Exploring the Effect of 1-MCP Treatment on the Post-Harvest Quality and Electronic Nose Characteristics of ‘Jizaohong’ Apricots
Abstract
1. Introduction
2. Results
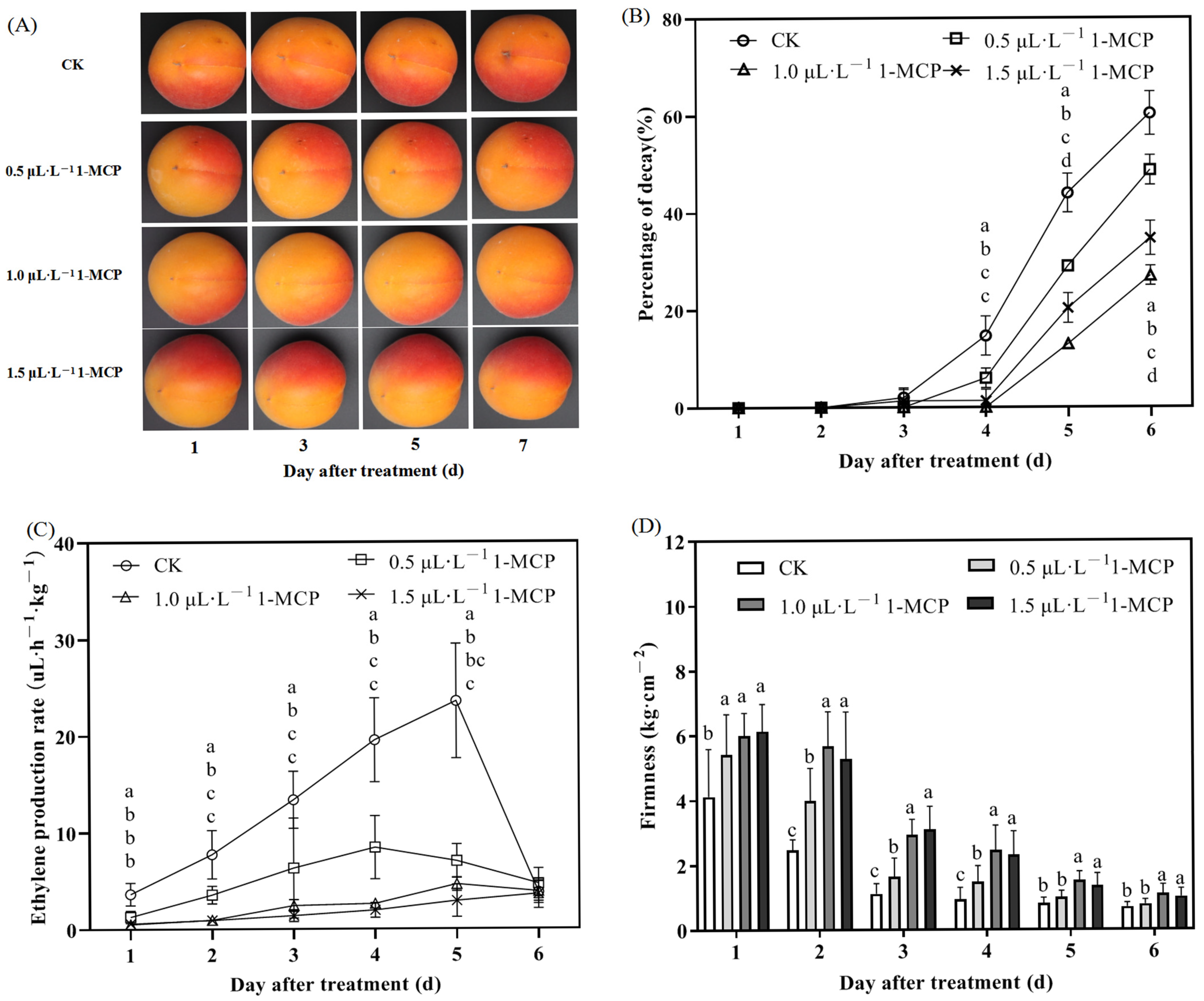
2.1. Effect of 1-MCP Treatment on the Flesh Percentage of Decay, Respiration Rate, Ethylene Release Rate, Firmness, and Soluble Solid Content (SSC) of ‘Jizaohong’ Apricot
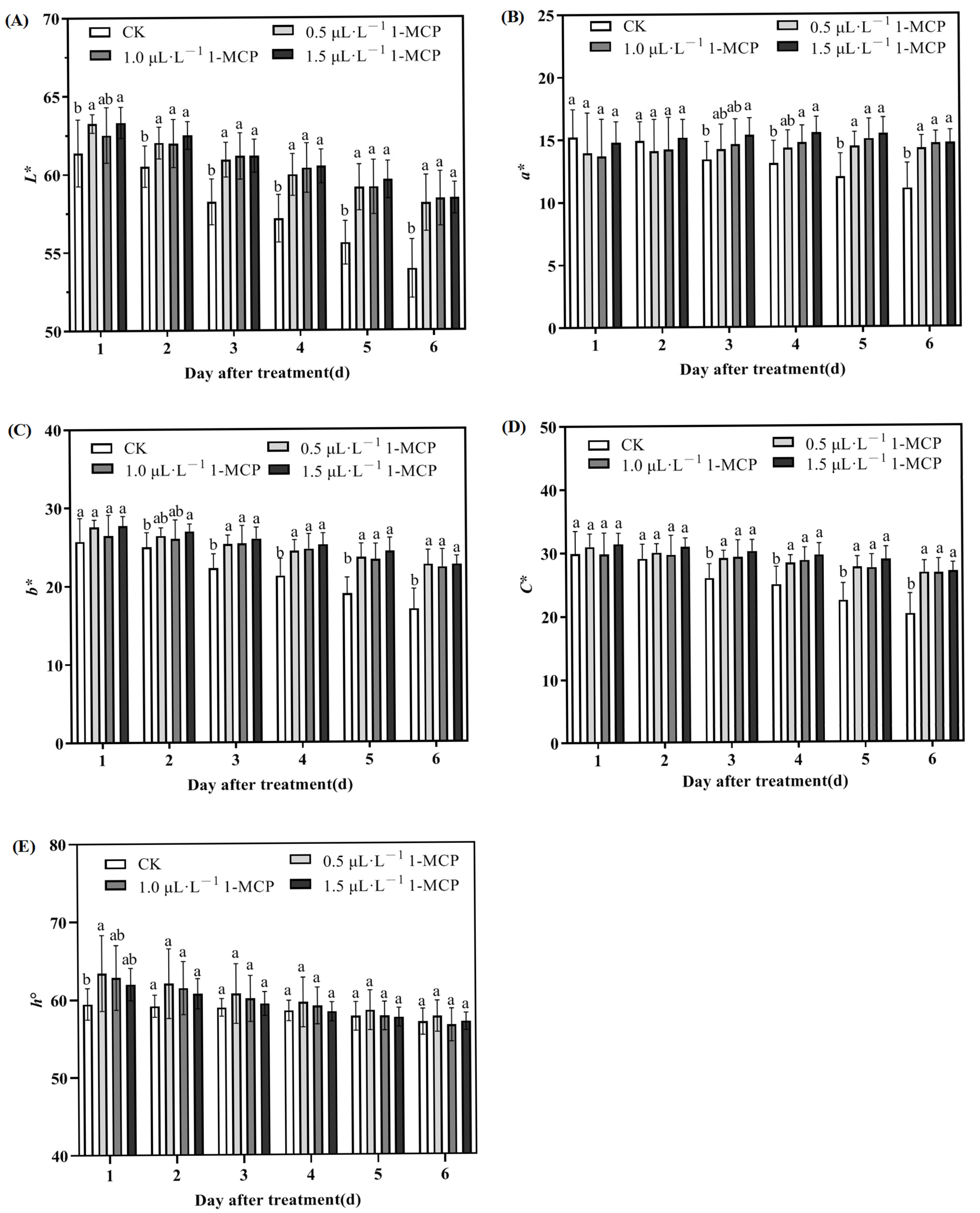
2.2. Effect of 1-MCP Treatment on the Color Parameters of ‘Jizaohong’ Apricots
2.3. Effect of 1-MCP Treatment on the Electronic Nose Characteristics of ‘Jizaohong’ Apricots
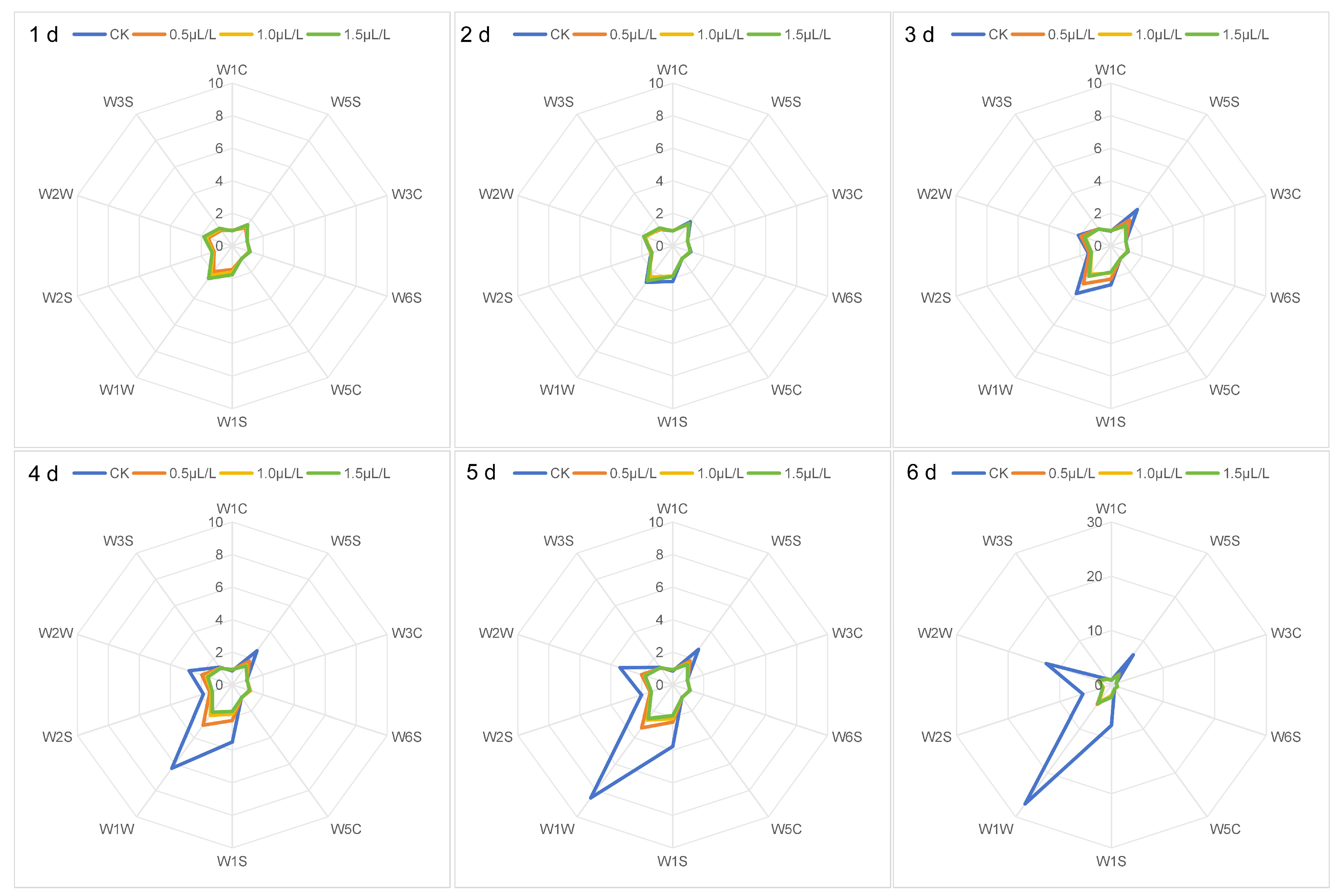
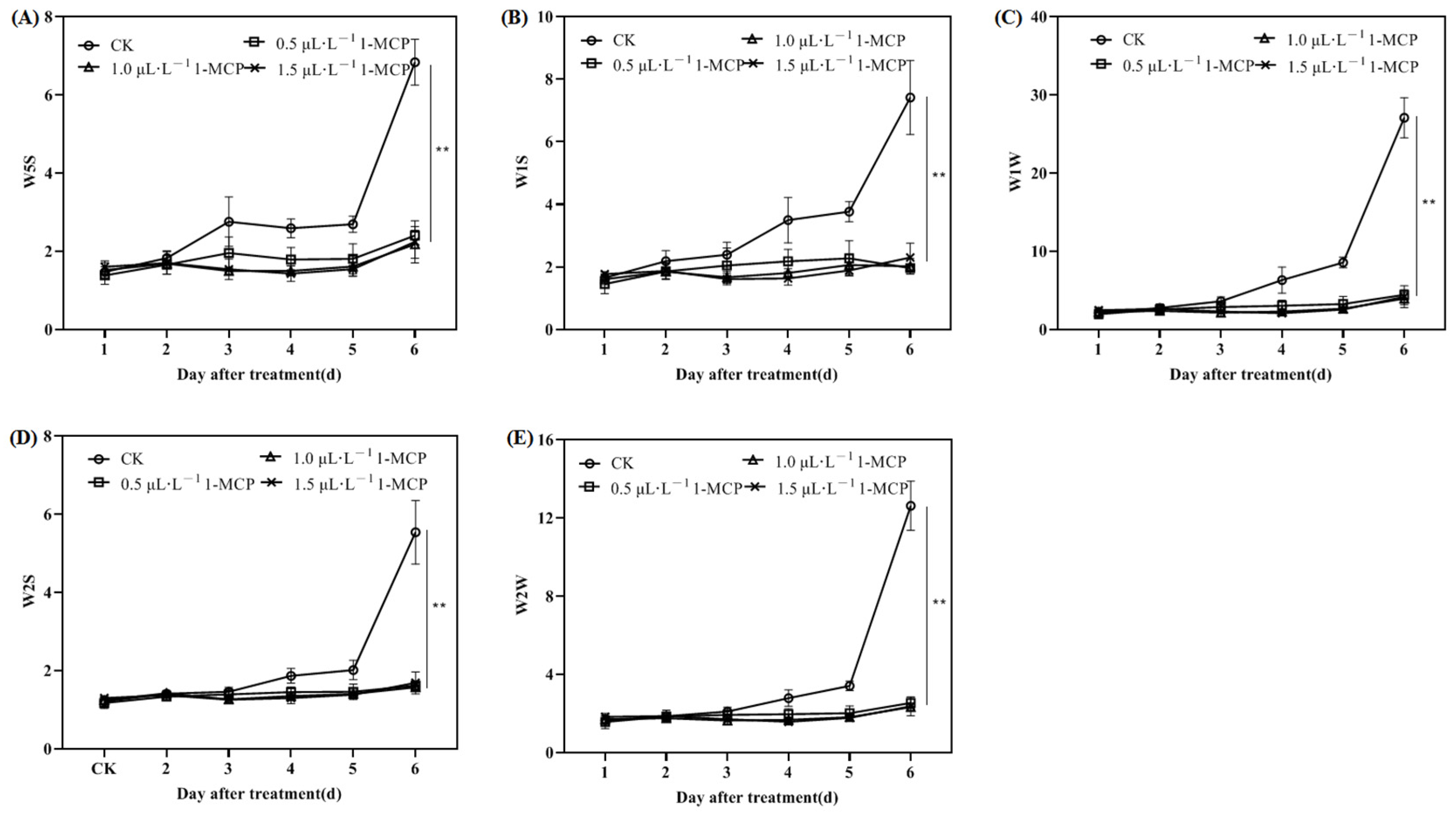
2.3.1. Effect of Different Concentrations of 1-MCP Treatment on the Sensor Response Values of the Electronic Nose
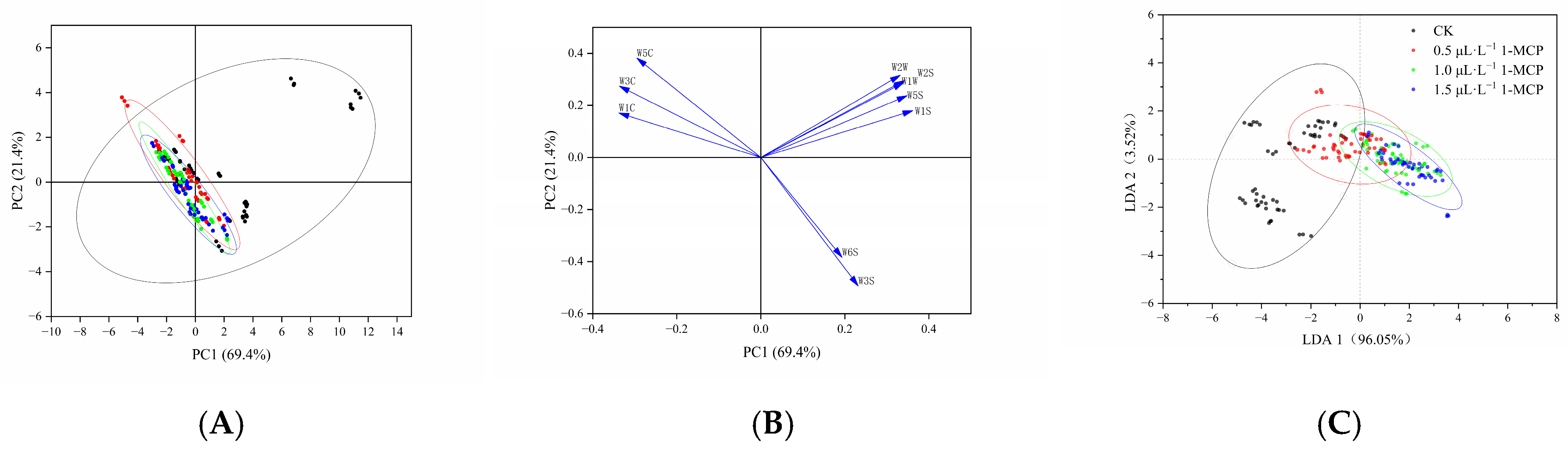
2.3.2. PCA of Electronic Nose Sensor Response Data
2.3.3. LA of Electronic Nose Sensor Response Values
2.3.4. LDA of Electronic Nose Sensor Response Values
2.4. Correlation Analysis of Quality, Color Difference, and Electronic Nose Features of ‘Jizaohong’ Apricots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Firmness and SSC
4.3. Color Parameter Measurement
4.4. Respiration Rate, Ethylene Production Rate, and Electronic Nose Analyses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCA | principal component analysis |
| LA | loading analysis |
| LDA | linear discriminant analysis |
| SSC | soluble solid content |
| 1-MCP | 1-methylcyclopropene |
Appendix A
| Number | Sensor | Performance | Notes |
|---|---|---|---|
| S1 | W1C | Aromatic Compounds | Methylbenzene, 10 ppm |
| S2 | W5S | Nitrogen Oxides | NO2, 1 ppm |
| S3 | W3C | Ammonia and Aromatic Compounds | Benzene, 10 ppm |
| S4 | W6S | Hydrogen | H2, 100 ppm |
| S5 | W5C | Short-chain Aliphatic Compounds | Dimethylmethane, 1 ppm |
| S6 | W1S | Methyl Aromatic Compounds | CH3, 100 ppm |
| S7 | W1W | Sulfides and Terpenes | H2S, 1 ppm |
| S8 | W2S | Alcohols and Aldehyde Compounds | CO, 100 ppm |
| S9 | W2W | Organic Sulfides and Compounds | H2S, 1 ppm |
| S10 | W3S | Long-chain Alkanes | CH3, 100 ppm |
References
- Cui, K.; Zhao, H.; Sun, L.; Yang, L.; Cao, J.; Jiang, W. Impact of near Freezing Temperature Storage on Postharvest Quality and Antioxidant Capacity of Two Apricot (Prunus armeniaca L.) Cultivars. J. Food Biochem. 2019, 43, e12857. [Google Scholar] [CrossRef]
