation Feedback Loop and on to Output. Trends Biochem. Sci. 2016, 41, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Kim, J.Y. Mammalian circadian networks mediated by the suprachiasmatic nucleus. FEBS J. 2022, 289, 6589–6604. [Google Scholar] [CrossRef]
- Le Minh, N.; Damiola, F.; Tronche, F.; Schütz, G.; Schibler, U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001, 20, 7128–7136. [Google Scholar] [CrossRef]
- Cailotto, C.; Lei, J.; van der Vliet, J.; van Heijningen, C.; van Eden, C.G.; Kalsbeek, A.; Pévet, P.; Buijs, R.M. Effects of nocturnal light on (clock) gene expression in peripheral organs: A role for the autonomic innervation of the liver. PLoS ONE 2009, 4, e5650. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Wang, Y.; Anderson, C.; Bucchi, A.; Baruscotti, M.; Olieslagers, S.; Mesirca, P.; Johnsen, A.B.; Mastitskaya, S.; Ni, H.; et al. A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate. Heart Rhythm. 2021, 18, 801–810. [Google Scholar] [CrossRef]
- Zhu, C.; Li, S.; Zhang, H. Heart Failure and Arrhythmias: Circadian and Epigenetic Interplay in Myocardial Electrophysiology. Int. J. Mol. Sci. 2025, 26, 96–99. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–99. [Google Scholar] [CrossRef]
- Zhang, L.; Prosdocimo, D.A.; Bai, X.; Fu, C.; Zhang, R.; Campbell, F.; Liao, X.; Coller, J.; Jain, M.K. KLF15 Establishes the Landscape of Diurnal Expression in the Heart. Cell Rep. 2015, 13, 2368–2375. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.A.; Hosseiny, S.; Aaliya, A.; Adel, M.; Yasseen, B.; Al-Okda, A.; Radwan, Y.; Saber, S.H.; Elkholy, N.; Elhanafy, E.; et al. Sleep/wake calcium dynamics, respiratory function, and ROS production in cardiac mitochondria. J. Adv. Res. 2021, 31, 35–47. [Google Scholar] [CrossRef]
- Molnar, J.; Zhang, F.; Weiss, J.; Ehlert, F.A.; Rosenthal, J.E. Diurnal pattern of QTc interval: How long is prolonged? Possible relation to circadian triggers of cardiovascular events. J. Am. Coll. Cardiol. 1996, 27, 76–83. [Google Scholar] [CrossRef]
- Culić, V. Acute risk factors for myocardial infarction. Int. J. Cardiol. 2007, 117, 260–269. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. Circadian clock and the onset of cardiovascular events. Hypertens. Res. 2016, 39, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Yamaoki, K.; Nagai, R.; Yoshizumi, M.; Takaku, F.; Satoh, H.; Inui, J.; Yazaki, Y. Endothelin: A potent vasoconstrictor associated with coronary vasospasm. Life Sci. 1989, 44, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Shea, S.A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014, 123, 590–593. [Google Scholar] [CrossRef]
- Scheer, F.A.; Michelson, A.D.; Frelinger, A.L., 3rd; Evoniuk, H.; Kelly, E.E.; McCarthy, M.; Doamekpor, L.A.; Barnard, M.R.; Shea, S.A. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE 2011, 6, e24549. [Google Scholar] [CrossRef]
- Knutsson, A. Increased risk of ischaemic heart disease in shift workers. Occup. Med. 1995, 45, 55. [Google Scholar] [CrossRef]
- Kirshenbaum, L.A.; Hill, M.; Singal, P.K. Endogenous antioxidants in isolated hypertrophied cardiac myocytes and hypoxia-reoxygenation injury. J. Mol. Cell. Cardiol. 1995, 27, 263–272. [Google Scholar] [CrossRef]
