Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases
Abstract
1. Introduction
2. Vitamin D Metabolism
- –
- 1α,25(OH)2D (calcitriol), which is the biologically active form of vitamin D. It is essential for calcium metabolism and the regulation of many cellular processes. It is associated with osteoporosis, rickets, hypocalcemia, immune dysfunction, and some autoimmune diseases.
- –
- 3-epi-1α,25(OH)2D (3-epi-calcitriol), which has a different configuration at carbon 3 of ring A. It has reduced affinity for the VDR and reduced biological effects. Its role is not fully understood, but it may be less calcium-affecting than calcitriol, with therapeutic implications in conditions requiring control of calcium metabolism. Do not alter the core message.
- –
- 24R,25(OH)D, which is a less well-understood metabolite of vitamin D involved in bone repair and regulation of vitamin D metabolism. It has been linked to a protective role in certain bone diseases and the regulation of vitamin D excess [30].
3. Molecular Effects of Vitamin D on Immune Cells
4. Allergic Diseases
4.1. Vitamin D, Asthma, and Rhinosinusitis
4.2. Vitamin D and Atopic Dermatitis
5. Vitamin D and Autoimmune Diseases
5.1. Vitamin D and Rheumatoid Arthritis
5.2. Vitamin D and Psoriasis
5.3. Vitamin D and Systemic Lupus Erythematosus
5.4. Vitamin D and Autoimmune Thyroid Diseases
5.5. Vitamin D and Type 1 and Type 2 Diabetes Mellitus
6. Vitamin D and Infectious Diseases
Vitamin D and Acquired Immunodeficiency Syndrome (AIDS)
7. Vitamin D and Multiple Sclerosis
8. Conclusions
| Author | Design | Duration | Participants (CA/CO) | Dose of Vitamin D | Results |
|---|---|---|---|---|---|
| VITAMIN D AND ASTHMA | |||||
| Majak et al. [295] | RCT | 6 months | 48 children (aged 5 to 18 years) with new diagnoses of asthma (24/24) | Participants were randomly assigned to: - Budesonide 800 mg/day administered as a dry powder and vitamin D placebo or - Budesonide 800 mg/day administered as a dry powder and 500 IU vitamin D cholecalciferol | The vitamin D group showed a significantly lower number of children who experienced asthma exacerbation and a lower number of children with a decrease of 25(OH)D than the steroid group (p = 0.029), so vitamin D supplementation prevented the reduction of serum concentrations of 25(OH)D and reduced the risk of asthma exacerbation triggered by acute RTIs. |
| Nanzer et al. [296] | RCT | 4 weeks | 24 patients (aged 18 to 75 years) with glucocorticoid-resistant asthma (13/11) | In the screening period, patients received a 2-week course of oral prednisolone; after a 4-week washout period, patients were randomly divided into two groups that received calcitriol 0.25 μg soft capsules or placebo twice daily for 4 weeks with a second course of oral prednisolone identical to the first repeated during the final 2 weeks. | The calcitriol group showed a modest but significant improvement in absolute and predicted FEV1 compared to the placebo group (p = 0.03), so 1,25(OH)2D supplementation improved the clinical response to oral steroids. |
| Castro et al. [90] | RCT | 28 weeks | 408 adults (aged 18 years or older) with asthma and a serum 25(OH)D level of less than 30 ng/mL (201/207) | Participants were randomly assigned to either a placebo or a high-dose of vitamin D (100,000 IU once), followed by 4000 IU/day for 28 weeks added to inhaled ciclesonide (320 µg/d; 2 puffs twice/day) and levalbuterol; subsequently, inhaled corticosteroids were tapered by 50% if the participant’s asthma symptoms were controlled. | Treatment with vitamin D did not alter the rate of first treatment failure or exacerbation compared with the placebo group; however, among secondary outcomes, the only statistically significant one was a small difference in the overall dose of ciclesonide required to maintain asthma control in the vitamin D group compared to the placebo group (111.3 µg/d [95% CI, 102.2–120.4 µg/d] in the vitamin D group vs. 126.2 µg/d [95% CI, 117.2–135.3 µg/d] in the placebo group; difference of 14.9 µg/d [95% CI, 2.1–27.7 µg/d]). |
| Mahboub et al. [88] | RCT | 8 weeks | 54 adults (aged 18 to 65 years) with moderate-to-severe asthma and 25(OH)D levels less than 20 ng/mL (34/20) | Participants were randomly assigned to: - The experimental group received 50,000 IU of vitamin D orally weekly - The placebo group received the placebo | There was a significant increase in GR-α expression in the experimental group compared to the placebo control (p < 0.05), while no change in GR-β expression was observed; consequently, the GR-α/GR-β ratio increased significantly (p < 0.05). |
