Loxl3 Affects Palatal Shelf Elevation by Regulating Cell Proliferation and Collagen Deposition
Abstract
1. Introduction
2. Result
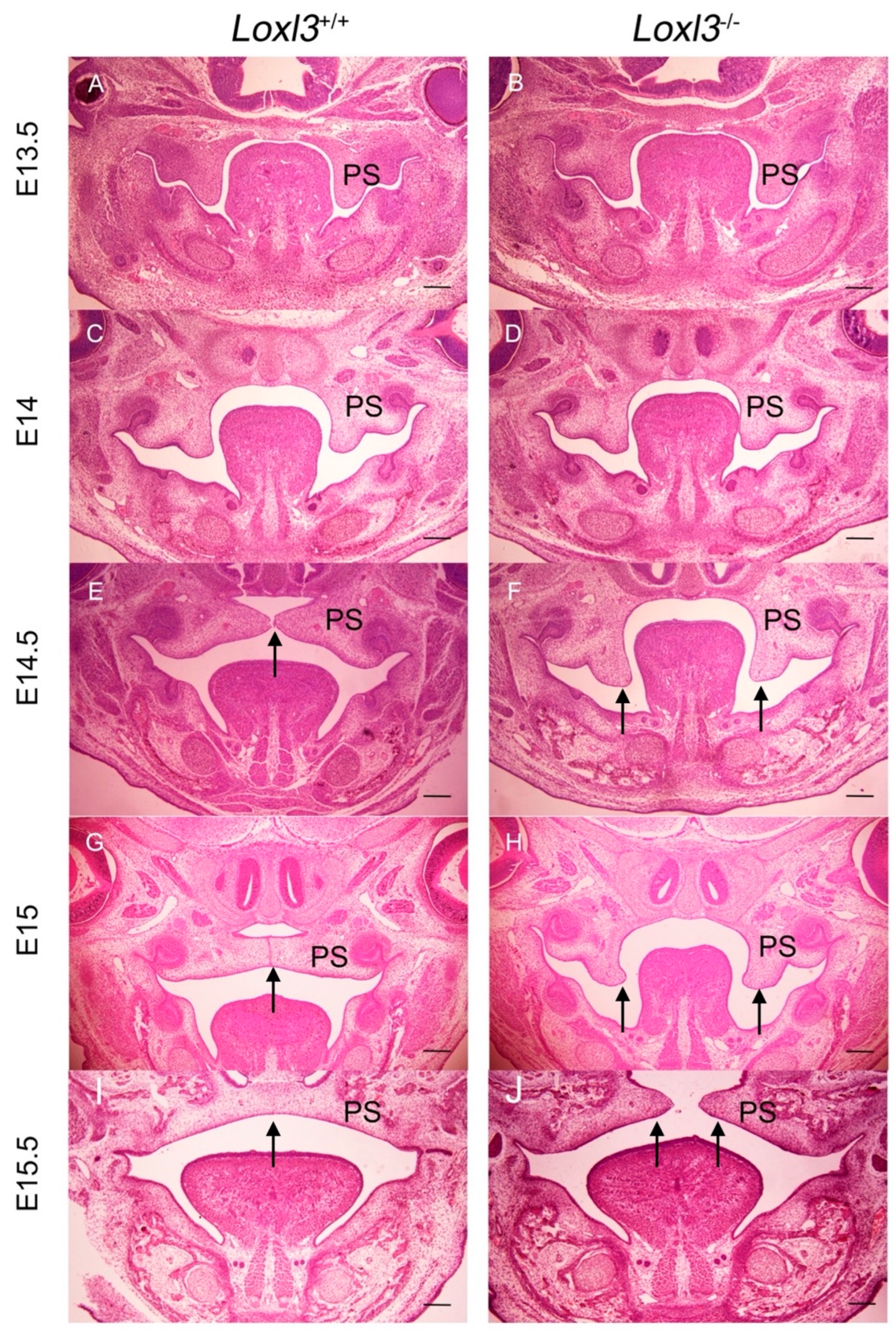
2.1. Delayed Palatal Shelf Elevation Due to Loxl3 Deletion Occurred Between E14 and E14.5
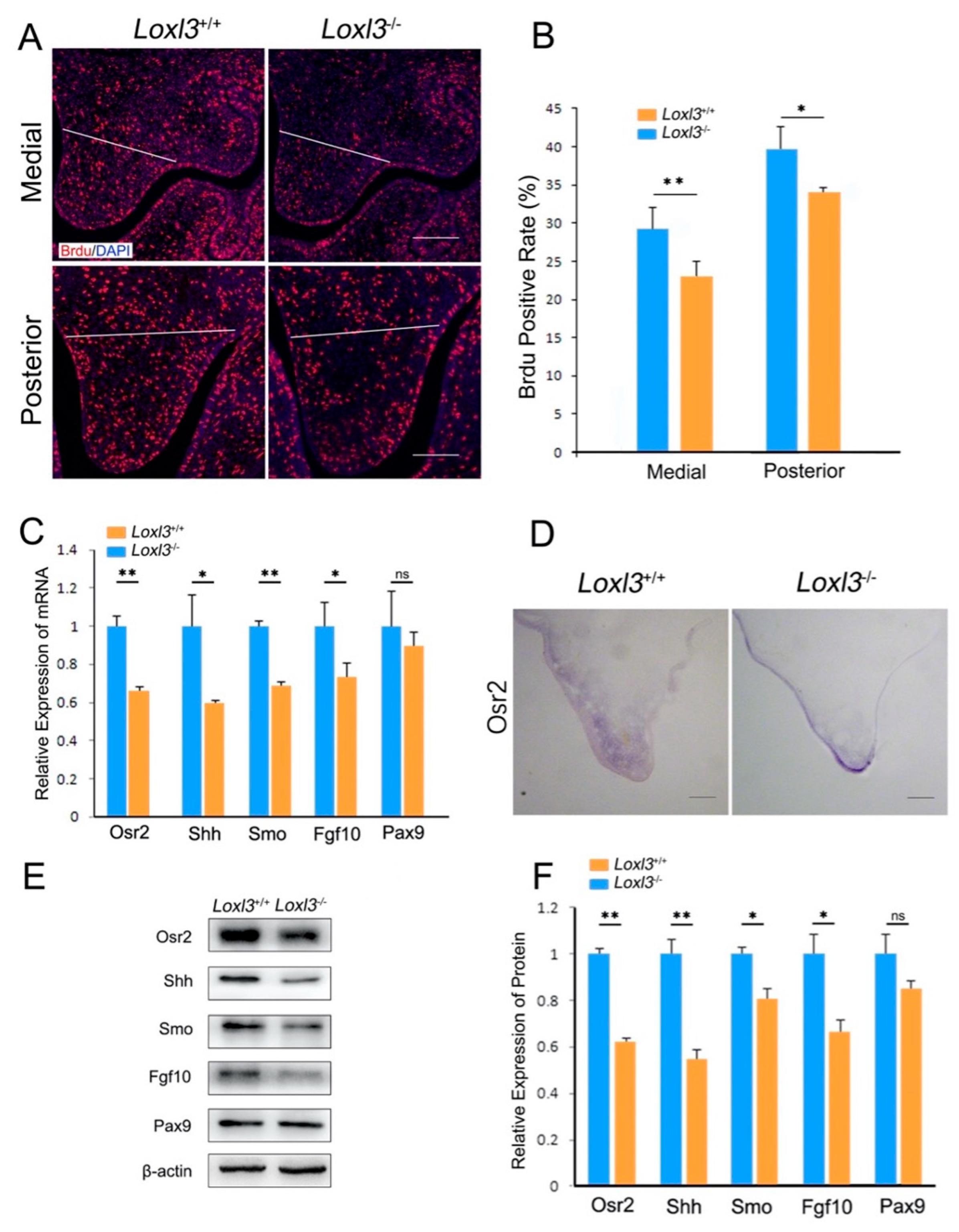
2.2. Loxl3 Deletion Caused Reduced Cell Proliferation and Expression of Proliferation-Related Genes In Vivo
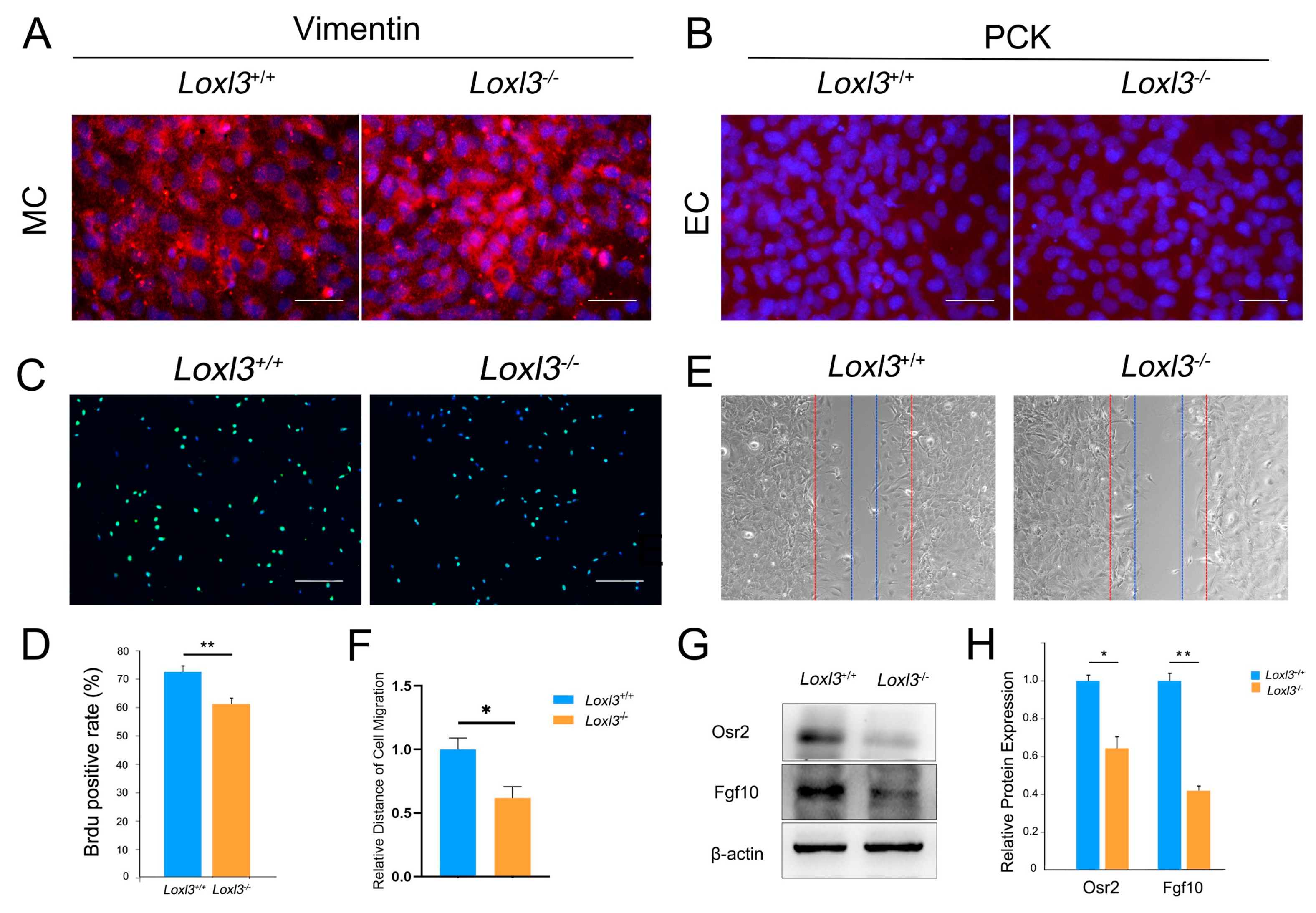
2.3. Loxl3 Deletion Resulted in Reduced Cell Proliferation and Migration In Vitro
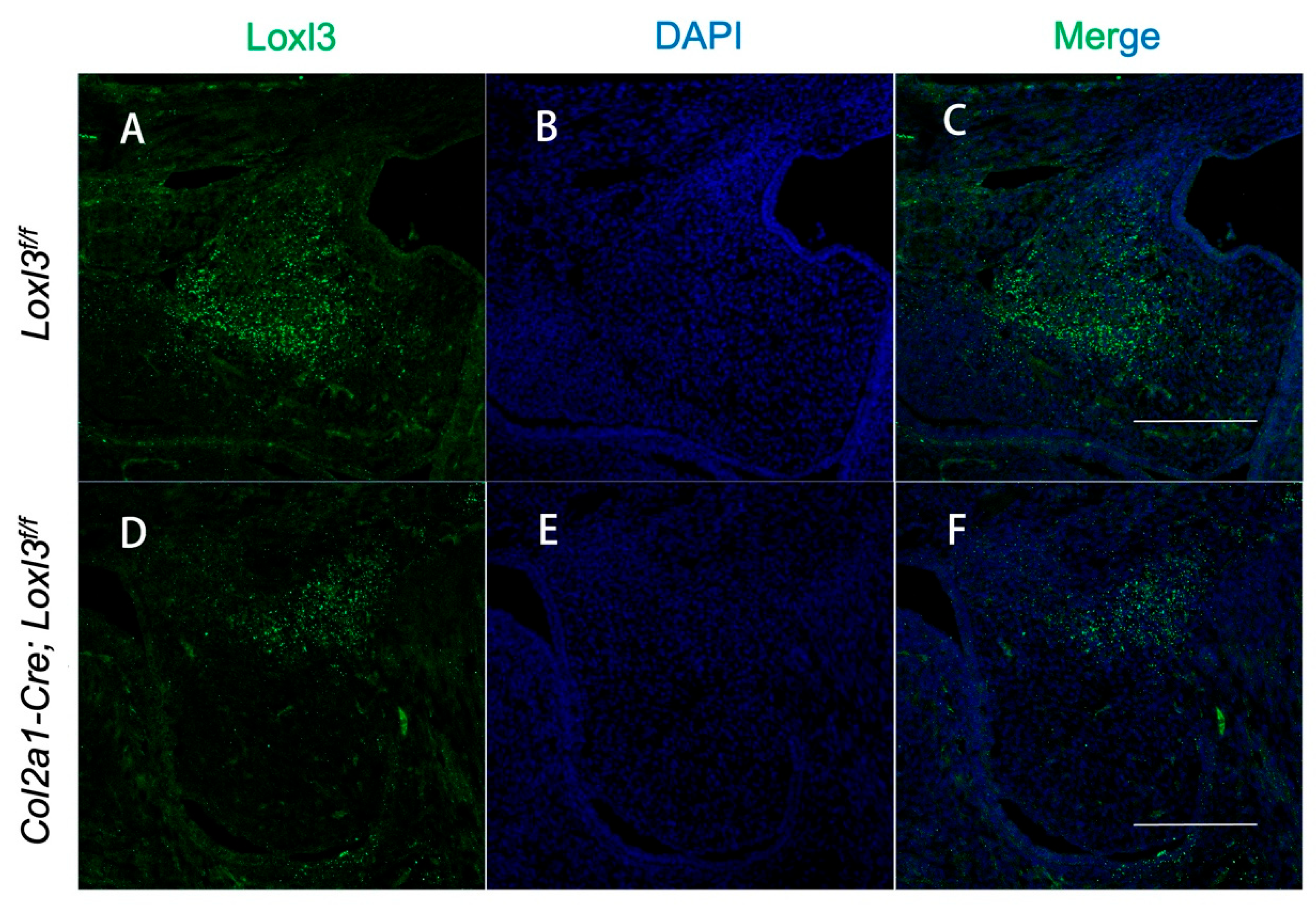
2.4. Col2a1-Cre Mediated Ablation of the Loxl3 in the Palatal Mesenchyme
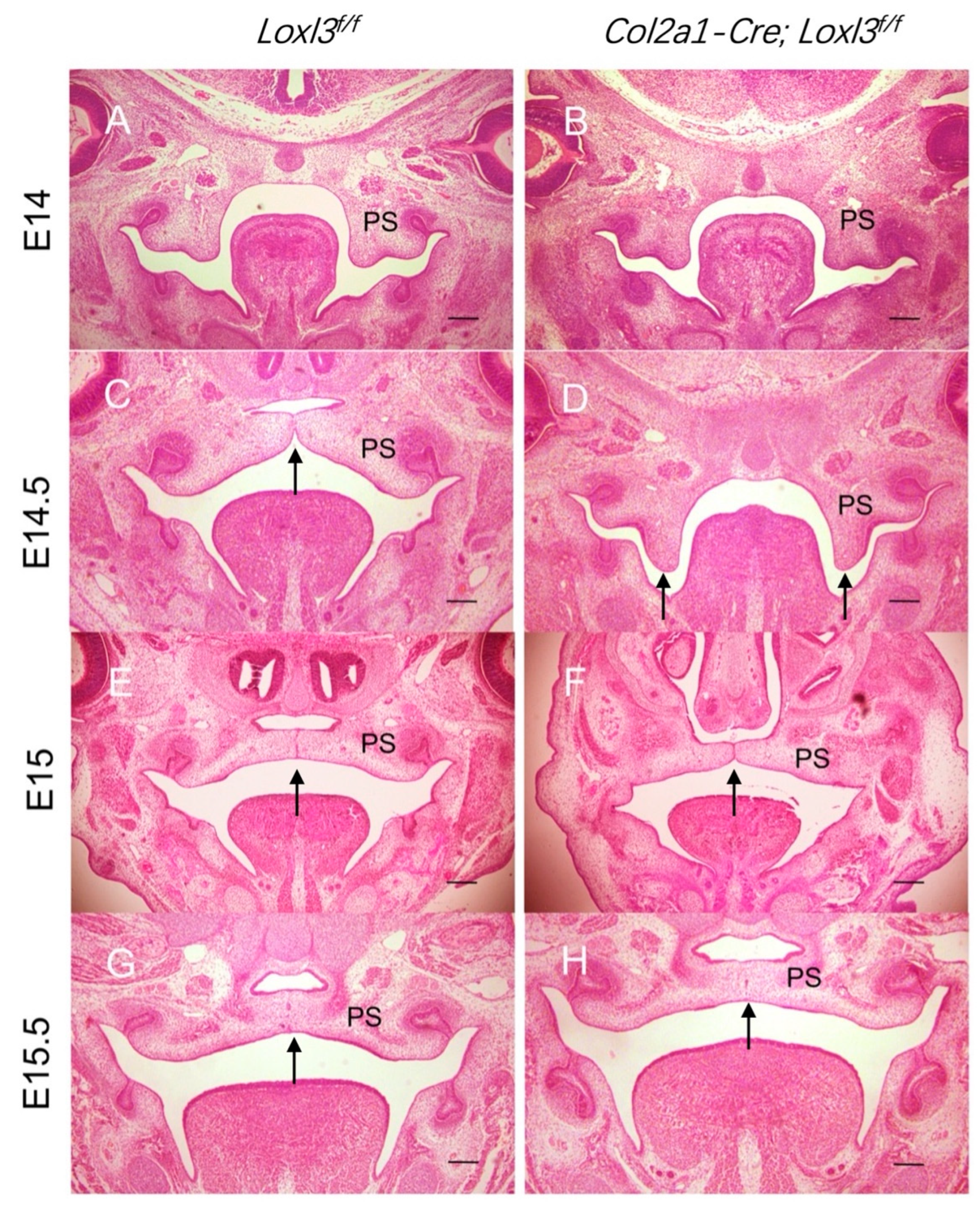
2.5. Col2a1-Cre-Mediated Loxl3 Ablation Led to Delayed Elevation but Normal Fusion of Palatal Shelves
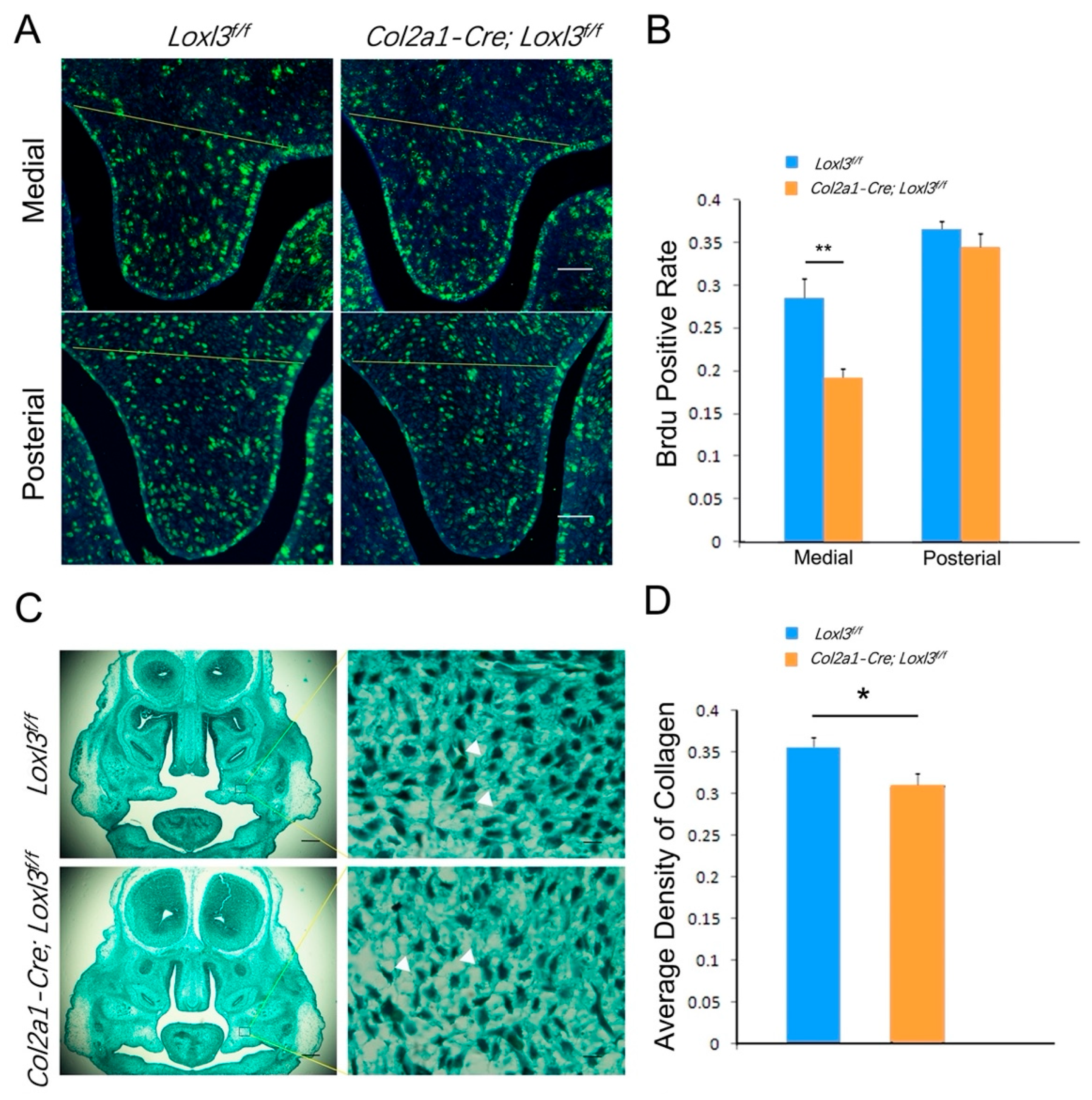
2.6. Col2a1-Cre; Loxl3f/f Embryos Exhibited Reduced Cell Proliferation and Collagen Deposition
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Histological Analysis
4.3. BrdU Labeling and Proliferation Analysis