- Mann, D.L. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circ. Res. 2011, 108, 1133–1145. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Rasouli, M.; Reitz, C.J.; Posen, I.; Margulets, V.; Dhingra, R.; Khatua, T.N.; Thliveris, J.A.; Martino, T.A.; Kirshenbaum, L.A. Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress. Autophagy 2021, 17, 3794–3812. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, F.J.; Tsimakouridze, E.V.; Chinnappareddy, N.; Wright, D.C.; Billia, F.; O’Sullivan, M.L.; Pyle, W.G.; Sole, M.J.; Martino, T.A. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ. Res. 2014, 114, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Das, P.; Hashimoto, I.; Nakao, T.; Deguchi, Y.; Gouraud, S.S.; Waki, H.; Muragaki, Y.; Maeda, M. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS ONE 2014, 9, e112811. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tien, C.L.; Jain, M.K.; Zhang, L. Kruppel-Like Factor 15 Regulates the Circadian Susceptibility to Ischemia Reperfusion Injury in the Heart. Circulation 2020, 141, 1427–1429. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Scheer, F.A.; Ripperger, J.A.; Haldar, S.M.; Lu, Y.; Prosdocimo, D.A.; Eapen, S.J.; Eapen, B.L.; Cui, Y.; Mahabeleshwar, G.H.; et al. KLF15 orchestrates circadian nitrogen homeostasis. Cell Metab. 2012, 15, 311–323. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lu, Y.; Guo, Y.; Li, S.; Lu, X.; Shao, S.; Zhou, H.; Wang, R.; Wang, J.; Gao, P.; et al. Krüppel-Like Factor 15 Modulates CXCL1/CXCR2 Signaling-Mediated Inflammatory Response Contributing to Angiotensin II-Induced Cardiac Remodeling. Front. Cell Dev. Biol. 2021, 9, 644954. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, H.; Kong, J.; Liu, D.; Qin, W.; Bai, W. Krüppel-Like Factor 15 Reduces Ischemia-Induced Apoptosis Involving Regulation of p38/MAPK Signaling. Hum. Gene Ther. 2021, 32, 1471–1480. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Q.; Chen, A.; Liu, N.; Chen, N.; Chen, X.; Zhu, L.; Xia, B.; Gong, Y.; Chen, X. KLF15-activating Twist2 ameliorated hepatic steatosis by inhibiting inflammation and improving mitochondrial dysfunction via NF-κB-FGF21 or SREBP1c-FGF21 pathway. FASEB J. 2019, 33, 14254–14269. [Google Scholar] [CrossRef]
- Liao, B.; Tian, X. CTRP12 alleviates cardiomyocyte ischemia-reperfusion injury via regulation of KLF15. Mol. Med. Rep. 2022, 26, 247. [Google Scholar] [CrossRef]
- Zheng, H.; Ye, W.; Huang, K.; Chen, Q.; Yang, J.; Luo, L. KLF15 alleviates oxidative stress and apoptosis of H/R-induced trophoblast cells to improve invasion and migration capacity via the activation of IGF1R. Tissue Cell 2024, 90, 102485. [Google Scholar] [CrossRef]
- Prosdocimo, D.A.; Anand, P.; Liao, X.; Zhu, H.; Shelkay, S.; Artero-Calderon, P.; Zhang, L.; Kirsh, J.; Moore, D.; Rosca, M.G.; et al. Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J. Biol. Chem. 2014, 289, 5914–5924. [Google Scholar] [CrossRef] [PubMed]
- Nagare, T.; Sakaue, H.; Matsumoto, M.; Cao, Y.; Inagaki, K.; Sakai, M.; Takashima, Y.; Nakamura, K.; Mori, T.; Okada, Y.; et al. Overexpression of KLF15 transcription factor in adipocytes of mice results in down-regulation of SCD1 protein expression in adipocytes and consequent enhancement of glucose-induced insulin secretion. J. Biol. Chem. 2011, 286, 37458–37469. [Google Scholar] [CrossRef]