| Wang Q. et al. [78] | MT (12 RCTs) | From 6 weeks to 6 months | 1871 children with asthma (898/973) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 200 IU/day to 60,000 IU/month) - The placebo group received the placebo | The experimental group had a significantly lower recurrence rate than the placebo group (18.4% versus 35.9%, RR = 0.35, 95% CI = 0.35–0.79, p = 0.002). |
| Wang M. et al. [297] | MT (14 RCTs) | From 3 to 12 months | 1421 patients with asthma (711/710) | Four studies compared vitamin D (from 500 IU/day to 50,000 IU/week) to placebo as a treatment individually, while other studies received vitamin D as an adjunct treatment (corticosteroids/SABA/LABA/Montelukast/conventional therapy not described). | Vitamin D supplementation was associated with: - a significant improvement of FEV1% in patients with vitamin D insufficiency and airflow limitation (baseline FEV1% < 80%) (MD: 8.30, 95% Cl (5.95, 10.64) - a reduction in the rate of exacerbation compared with placebo (RR: 0.7395% Cl (0.58,0.92)). |
| Wang Y. et al. [77] | MT (19 RCTs) | From 6 weeks to 48 weeks | 2063 patients with asthma (1039/1024) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 100 IU/day to 60,000 IU/week) - The placebo group received placebo | In the vitamin D supplementation group: -Number of exacerbations was less, while there was no statistical difference (OR = 0.73, p = 0.06, I2 = 59%) - The FEV1/FVC was significantly improved (OR = 4.33, p = 0.02, I2 = 99%) - Length of hospital stay and mortality were not changed - Levels of IL-5 and IgE were significantly decreased (OR = −9.18, p = 0.0004, I2 = 99%; OR = −100.85, p < 0.00001, I2 = 0%) - Levels of IL-6 and IL-10 and eosinophil counts were not significantly different, while in subgroup analysis based on serum vitamin D, the IL-10 level of the vitamin D deficiency group was significantly increased (OR = 2.51, p < 0.00001, I2 = 32%). |
| Fedora et al. [86] | MT (10 RCTs) | From 6 weeks to 12 months | 1243 asthmatic patients from 0 to 18 years old (631/612) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 150 IU/day up to 4000 IU/day) - The placebo group received placebo | In the vitamin D supplementation group there was: - A significant reduction of asthma exacerbations (RR 0.62; 95% CI: 0.44, 0.87; p = 0.006) (primary outcome) - A significant improvement in predicted percentage of FEV1 levels |
| Pojsupap et al. [298] | MT (5 RCTs) | From 4 to 52 weeks | 625 asthmatic patients (from 5 to 18 years old) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 500 IU/day up to 2000 IU/day) - The placebo group received placebo or budesonide ICS | In vitamin D supplementation group, there was a statistically significant reduction in asthma exacerbation (RR 0.41, CI 0.27–0.63). |
| Jolliffe et al. [89] | MT (7 RCTs) | From 15 weeks to 1 year | 955 asthmatic patients | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 500 IU/day up to 2000 IU/day) - The placebo group received placebo | Vitamin D supplementation reduced the rate of asthma exacerbation requiring treatment with systemic corticosteroids among all participants (adjusted incidence rate ratio [aIRR] 0.74, 95% CI 0.56–0.97; p = 0.03). There were no significant differences between vitamin D and placebo in the proportion of participants with at least one exacerbation or time to first exacerbation. |
| Niu et al. [299] | MT (12 RCTs) | From 9 weeks to 9 months | 1295 asthmatic patients (649/646) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 400 IU up to 100,000 IU) - The placebo group received placebo | Vitamin D supplementation significantly reduced the number of asthma exacerbations, including the rate of exacerbations requiring systemic corticosteroid therapy and the rate of acute exacerbations requiring emergency department or hospital visits or both. |
| Riverin et al. [300] | MT (8 RCTs) | From 1 to 12 months | 573 asthmatic children aged 3 to 18 years | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 1000 IU/week up to 60,000 IU/month) - The placebo group received placebo, budesonide, fluticasone, or prednisone | In vitamin D supplementation group there were: - a reduced risk of asthma exacerbations (RR 0.41, 95% CI 0.27 to 0.63, 3 studies, n = 378). - no significant effect for asthma symptom scores and lung function. |