4.4. RNA Extraction and Quantitative Real-Time RT-PCR
4.5. Western Blot Analysis
4.6. Cell Culture
4.7. Immunofluorescence
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Worley, M.L.; Patel, K.G.; Kilpatrick, L.A. Cleft Lip and Palate. Clin. Perinatol. 2018, 45, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Li, A.; Jia, P.; Mallik, S.; Fei, R.; Yoshioka, H.; Suzuki, A.; Iwata, J.; Zhao, Z. Critical microRNAs and regulatory motifs in cleft palate identified by a conserved miRNA-TF-gene network approach in humans and mice. Brief. Bioinform. 2020, 21, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Kaartinen, V. Signaling networks in palate development. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 271–278. [Google Scholar] [CrossRef]
- Nixon, T.R.W.; Richards, A.J.; Martin, H.; Alexander, P.; Snead, M.P. Autosomal Recessive Stickler Syndrome. Genes 2022, 13, 1135. [Google Scholar] [CrossRef]
- Alzahrani, F.; Al Hazzaa, S.A.; Tayeb, H.; Alkuraya, F.S. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Hum. Genet. 2015, 134, 451–453. [Google Scholar] [CrossRef]
- Chan, T.K.; Alkaabi, M.K.; ElBarky, A.M.; El-Hattab, A.W. LOXL3 novel mutation causing a rare form of autosomal recessive Stickler syndrome. Clin. Genet. 2019, 95, 325–328. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Liu, Z.; Hou, C.; Zong, W.; Zhang, A.; Sun, X.; Gao, J. Loss of lysyl oxidase-like 3 causes cleft palate and spinal deformity in mice. Hum. Mol. Genet. 2015, 24, 6174–6185. [Google Scholar] [CrossRef]
- Li, J.; Rodriguez, G.; Han, X.; Janečková, E.; Kahng, S.; Song, B.; Chai, Y. Regulatory Mechanisms of Soft Palate Development and Malformations. J. Dent. Res. 2019, 98, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, Y.; Zhao, X.; Zhang, X.; Fermin, C.; Chen, Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 2002, 129, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yeo, J.Y.; Chytil, A.; Han, J.; Bringas, P., Jr.; Nakajima, A.; Shuler, C.F.; Moses, H.L.; Chai, Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 2003, 130, 5269–5280. [Google Scholar] [CrossRef]