- Cui, K.; Zhu, Z.; Yang, L.; Zhu, Z.; Ma, W.; Abulizi, B.; Shen, X.; Jiang, W. Research on status and prospects of Xinjiang apricot storage. Food Ferment. Ind. 2022, 48, 280–286. [Google Scholar] [CrossRef]
- Wu, X.; Wang, D.; Chen, X.; Liu, Z.; Zhao, X.; Jing, C. “Jizaohong”—An Early-Ripening Apricot Cultivar. HortScience 2024, 59, 262–263. [Google Scholar] [CrossRef]
- An, L.; Liu, Y. China’s National Forestry and Grassland Administration Recommends Four Elite New Plant Varieties for Global Cultivation. For. Sci. Technol. 2023, 6, 19. [Google Scholar]
- Wang, D.; Jing, C.; Chen, X.; Liu, Z.; Li, Y.; Wu, X. Analysis on Aroma Components in Fruits of Different Cultivars Lines of North China Apricot During Developmental Stages. Jiangsu J. Agr. Sci. 2021, 37, 974–981. [Google Scholar] [CrossRef]
- Fan, X.; Argenta, L.; Mattheis, J.P. Inhibition of Ethylene Action by 1-Methylcyclopropene Prolongs Storage Life of Apricots. Postharvest Biol. Technol. 2000, 20, 135–142. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A Review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Du, M.; Li, X.; Wang, J.; Ban, Z.; Jiang, Y. Practical 1-Methylcyclopropene Technology for Increasing Apple (Malus Domestica Borkh) Storability in the Aksu Region. Foods 2024, 13, 2918. [Google Scholar] [CrossRef]
- Qin, K.; Ge, S.; Xiao, G.; Chen, F.; Ding, S.; Wang, R. 1-MCP Treatment Improves the Postharvest Quality of Jinxiu Yellow Peach by Regulating Cuticular Wax Composition and Gene Expression During Cold Storage. J. Food Sci. 2024, 89, 2787–2802. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Lin, Q.; Duan, Y. Exploring the Effects of Different 1-MCP Concentration Treatment on Chilling Injury of Postharvest Peach Fruit. J. Food Biochem. 2024, 2024, 9917257. [Google Scholar] [CrossRef]
- Bai, J.; Mattheis, J.P.; Reed, N. Re-Initiating Softening Ability of 1-Methylcyclopropene-Treated ‘Bartlett’ and ‘d’Anjou’ Pears after Regular Air or Controlled Atmosphere Storage. J. Hortic. Sci. Biotechnol. 2006, 81, 959–964. [Google Scholar] [CrossRef]