| Chen et al. [292] | MT (12 RCTs) | From 2 to 12 months | 1543 asthmatic patients (769/774) | Participants were randomly assigned to: - The experimental group received vitamin D supplements (from 1000 IU/week up to 60,000 IU/week) - The placebo group received placebo | Vitamin D supplementation significantly reduced the risk of asthma exacerbation (pooled risk ratio (RR) 0.70, 95% confidence interval (CI), 0.59, 0.83; p < 0.05), but did not improve ACT score or lung function among patients with asthma treated with corticosteroids. |
| VITAMIN D AND ALLERGIC RHINITIS | |||||
| Bhardwaj et al. [94] | RCT | 2 years and 5 months | 87 patients (aged 16 to 60 years) with allergic rhinitis and serum vitamin D levels less than 20 ng/mL | Participants were randomly divided into two groups: - Group A received Fluticasone 50 mcg nasal spray, two puffs BID for 4 weeks - Group B received Fluticasone 50 mcg nasal spray, two puffs BID, and Oral vitamin D cholecalciferol 60,000 IU once a week for 4 weeks | The pre-treatment TNSS score in Group A was 12.5 ± 2.68, while the post-treatment score was 8.98 ± 1.009, with the difference in both scores of Group A as 3.52 (the t score was 117.387 and p < 0.000). The pre-treatment TNSS score in Group B was 11.64 ± 3.09 while the post-treatment score was 6.3 ± 1.45, with the difference in both scores of Group B as 5.34 (the t score was 131.1403 and p < 0.0001). The post-treatment RCAT in Group A and Group B was 19.72 ± 2.84 and 28.2 ± 1.53, respectively, with the difference between the two groups as 8.48 (t score as 135.27 and p < 0.05). |
| Guo [95] | RCT | 5 years | 140 patients (aged 16 to 60 years) with moderate-to-severe allergic rhinitis (70/70) | Participants were randomly divided into two groups: - The control group received 200 µg mometasone nasal spray and two puffs of BID for 4 weeks. - The experimental group received 200 µg mometasone nasal spray, two puffs BID, and two oral vitamin D capsules (400 IU vitamin D per capsule) for 4 weeks. | Total TNSS scores were significantly reduced compared with those before treatment in both groups for all four main symptoms assessed (p < 0.05), and the improvements with vitamin D supplementation were significantly greater than those of the control group in the symptoms assessed, except for sneezing (p < 0.05). RQLQ scores in both groups were significantly lower after the therapy than before the therapy in all aspects (p < 0.05). The improvements with vitamin D supplementation were significantly higher than those in the control group, except for eye symptoms, which were improved. Still, the difference was not statistically significant compared to the control (p < 0.05). |
| Bakhshaee et al. [96] | RCT | 1 year | 80 patients (aged 18 to 40 years) with allergic rhinitis and vitamin D level of 10–20 ng/mL (40/40) | Participants were randomly divided into two groups: - The control group received a placebo plus cetirizine for eight weeks - Intervention group received vitamin D (weekly 50,000 IU) plus cetirizine for eight weeks | There was no significant difference between the two groups at the onset of the study and 4 weeks later regarding the mean scores of symptom severity (p = 0.073), whereas a significant difference was obtained between the two groups in terms of symptom severity at baseline and 8 weeks post-treatment (p = 0.007). |
| Jerzynska et al. [97] | RCT | 5 months | 50 children (aged 5 to 12 years) with grass-related moderate-to-severe rhinoconjunctivitis; eight patients had concomitant asthma (25/25) | Participants were randomly divided into two groups: - The control group received SLIT with a placebo - The experimental group received SLIT with vitamin D 1000 IU daily supplementation | The experimental group shows a reduction of nasal symptoms (p = 0.04), asthma symptoms (p = 0.001), and combined symptom-medication score (p = 0.001); there was no significant difference between groups in medication and ocular scores. |
| VITAMIN D AND ATOPIC DERMATITIS (AD) | |||||