- Logan, S.M.; Ruest, L.B.; Benson, M.D.; Svoboda, K.K.H. Extracellular Matrix in Secondary Palate Development. Anat. Rec. 2020, 303, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Zhu, Z.; Yuan, L.; Chan, W.Y.; Sha, O. Extracellular Matrix Remodeling During Palate Development. Organogenesis 2020, 16, 43–60. [Google Scholar] [CrossRef]
- Ferguson, M.W. Palate development. Development 1988, 103, 41–60. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Lan, Y.; Jia, S.; Jiang, R. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 2013, 140, 4709–4718. [Google Scholar] [CrossRef]
- Jia, S.; Kwon, H.E.; Lan, Y.; Zhou, J.; Liu, H.; Jiang, R. Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Dev. Biol. 2016, 420, 110–119. [Google Scholar] [CrossRef]
- Fu, X.; Xu, J.; Chaturvedi, P.; Liu, H.; Jiang, R.; Lan, Y. Identification of Osr2 Transcriptional Target Genes in Palate Development. J. Dent. Res. 2017, 96, 1451–1458. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H. Fgf10: A paracrine-signaling molecule in development, disease, and regenerative medicine. Curr. Mol. Med. 2014, 14, 504–509. [Google Scholar] [CrossRef]
- Lan, Y.; Ovitt, C.E.; Cho, E.-S.; Maltby, K.M.; Wang, Q.; Jiang, R. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 2004, 131, 3207–3216. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, X.; Wan, P.; Mo, F.; Chen, G.; Zhang, J.; Gao, J. Targeted Deletion of Loxl3 by Col2a1-Cre Leads to Progressive Hearing Loss. Front. Cell Dev. Biol. 2021, 9, 683495. [Google Scholar] [CrossRef] [PubMed]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Robin, N.H.; Moran, R.T.; Ala-Kokko, L. Stickler Syndrome. In GeneReviews(®); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2000. [Google Scholar]
- Baek, J.A.; Lan, Y.; Liu, H.; Maltby, K.M.; Mishina, Y.; Jiang, R. Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev. Biol. 2011, 350, 520–531. [Google Scholar] [CrossRef]
- Enomoto, H.; Nelson, C.M.; Somerville, R.; Mielke, K.; Dixon, L.J.; Powell, K.; Apte, S.S. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development 2010, 137, 4029–4038. [Google Scholar] [CrossRef]