- Dou, T.-X.; Shi, J.-F.; Li, Y.; Bi, F.-C.; Gao, H.-J.; Hu, C.-H.; Li, C.-Y.; Yang, Q.-S.; Deng, G.-M.; Sheng, O.; et al. Influence of Harvest Season on Volatile Aroma Constituents of Two Banana Cultivars by Electronic Nose and HS-SPME Coupled with GC-MS. Sci. Hortic. 2020, 265, 109214. [Google Scholar] [CrossRef]
- Shu, C.; Wall, M.M.; Follett, P.A.; Sugimoto, N.; Bai, J.; Sun, X. Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit. Horticulturae 2023, 9, 1062. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, S.; Su, J.; Cao, Z.; Wang, X.; Shen, W.; Li, T.; Ge, X. Studies on the Effect of Cinnamon Essential Oil-Micelles Combined with 1-MCP/PVA Film on Postharvest Preservation of Apricots. Food Control 2024, 162, 110420. [Google Scholar] [CrossRef]
- Meng, X.; Fang, J.; Fu, M.; Jiao, W.; Ren, P.; Yang, X. The Role of 1-Methylcyclopropylene (1-MCP) and Salicylic Acid (SA) in Induced Resistance of Postharvest Fruits. Horticulturae 2023, 9, 108. [Google Scholar] [CrossRef]
- Maqbool, A.; Beigh, M.A.; Hussain, S.Z.; Bhat, T.A.; Zargar, I.A.; Akhter, S.; Wani, N.; Qadri, T. Effect of 1-MCP and KMnO4 Treatments with Different Packaging on Quality Preservation of Golden Delicious Apples. Food Chem. X 2024, 23, 101768. [Google Scholar] [CrossRef]
- Dou, H.; Jones, S.; Ritenour, M. Influence of 1-MCP Application and Concentration on Post-Harvest Peel Disorders and Incidence of Decay in Citrus Fruit. J. Hortic. Sci. Biotechnol. 2005, 80, 786–792. [Google Scholar] [CrossRef]
- Hasan, M.K.; Alam, A.; Islam, M.R.; Akhtaruzzaman, M.; Biswas, M. Evaluating the Potential of 1-Methylcyclopropene Treatments on Physicochemical Properties, Bioactive Compounds, and Shelf Life of Mango Fruits under Different Storage Conditions. Heliyon 2024, 10, e34695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, S.; Yu, R.; Li, P.; Zhou, C.; Qu, Y.; Li, H.; Luo, H.; Yu, L. Co-Application of 1-MCP and Laser Microporous Plastic Bag Packaging Maintains Postharvest Quality and Extends the Shelf-Life of Honey Peach Fruit. Foods 2022, 11, 1733. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Zapata, P.; Martínez-García, P.J.; Martínez-Gómez, P. Whole Transcriptome Analyses of Apricots and Japanese Plum Fruits after 1-MCP (Ethylene-Inhibitor) and Ethrel (Ethylene-Precursor) Treatments Reveal New Insights into the Physiology of the Ripening Process. Int. J. Mol. Sci. 2022, 23, 11045. [Google Scholar] [CrossRef]