- Durumutla, H.B.; Prabakaran, A.D.; El Abdellaoui Soussi, F.; Akinborewa, O.; Latimer, H.; McFarland, K.; Piczer, K.; Werbrich, C.; Jain, M.K.; Haldar, S.M.; et al. Glucocorticoid chronopharmacology promotes glucose metabolism in heart through a cardiomyocyte-autonomous transactivation program. JCI Insight 2024, 9, e182599. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zou, S.; Jian, C.; Tang, F.; Xiao, Y.; Chen, L. Regulatory Effects of Krüppel-like Factor 15 on Lipid Deposition and Cardiac Function in Rats Following Pressure Overload/Unloading. J. Third Mil. Med. Univ. 2015, 37, 1813–1817. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Gao, R.; Xu, Y.; Huangfu, B.; Asakiya, C.; Huang, X.; Zhang, F.; Huang, K.; He, X.; et al. Diallyl Trisulfide Prevents Adipogenesis and Lipogenesis by Regulating the Transcriptional Activation Function of KLF15 on PPARγ to Ameliorate Obesity. Mol. Nutr. Food Res. 2022, 66, e2200173. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Cai, M.; Bao, L.; Pan, Y.; Wu, P.; Chu, W.; Zhang, J. The circadian rhythm regulates branched-chain amino acids metabolism in fast muscle of Chinese perch (Siniperca chuatsi) during short-term fasting by Clock-KLF15-Bcat2 pathway. Br. J. Nutr. 2023, 130, 604–615. [Google Scholar] [CrossRef]
- Millar-Craig, M.W.; Bishop, C.N.; Raftery, E.B. Circadian variation in blood-pressure. Lancet 1978, 1, 1210–1211. [Google Scholar]
- Thosar, S.S.; Shea, S.A. Circadian control of human cardiovascular function. Curr. Opin. Pharmacol. 2021, 57, 89–97. [Google Scholar] [CrossRef]
- Kirshenbaum, L.A.; Singal, P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. 1993, 265, H484–H493. [Google Scholar] [CrossRef]
- Teixeira, K.R.C.; Dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci. Rep. 2019, 9, 4455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, X.; Wan, F.; Gao, L.; Lin, N.; He, J.; Wei, L.; Dong, J.; Qin, Z.; Zhong, F.; et al. Disruption of Circadian Rhythms by Shift Work Exacerbates Reperfusion Injury in Myocardial Infarction. J. Am. Coll. Cardiol. 2022, 79, 2097–2115. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; De Gioia, S.; Mezzetti, A.; Porreca, E.; Ciofani, G.; Marzio, L.; Capani, F.; Di Ilio, C.; Cuccurullo, F. Circadian variations in antioxidant defences and lipid peroxidation in the rat heart. Free Radic. Res. Commun. 1992, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Tsimakouridze, E.V.; Reitz, C.J.; Pyle, W.G.; Martino, T.A. Consequences of Circadian and Sleep Disturbances for the Cardiovascular System. Can. J. Cardiol. 2015, 31, 860–872. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cell Signal 2010, 22, 1282–1290. [Google Scholar] [CrossRef]
- Zhao, T.; Qiu, Z.; Gao, Y. MiR-137-3p exacerbates the ischemia-reperfusion injured cardiomyocyte apoptosis by targeting KLF15. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1013–1024. [Google Scholar] [CrossRef]
- Tang, Q.; Li, M.Y.; Su, Y.F.; Fu, J.; Zou, Z.Y.; Wang, Y.; Li, S.N. Absence of miR-223-3p ameliorates hypoxia-induced injury through repressing cardiomyocyte apoptosis and oxidative stress by targeting KLF15. Eur. J. Pharmacol. 2018, 841, 67–74. [Google Scholar] [CrossRef]
- Lu, X.; Yang, B.; Qi, R.; Xie, Q.; Li, T.; Yang, J.; Tong, T.; Niu, K.; Li, M.; Pan, W.; et al. Targeting WWP1 ameliorates cardiac ischemic injury by suppressing KLF15-ubiquitination mediated myocardial inflammation. Theranostics 2023, 13, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Xi, S.; Pan, X.; Fang, X. Exploring the Mechanism of KLF15 on the Biological Activity and Autophagy of Gastric Cancer Cells based on PI3K/Akt/Mtor Signaling Pathway. Comb. Chem. High Throughput Screen. 2024, 27, 1515–1523. [Google Scholar] [CrossRef]
- Shao, Y.; Li, X.; Zhou, W.; Qian, S.; Wang, L.; Fang, X. KLF15 attenuates lipopolysaccharide-induced apoptosis and inflammatory response in renal tubular epithelial cells via pparδ. Shock 2024, 62, 574–581. [Google Scholar] [CrossRef]