- Yoshioka, H.; Jun, G.; Suzuki, A.; Iwata, J. Dexamethasone Suppresses Palatal Cell Proliferation through miR-130a-3p. Int. J. Mol. Sci. 2021, 22, 12453. [Google Scholar] [CrossRef] [PubMed]
- Cobourne, M.T.; Green, J.B. Hedgehog signalling in development of the secondary palate. Front. Oral. Biol. 2012, 16, 52–59. [Google Scholar]
- Rice, R.; Spencer-Dene, B.; Connor, E.C.; Gritli-Linde, A.; McMahon, A.P.; Dickson, C.; Thesleff, I.; Rice, D.P.C. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Investig. 2004, 113, 1692–1700. [Google Scholar] [CrossRef]
- Lan, Y.; Jiang, R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development 2009, 136, 1387–1396. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Salybekova, A.K.; Pola, R.; Asahara, T. Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. Int. J. Mol. Sci. 2018, 19, 3040. [Google Scholar] [CrossRef]
- Rubinstein, T.J.; Weber, A.C.; Traboulsi, E.I. Molecular biology and genetics of embryonic eyelid development. Ophthalmic Genet. 2016, 37, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Mansell, J.P.; Kerrigan, J.; McGill, J.; Bailey, J.; TeKoppele, J.; Sandy, J.R. Temporal changes in collagen composition and metabolism during rodent palatogenesis. Mech. Ageing Dev. 2000, 119, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Liu, R.-K.; Shi, B. A possible explanation of what’s happening at the moment of palatal shelf elevation. Biosci. Hypotheses 2009, 2, 372–374. [Google Scholar] [CrossRef]
- Hutson, M.S.; Leung, M.C.K.; Baker, N.C.; Spencer, R.M.; Knudsen, T.B. Computational Model of Secondary Palate Fusion and Disruption. Chem. Res. Toxicol. 2017, 30, 965–979. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, H.F.; Wei, Y.; Zhu, W.L. Effect of Smo SiRNA-mediated Hedgehog Signaling Pathway Inhibition on Palatal Fusion. Biomed. Environ. Sci. 2016, 29, 594–598. [Google Scholar]
- Alappat, S.R.; Zhang, Z.; Suzuki, K.; Zhang, X.; Liu, H.; Jiang, R.; Yamada, G.; Chen, Y. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev. Biol. 2005, 277, 102–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Mo, F.; Dong, X.; Chen, G.; Gao, J.; Zhang, J. Loxl3 Affects Palatal Shelf Elevation by Regulating Cell Proliferation and Collagen Deposition. Int. J. Mol. Sci. 2025, 26, 4815. https://doi.org/10.3390/ijms26104815
Liu Z, Mo F, Dong X, Chen G, Gao J, Zhang J. Loxl3 Affects Palatal Shelf Elevation by Regulating Cell Proliferation and Collagen Deposition. International Journal of Molecular Sciences. 2025; 26(10):4815. https://doi.org/10.3390/ijms26104815
Chicago/Turabian StyleLiu, Ziyi, Fan Mo, Xinyu Dong, Ge Chen, Jiangang Gao, and Jian Zhang. 2025. "Loxl3 Affects Palatal Shelf Elevation by Regulating Cell Proliferation and Collagen Deposition" International Journal of Molecular Sciences 26, no. 10: 4815. https://doi.org/10.3390/ijms26104815
APA StyleLiu, Z., Mo, F., Dong, X., Chen, G., Gao, J., & Zhang, J. (2025). Loxl3 Affects Palatal Shelf Elevation by Regulating Cell Proliferation and Collagen Deposition. International Journal of Molecular Sciences, 26(10), 4815. https://doi.org/10.3390/ijms26104815