- Kirasak, K.; Kunyamee, S.; Ketsa, S. 1-MCP Prevents Ultrastructural Changes in the Organelles of Dendrobium Petals That Are Induced by Exogenous Ethylene. Plant Physiol. Biochem. 2023, 200, 107758. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Cui, X.; Sun, C.; Ye, S.; Huang, N.; Chen, R.; Zhang, A.; Yang, Y.; Gong, H.; Sun, S.; et al. Synergistic and Antagonistic Effects of Preharvest Salicylic Acid and Postharvest 1-Methylcyclopropene Treatments on the Storage Quality of Apricot. Food Chem. 2023, 405, 134764. [Google Scholar] [CrossRef]
- Tong, X.; Song, H.; Chen, Y.; Shi, N.; Shi, P.; Yu, L.; Kong, X.; Luo, H. 1-MCP Combined with Laser Microporous Film Packaging Maintains the Quality and Prolongs the Storage Period of Xiahei Grapes. Int. J. Food Sci. Technol. 2024, 59, 1550–1559. [Google Scholar] [CrossRef]
- Rahman, W.U.; Hashmi, M.S.; Durrani, Y.; Shah, S.; Ahmad, A.; Alam, S.; Ali, W. Hypobaric Treatment Augments the Efficacy of 1-MCP in Apple Fruit. J. Food Sci. Technol. 2022, 59, 4221–4229. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, G.; Ouyang, H.; Sang, Y.; Jiang, Y.; Cheng, S. Effects of 1-MCP Treatment on Volatile Compounds and Quality in Xiaobai Apricot during Storage at Low Temperature. J. Food Process. Preserv. 2021, 45, e15452. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Ye, S. Prediction of Soluble Solids Content, Firmness and pH of Pear by Signals of Electronic Nose Sensors. Anal. Chim. Acta 2008, 606, 112–118. [Google Scholar] [CrossRef]
- Cervellieri, S.; Lippolis, V.; Mancini, E.; Pascale, M.; Logrieco, A.F.; De Girolamo, A. Mass Spectrometry-Based Electronic Nose to Authenticate 100% Italian Durum Wheat Pasta and Characterization of Volatile Compounds. Food Chem. 2022, 383, 132548. [Google Scholar] [CrossRef]
- Gao, Q.; Tian, Y.; Zhang, J.; Zhang, P.; Zhang, M.; Bi, J.; Li, J.; Xue, Y. Discriminant Analysis of ‘Fuji’ and ‘Hanfu’ Apples under 1-Methylcyclopropene (1-MCP) and Cold Storage Conditions Based on Their Postharvest Quality and Aroma Properties. Food Meas. 2024, 18, 9492–9507. [Google Scholar] [CrossRef]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential Expression Levels of Aroma-Related Genes During Ripening of Apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Zhou, H.; Leng, F.