- Prosdocimo, D.A.; Sabeh, M.K.; Jain, M.K. Kruppel-like factors in muscle health and disease. Trends Cardiovasc. Med. 2015, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Wang, B.; Orihuela, Y.; Hong, E.G.; Fisch, S.; Haldar, S.; Cline, G.W.; Kim, J.K.; Peroni, O.D.; Kahn, B.B.; et al. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.M.; Jeyaraj, D.; Anand, P.; Zhu, H.; Lu, Y.; Prosdocimo, D.A.; Eapen, B.; Kawanami, D.; Okutsu, M.; Brotto, L.; et al. Kruppel-like factor 15 regulates skeletal muscle lipid flux and exercise adaptation. Proc. Natl. Acad. Sci. USA 2012, 109, 6739–6744. [Google Scholar] [CrossRef]
- Li, H.; An, X.; Bao, L.; Li, Y.; Pan, Y.; He, J.; Liu, L.; Zhu, X.; Zhang, J.; Cheng, J.; et al. MiR-125a-3p-KLF15-BCAA Regulates the Skeletal Muscle Branched-Chain Amino Acid Metabolism in Nile Tilapia (Oreochromis niloticus) During Starvation. Front. Genet. 2020, 11, 852. [Google Scholar] [CrossRef]
- Han, S.; Ray, J.W.; Pathak, P.; Sweet, D.R.; Zhang, R.; Gao, H.; Jain, N.; Koritzinsky, E.H.; Matoba, K.; Xu, W.; et al. KLF15 regulates endobiotic and xenobiotic metabolism. Nat. Metab. 2019, 1, 422–430. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Wang, L.; Rane, M.J.; Han, F.; Cai, L. Multiple roles of KLF15 in the heart: Underlying mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2019, 129, 193–196. [Google Scholar] [CrossRef]
- Fan, L.; Hsieh, P.N.; Sweet, D.R.; Jain, M.K. Krüppel-like factor 15: Regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol. Res. 2018, 130, 123–126. [Google Scholar] [CrossRef]
- Fan, L.; Sweet, D.R.; Prosdocimo, D.A.; Vinayachandran, V.; Chan, E.R.; Zhang, R.; Ilkayeva, O.; Lu, Y.; Keerthy, K.S.; Booth, C.E.; et al. Muscle Krüppel-like factor 15 regulates lipid flux and systemic metabolic homeostasis. J. Clin. Investig. 2021, 131, e139496. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sweet, D.R.; Fan, E.K.; Prosdocimo, D.A.; Madera, A.; Jiang, Z.; Padmanabhan, R.; Haldar, S.M.; Vinayachandran, V.; Jain, M.K. Transcription factors KLF15 and PPARδ cooperatively orchestrate genome-wide regulation of lipid metabolism in skeletal muscle. J. Biol. Chem. 2022, 298, 101926. [Google Scholar] [CrossRef] [PubMed]
- Gire, D.; Acharya, J.; Malik, S.; Inamdar, S.; Ghaskadbi, S. Molecular mechanism of anti-adipogenic effect of vitexin in differentiating hMSCs. Phytother. Res. 2021, 35, 6462–6471. [Google Scholar] [CrossRef]
- Suzuki, J.; Shen, W.J.; Nelson, B.D.; Selwood, S.P.; Murphy, G.M., Jr.; Kanehara, H.; Takahashi, S.; Oida, K.; Miyamori, I.; Kraemer, F.B. Cardiac gene expression profile and lipid accumulation in response to starvation. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E94–E102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugi, K.; Hsieh, P.N.; Ilkayeva, O.; Shelkay, S.; Moroney, B.; Baadh, P.; Haynes, B.; Pophal, M.; Fan, L.; Newgard, C.B.; et al. Kruppel-like factor 15 is required for the cardiac adaptive response to fasting. PLoS ONE 2018, 13, e0192376. [Google Scholar] [CrossRef]
- Walter, L.M.; Deguise, M.O.; Meijboom, K.E.; Betts, C.A.; Ahlskog, N.; van Westering, T.L.E.; Hazell, G.; McFall, E.; Kordala, A.; Hammond, S.M.; et al. Interventions Targeting Glucocorticoid-Krüppel-like Factor 15-Branched-Chain Amino Acid Signaling Improve Disease Phenotypes in Spinal Muscular Atrophy Mice. EBioMedicine 2018, 31, 226–242. [Google Scholar] [CrossRef]
- Hsieh, P.N.; Zhang, L.; Jain, M.K. Coordination of cardiac rhythmic output and circadian metabolic regulation in the heart. Cell. Mol. Life Sci. 2018, 75, 403–416. [Google Scholar] [CrossRef]