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Gao, Y.; Ye, Z. Effect of 1-Methylcyclopropene on Flat Peach Fruit Quality Based on Electronic Senses, LC-MS, and HS-SPME-GC-MS during Shelf Storage. LWT 2023, 173, 114388. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, P.; Zhang, X.; Luo, W.; Qiao, X.; Wang, L.; Zhang, S. Metabolome and Transcriptome Profiling Reveal the Effect of 1-MCP (1-Methylcyclopropene) and Ethrel (2-Chloroethyl Phosphonic Acid) Treatments on Volatile Metabolism in Postharvest Pear Fruit. Sci. Hortic. 2024, 338, 113638. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative Analysis on Difference of Apple Fruits Flavour Using Electronic Nose and Electronic Tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Li, Q.; Li, J.; Chen, W.; Li, X. Physiological and Transcriptomic Analysis Reveals the Roles of 1-MCP in the Ripening and Fruit Aroma Quality of Banana Fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Wang, M.; Liu, Y.; Li, L.; He, F.; Xiao, J. 1-Methylcyclopropene and Bacillus Treatment Affects Blueberry Storage Quality and Antioxidant Levels. Horticulturae 2024, 10, 859. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, T.; Ran, Y.; Zhou, Y. Effects of 1-MCP Treatment on‘Bluecrop’ Blueberry Fruits Quality and Physiology Based on Principal Component Analysis. Storage Process 2024, 24, 8–18. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, L.; Lin, H.; Lin, Y.; Lin, Y.; Wang, H. Paper-Containing 1-Methylcyclopropene Treatment Suppresses Fruit Decay of Fresh Anxi Persimmons by Enhancing Disease Resistance. Food Qual. Saf. 2021, 5, fyab007. [Google Scholar] [CrossRef]
- Ortuño-Hernández, G.; Fernández, M.; Martínez-Gómez, P.; Ruiz, D.; Salazar, J.A. Ripening-Related Gene Expression Analysis Revealed the Molecular Impact of 1-MCP Application on Apricot Fruit Softening, Color, Aroma, and Antioxidant Capacity. Postharvest Biol. Technol. 2024, 216, 113037. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and Fruit Ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Bound, S.A.; Hunt, I.; Swarts, N.D. Impact of Pre-Harvest Application of 1-Methylcyclopropene (1-MCP) on Fruit Quality, Physiological Disorders and Respiration Rate of ‘Scilate’ Apple. Hortic. Environ. Biotechnol. 2024, 65, 877–890. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Wang, X.; Yu, M.; Ma, R.; Yu, Z. Synergy of Nitric Oxide and 1-Methylcyclopropene Treatment in Prolong Ripening and Senescence of Peach Fruit. Foods 2021, 10, 2956. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Bekele, E.A.; Beshir, W.F.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A.H. Metabolomics of the Effect of 1-MCP and CA during Ripening and Storage in Apples. Acta Hortic. 2015, 1079, 223–228. [Google Scholar] [CrossRef]
- Majoni, T.J.; Jooste, M.; Crouch, E.M. The Effect of 1-MCP and Storage Duration on the Storage Potential and Flesh Browning Development on ‘Cripps’ Pink’ Apples Stored under Controlled Atmosphere Conditions. Acta Hortic. 2013, 1007, 49–56. [Google Scholar] [CrossRef]
- Chigwaya, K.; Plessis, A.D.; Viljoen, D.W.; Crouch, I.J.; Crouch, E.M. Use of X-Ray Computed Tomography and 3D Image Analysis to Characterize Internal Browning in ‘Fuji’ Apples after Exposure to CO2 Stress. Sci. Hortic. 2021, 277, 109840. [Google Scholar] [CrossRef]
- Chai, J.; Wang, Y.; Liu, Y.; Yong, K.; Liu, Z. 1-MCP Extends the Shelf Life of Ready-to-Eat ‘Hayward’ and ‘Qihong’ Kiwifruit Stored at Room Temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Wu, L.; Liu, C.; Yan, Z.; Tang, H.; Sun, H.; Zhu, Z. Effect of 1-MCP Treatment on Cellulase Activity and Its Gene Expression to Improve Postharvest Quality of Custard Apple Fruit. Process. Biochem. 2024, 139, 58–69. [Google Scholar] [CrossRef]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-Methylcyclopropene (1-MCP) on Ripening and Cell-Wall Matrix Polysaccharides of Avocado (Persea americana) Fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P. Yellowing of Broccoli in Storage Is Reduced by 1-Methylcyclopropene. HortScience 2000, 35, 885–887. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, J.; Zhou, H.; Tian, M.; Huang, W.; Luo, S.; Hu, H.; Li, P. 1-Methylcyclopropene Counteracts Ethylene Inhibition of Anthocyanin Accumulation in Peach Skin after Harvest. Postharvest Biol. Technol. 2022, 183, 111737. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Rudell, D.; Park, D.; Algul, B.E.; Qin, M.; Shi, M.; Dando, R.; Watkins, C.B. Aroma Volatiles and Sensory Quality of Organically-Grown Apple Cultivars After Dynamic Controlled Atmosphere (DCA) Storage and Comparison with CA-Stored Fruit with and without 1-Methylcyclopropene (1-MCP). Postharvest Biol. Technol. 2024, 218, 113162. [Google Scholar] [CrossRef]