- Feigin, R.D.; Klainer, A.S.; Beisel, W.R. Circadian periodicity of blood amino-acids in adult men. Nature 1967, 215, 512–514. [Google Scholar] [CrossRef]
- Reddy, A.B.; Karp, N.A.; Maywood, E.S.; Sage, E.A.; Deery, M.; O’Neill, J.S.; Wong, G.K.; Chesham, J.; Odell, M.; Lilley, K.S.; et al. Circadian orchestration of the hepatic proteome. Curr. Biol. 2006, 16, 1107–1115. [Google Scholar] [CrossRef]
- Chin, W.C.; Chu, P.H.; Wu, L.S.; Lee, K.T.; Lin, C.; Ho, C.T.; Yang, W.S.; Chung, I.H.; Huang, Y.S. The Prognostic Significance of Sleep and Circadian Rhythm for Myocardial Infarction Outcomes: Case-Control Study. J. Med. Internet Res. 2025, 27, e63897. [Google Scholar] [CrossRef]
- Yao, L.; Liu, Y.; Li, M.; Zheng, H.; Sun, M.; He, M.; Zhong, Z.; Ma, S.; Huang, H.; Wang, H. The central regulatory effects of acupuncture in treating primary insomnia: A review. Front. Neurol. 2024, 15, 1406485. [Google Scholar] [CrossRef] [PubMed]
| Reference | Year | Author | Key Findings |
|---|---|---|---|
| [1] | 2024 | Liu, M.; He, X.; Wang, Z.; Hu, S. | Provides data on the epidemiology of cardiovascular diseases and the current status of interventional diagnosis and treatment in China. |
| [3] | 2007 | Thygesen, K.; Alpert, J. S.; White, H. D.; Jaffe, A. S.; Apple, F. S.; Galvani, M.; Katus, H. A.; Newby, L. K.; Ravkilde, J.; Chaitman, B.; et al. | International diagnostic criteria for myocardial infarction. |
| [5] | 2022 | Sagris, M.; Antonopoulos, A. S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E. S.; Kaski, J. C.; Tousoulis, D. | Adverse lifestyle behaviors, including chronic sleep deprivation, markedly elevate the incidence of acute myocardial infarction among young adults. |
| [6] | 2023 | Rabinovich-Nikitin, I.; Kirshenbaum, E.; Kirshenbaum, L. A. | Reveals the critical role of the biological clock-autophagy axis in cardiovascular diseases, providing a theoretical foundation for chronotherapy. |
| [7] | 2025 | Delisle, B. P.; Prabhat, A.; Burgess, D. E.; Stumpf, I. G.; McCarthy, J. J.; Procopio, S. B.; Zhang, X.; Esser, K. A.; Schroder, E. A. | elucidates the molecular mechanism by which the biological clock regulates ion channels via KLF15, thereby explaining the morning peak incidence of myocardial infarction and cardiac arrhythmias. |
| [10] | 2011 | Wu, X.; Liu, Z.; Shi, G.; Xing, L.; Wang, X.; Gu, X.; Qu, Z.; Dong, Z.; Xiong, J.; Gao, X.; et al. | This study provides direct evidence in animal models that biological clock genes dynamically regulate cardiac function in real time. |
| [19] | 2015 | Zhang, L.; Prosdocimo, D. A.; Bai, X.; Fu, C.; Zhang, R.; Campbell, F.; Liao, X.; Coller, J.; Jain, M. K. | KLF15 orchestrates cardiac metabolism and repair through a biphasic regulatory mode, thereby providing mechanistic evidence for the circadian nature of myocardial infarction. |
| [21] | 1996 | Molnar, J.; Zhang, F.; Weiss, J.; Ehlert, F. A.; Rosenthal, J. E. | This study establishes a clinically relevant link between QTc rhythm variations and temporal patterns of cardiovascular events, providing evidence for the dual regulation of cardiac electrophysiology by both the autonomic nervous system and biological clock. |
| [22] | 2007 | Culić, V. | Myocardial infarction exhibits circadian rhythmicity, correlating with neural activation and blood pressure fluctuations. |
| [23] | 2016 | Takeda, N.; Maemura, K. | The Bmal1/CLOCK genes regulate circadian blood pressure rhythms, and their deficiency causes rhythm disruption, thereby increasing myocardial infarction risk. |