- Wu, X.; Jing, C.; Chen, X.; Zhao, X.; Ji, W.; Li, L.; Yuan, L.; Wang, D. Research on Aromatic Substances in Two Strains Apricot and Their Parents. S. China J. Agric. Sci. 2020, 33, 2345–2351. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on Ripening and Volatile Compounds of Blueberry Fruit. J. Food Process. Preserv. 2020, 44, e14840. [Google Scholar] [CrossRef]
- Moazzem, M.S.; Hayden, M.; Kim, D.-J.; Cho, S. Assessment of Changes in Sensory Characteristics of Strawberries during 5-Day Storage through Correlation between Human Senses and Electronic Senses. Foods 2024, 13, 3269. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Sun, K.; Liu, Q.; Pan, L.; Tu, K. Development of Novel Electronic Nose Applied for Strawberry Freshness Detection during Storage. Int. J. Food Eng. 2018, 14, 20180111. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Shivashankar, S.; Chhetri, K.B.; Kumar, A.; Rao, B.B.; Aravind, J.; Swami, D.V. Enhancing Food Authentication through E-Nose and E-Tongue Technologies: Current Trends and Future Directions. Trends Food Sci. Technol. 2024, 150, 104574. [Google Scholar] [CrossRef]
- Guo, F.; Jin, M.; Xie, Y.; Feng, L.; Jiang, L. Combined Application of Sucrose and 1-MCP Alleviated the Senescence of Gynura Bicolor DC Through Regulating Hexose Accumulation. Plant Physiol. Biochem. 2024, 212, 108745. [Google Scholar] [CrossRef]
| Percentage of Decay | Ethylene Production Rate | Respiration Rate | Firmness | SSC | L* | a* | b* | h° | C* | ΔE* | W5S | W1S | W1W | W2S | W2W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of Decay | 1.00 | |||||||||||||||
| Ethylene Production Rate | 0.55 ** | 1.00 | ||||||||||||||
| Respiration Rate | 0.65 ** | 0.52 ** | 1.00 | |||||||||||||
| Firmness | −0.90 ** | −0.77 ** | −0.66 ** | 1.00 | ||||||||||||
| SSC | 0.82 ** | 0.54 ** | 0.59 ** | −0.86 ** | 1.00 | |||||||||||
| L* | −0.87 ** | −0.77 ** | −0.62 ** | 0.97 ** | −0.88 ** | 1.00 | ||||||||||
| a* | −0.23 | −0.37 | −0.2 | 0.31 | −0.25 | 0.33 | 1.00 | |||||||||
| b* | −0.85 ** | −0.78 ** | −0.64 ** | 0.95 ** | −0.88 ** | 1.00 ** | 0.34 | 1.00 | ||||||||
| h° | −0.86 ** | −0.50 * | −0.65 ** | 0.85 ** | −0.85 ** | 0.87 ** | −0.08 | 0.87 ** | 1.00 | |||||||
| C* | −0.81 ** | −0.77 ** | −0.64 ** | 0.92 ** | −0.87 ** | 0.97 ** | 0.45 * | 0.98 ** | 0.80 ** | 1.00 | ||||||
| ΔE* | 0.80 ** | 0.39 | 0.52 ** | −0.74 ** | 0.68 ** | −0.73 ** | 0.24 | −0.70 ** | −0.89 ** | −0.59 ** | 1.00 | |||||
| W5S | 0.64 ** | 0.69 ** | 0.53 ** | −0.73 ** | 0.59 ** | −0.73 ** | −0.53 ** | −0.73 ** | −0.51 * | −0.76 ** | 0.35 | 1.00 | ||||
| W1S | 0.67 ** | 0.81 ** | 0.59 ** | −0.80 ** | 0.69 ** | −0.82 ** | −0.43 * | −0.82 ** | −0.60 ** | −0.84 ** | 0.46 * | 0.90 ** | 1.00 | |||
| W1W | 0.76 ** | 0.73 ** | 0.65 ** | −0.82 ** | 0.70 ** | −0.81 ** | −0.48 * | −0.80 ** | −0.62 ** | −0.82 ** | 0.47 * | 0.96 ** | 0.92 ** | 1.00 | ||
| W2S | 0.78 ** | 0.71 ** | 0.69 ** | −0.85 ** | 0.78 ** | −0.86 ** | −0.43 * | −0.86 ** | −0.70 ** | −0.88 ** | 0.53 ** | 0.91 ** | 0.92 ** | 0.95 ** | 1.00 | |
| W2W | 0.72 ** | 0.67 ** | 0.63 ** | −0.77 ** | 0.63 ** | −0.74 ** | −0.49 * | −0.73 ** | −0.56 ** | −0.76 ** | 0.42 * | 0.97 ** | 0.89 ** | 0.99 ** | 0.92 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, X.; Jing, C.; Wang, D.; He, J.; Hu, J.; Wu, X. Exploring the Effect of 1-MCP Treatment on the Post-Harvest Quality and Electronic Nose Characteristics of ‘Jizaohong’ Apricots. Int. J. Mol. Sci. 2025, 26, 4820. https://doi.org/10.3390/ijms26104820
Liu Z, Chen X, Jing C, Wang D, He J, Hu J, Wu X. Exploring the Effect of 1-MCP Treatment on the Post-Harvest Quality and Electronic Nose Characteristics of ‘Jizaohong’ Apricots. International Journal of Molecular Sciences. 2025; 26(10):4820. https://doi.org/10.3390/ijms26104820
Chicago/Turabian StyleLiu, Zhikun, Xuefeng Chen, Chenjuan Jing, Duan Wang, Jingang He, Jianfang Hu, and Xiaohong Wu. 2025. "Exploring the Effect of 1-MCP Treatment on the Post-Harvest Quality and Electronic Nose Characteristics of ‘Jizaohong’ Apricots" International Journal of Molecular Sciences 26, no. 10: 4820. https://doi.org/10.3390/ijms26104820
APA StyleLiu, Z., Chen, X., Jing, C., Wang, D., He, J., Hu, J., & Wu, X. (2025). Exploring the Effect of 1-MCP Treatment on the Post-Harvest Quality and Electronic Nose Characteristics of ‘Jizaohong’ Apricots. International Journal of Molecular Sciences, 26(10), 4820. https://doi.org/10.3390/ijms26104820