| [24] | 1989 | Kurihara, H.; Yamaoki, K.; Nagai, R.; Yoshizumi, M.; Takaku, F.; Satoh, H.; Inui, J.; Yazaki, Y. | Endothelin-1 levels exhibit a morning surge, which may contribute to the higher morning incidence of myocardial infarction. |
| [25] | 2006 | Förstermann, U.; Münzel, T. | Oxidative stress reduces endothelial nitric oxide synthase (eNOS) activity, leading to increased vascular tone in the morning and consequently exacerbating cardiovascular risk. |
| [26] | 2014 | Scheer, F. A.; Shea, S. A. | The morning peak of plasminogen activator inhibitor-1 (PAI-1) increases thrombotic risk and may underlie the morning predominance of myocardial infarction. |
| [27] | 2011 | Scheer, F. A.; Michelson, A. D.; Frelinger, A. L., 3rd; Evoniuk, H.; Kelly, E. E.; McCarthy, M.; Doamekpor, L. A.; Barnard, M. R.; Shea, S. A. | The surface activity of platelet GPIIb-IIIa complex peaks during circadian morning hours, enhancing thrombus formation and potentially explaining the morning peak incidence of myocardial infarction. |
| [28] | 1995 | Knutsson, A. | Circadian rhythm disruption elevates myocardial infarction risk. |
| [29] | 1995 | Kirshenbaum, L. A.; Hill, M.; Singal, P. K. | Circadian disruption exacerbates oxidative stress, rendering cardiomyocytes more vulnerable to injury. |
| [30] | 2011 | Mann, D. L. | Circadian regulation of immune responses affects post-MI repair. |
| [31] | 2021 | Rabinovich-Nikitin, I.; Rasouli, M.; Reitz, C. J.; Posen, I.; Margulets, V.; Dhingra, R.; Khatua, T. N.; Thliveris, J. A.; Martino, T. A.; Kirshenbaum, L. A. | The Bmal1 gene enhances mitophagy to protect ischemic cardiomyocytes and improve survival rates. |
| [32] | 2014 | Alibhai, F. J.; Tsimakouridze, E. V.; Chinnappareddy, N.; Wright, D. C.; Billia, F.; O’Sullivan, M. L.; Pyle, W. G.; Sole, M. J.; Martino, T. A. | Light/dark cycle disruption exacerbates post-MI cardiac remodeling and impairs functional recovery in mice. |
| [33] | 2014 | Kohsaka, A.; Das, P.; Hashimoto, I.; Nakao, T.; Deguchi, Y.; Gouraud, S. S.; Waki, H.; Muragaki, Y.; Maeda, M. | Bmal1 deficiency dysregulates cardiac oxidative stress-related gene expression and exacerbates myocardial infarction injury. |
| [34] | 2020 | Li, L.; Li, H.; Tien, C. L.; Jain, M. K.; Zhang, L. | KLF15 maintains normal ventricular rhythm by regulating the circadian oscillation of cardiac ion channels and QT interval. |
| [35] | 2012 | Jeyaraj, D.; Scheer, F. A.; Ripperger, J. A.; Haldar, S. M.; Lu, Y.; Prosdocimo, D. A.; Eapen, S. J.; Eapen, B. L.; Cui, Y.; Mahabeleshwar, G. H.; et al. | KLF15 serves as a pivotal link between circadian rhythms and cardiac function. |
| [36] | 2021 | He, S.; Lu, Y.; Guo, Y.; Li, S.; Lu, X.; Shao, S.; Zhou, H.; Wang, R.; Wang, J.; Gao, P.; et al. | KLF15 exerts anti-inflammatory effects via NF-κB/MAPK inhibition. |
| [37] | 2021 | Wang, B.; Xu, H.; Kong, J.; Liu, D.; Qin, W.; Bai, W. | KLF15 attenuates ischemic apoptosis via p38/MAPK inhibition. |
| [38] | 2019 | Zhou, L.; Li, Q.; Chen, A.; Liu, N.; Chen, N.; Chen, X.; Zhu, L.; Xia, B.; Gong, Y.; Chen, X. | KLF15-Twist2 axis inhibits inflammation and enhances mitochondrial homeostasis. |
| [39] | 2022 | Liao, B.; Tian, X. | KLF15 alleviates ischemia-reperfusion injury by modulating CTRP12 expression. |
| [40] | 2024 | Zheng, H.; Ye, W.; Huang, K.; Chen, Q.; Yang, J.; Luo, L. | KLF15 activates PI3K-AKT to enhance cytoprotection against oxidative damage. |
| [41] | 2014 | Prosdocimo, D. A.; Anand, P.; Liao, X.; Zhu, H.; Shelkay, S.; Artero-Calderon, P.; Zhang, L.; Kirsh, J.; Moore, D.; Rosca, M. G.; et al. | KLF15-p300 axis attenuates cardiac lipotoxicity by reprogramming lipid metabolism. |
| [42] | 2011 | Nagare, T.; Sakaue, H.; Matsumoto, M.; Cao, Y.; Inagaki, K.; Sakai, M.; Takashima, Y.; Nakamura, K.; Mori, T.; Okada, Y.; et al. | KLF15 optimizes cardiac energetics via insulin-GLUT4 axis. |
| [43] | 2024 | Durumutla, H. B.; Prabakaran, A. D.; El Abdellaoui Soussi, F.; Akinborewa, O.; Latimer, H.; McFarland, K.; Piczer, K.; Werbrich, C.; Jain, M. K.; Haldar, S. M.; et al. | Glucocorticoid-KLF15 circadian axis coordinates cardiac glucose utilization. |
| [44] | 2015 | Jiao, J.; Zou, S.; Jian, C.; Tang, F.; Xiao, Y.; Chen, L. | KLF15 enhances post-unloading cardiac recovery by suppressing lipid accumulation. |
| [45] | 2022 | Hu, Y.; Xu, J.; Gao, R.; Xu, Y.; Huangfu, B.; Asakiya, C.; Huang, X.; Zhang, F.; Huang, K.; He, X.; et al. | KLF15 attenuates lipid accumulation via PPARγ downregulation. |
| [46] | 2016 | Sun, H.; Olson, K. C.; Gao, C.; Prosdocimo, D. A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J. Y.; Ren, S.; Liu, Y.; et al. | KLF15 deficiency disrupts BCAA catabolism, causing BCKA-induced mitochondrial impairment in heart failure. |
| [47] | 2023 | Zhu, X.; Liu, J.; Cai, M.; Bao, L.; Pan, Y.; Wu, P.; Chu, W.; Zhang, J. | Fasting-induced Clock-KLF15-Bcat2 axis dysregulation impairs BCAA metabolism in fish muscle. |
| Key Point | Reference(s) | Observed Effect on MI | |
|---|---|---|---|
| KLF15-Inflammations | NF-κB | He, S et al. (2021) [36] | KLF15 is able to attenuate inflammatory responses by inhibiting NF-κB pathway activation |
| Huang, B et al. (2010) [58] | |||
| MAPK | Wang, B et al. (2021) [37] | KLF15 is able to attenuate inflammatory responses by inhibiting MAPK pathway activation | |
| Twist2 | Zhou, L et al. (2019) [38] | KLF15 ameliorates postmyocardial infarction injury by regulating Twist2 | |
| CTRP12 | Liao, B et al. (2022) [39] | KLF15 can alleviate post-ischemic reperfusion injury by regulating CTRP1250 | |
| KLF15-Apoptosis | PI3K-AKT | Zheng, H et al. (2024) [40] | KLF15 ameliorates apoptosis through the PI3K-AKT pathway |
| KLF15-EnergyMetabolism | Glucose Metabolism | Nagare, T et al. (2011) [42] | KLF15 promotes the entry of glucose into cardiomyocytes for cardiac energy supply by regulating insulin secretion |
| Lipid Metabolism | Fan, L et al. (2022) [72] | KLF15 maintains lipid homeostasis in the heart by regulating PPAR-δ and PPAR-γ | |
| Gire, D et al. (2021) [73] | |||
| Hu, Y et al. (2022) [45] | |||
| Branched-Chain AminoAcid Metabolism | Sun, H et al. (2016) [46] | KLF15 improves cardiac function by regulating BCKAs | |
| Jeyaraj, D et al. (2012) [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chen, Z.; Yang, J.; Duan, L.; Yang, H.; Cai, D.; Zhao, Z. Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 4831. https://doi.org/10.3390/ijms26104831
Zhao J, Chen Z, Yang J, Duan L, Yang H, Cai D, Zhao Z. Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(10):4831. https://doi.org/10.3390/ijms26104831
Chicago/Turabian StyleZhao, Junxin, Zhuoyang Chen, Jingyi Yang, Lincheng Duan, Hong Yang, Dingjun Cai, and Zhengyu Zhao. 2025. "Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review" International Journal of Molecular Sciences 26, no. 10: 4831. https://doi.org/10.3390/ijms26104831
APA StyleZhao, J., Chen, Z., Yang, J., Duan, L., Yang, H., Cai, D., & Zhao, Z. (2025). Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review. International Journal of Molecular Sciences, 26(10), 4831. https://doi.org/10.3390/ijms26104831