| Kim et al. [46] | MT (11 studies: 7 observational trials and 4 RCTs) | In observational trials: N/A; in clinical trials: from 60 days to 2 months | In observation trials, 1643 children and adults with AD or healthy subjects (986/657); in clinical trials, 194 children and adults with AD (104/90) | In observational trials: N/A; in clinical trials: from cholecalciferol or ergocalciferol 1000 IU daily to cholecalciferol 1600 IU daily vs. placebo | In observational trials, the AD group had lower serum 25(OH)D levels compared to the healthy group (statistically significant in the pediatric AD patients, not statistically significant in the adult AD group); in clinical trials, the SCORAD index or EASI score decreased significantly (mean difference: −5.85 points) in the vitamin D group. |
| Hattangdi-Haridas et al. [103] | MT (16 studies: 11 observational studies and five interventional studies, including RCT, not RCT, clinical intervention, and audit trials) | In observational trials: N/A; in interventional trials: from 60 days to 3 months | In observational trials: adults and children or adults only or children only with AD or healthy subjects (1221/982); in interventional trials: adults and children with AD (165/170) | In observational trials: N/A; in interventional trials: cholecalciferol 1000 IU/daily or 1600 IU/daily or 2000 IU/daily | In observational trials, the AD group had a statistically significantly lower vitamin D concentration compared to the healthy group (p = 0.02); in interventional trials, for the repeated measures interventions, the SCORAD index decreased significantly (mean difference: 21 points; p < 0.0001) in vitamin D group; for the randomized control trials, the SCORAD index reduced significantly (mean difference: 11 points; p < 0.0001) in the vitamin D group. |
| Huang et al. [105] | SR (21 articles: 4 RCTs, 5 cohort studies, 6 case-control studies, 6cCross-sectional studies; vitamin D supplementation was investigated in 6 studies: 4 RCTs and 2 cohort studies) | From 4 to 12.86 weeks | A total of 354 patients with an average age of 6.79 years; participants involved with an RCT are 227 (114/113). | Vitamin D doses ranged from 1000 to 2000 IU/daily; cholecalciferol was used in 2 articles, while 2 studies were supplemented with ergocalciferol, and the remaining 2 studies did not specify what type of vitamin D was used. | Significant improvement of AD when supplemented with vitamin D was found in 67% (4/6) of studies. |
| Nielsen et al. [107] | MT (12 RCTs) | From 4 to 12 weeks | 686 patients with AD (347/339) | From 1000 to 5000 IU/daily and 8000 to 60,000 IU/weekly; cholecalciferol was predominantly used, except for one study that utilized ergocalciferol. | Significant improvement in AD symptoms following vitamin D supplementation was found in 27% (3/11) of studies in the intervention group compared with the control group. |
| VITAMIN D AND RHEUMATOID ARTHRITIS (RA) | |||||
| Andjelkovic et al. [301] | RCT | 3 months | 19 patients treated with standard DMARD therapy for acute RA | Oral alfacalcidol (2 micrograms/day) | Positive effect on disease activity in 89% of the patients: - 45% or nine patients with complete remission - 44% or eight patients with a satisfactory effect - 11% no improvement, but no new symptoms occurred |
| Scharla et al. [121] | RCT | 4 weeks | 71 inpatients with RA and osteopenia | Patients were randomly assigned to: - Group 1 treated with 1000 IU plain vitamin D + 500 mg calcium daily - Group 2 treated with 1 µg of alfacalcidol + 500 mg calcium daily | In patients with RA, alfacalcidol, but not plain vitamin D, improved calcium and bone metabolism, modulated inflammation, improved muscular function, and decreased pain symptoms. |
| Forsblad et al. [122] | RCT | 2 years | 88 women with RA aged between 45 and 65 years | All patients were treated with a daily dose of 400 IU vitamin D; - 41 women were randomized to receive HRT (oestradiol and noretisterone acetate), vitamin D and calcium - 47 to the control group receiving vitamin D and calcium alone | There are no differences between the HRT and control groups concerning the concentration of Ig in serum and to B- and T-cell-dependent recall antigen reactions. Thus, HRT did not stimulate nor suppress these immunological responses. |
| VITAMIN D AND PSORIASIS | |||||
| Pinter et al. [146] | MT (2 Phase 3 RCTs) | 8 weeks | 1271 patients: 783 male and 488 female subjects above the age of 18 years with mild-to-moderate psoriasis according to PGA and with a treatment area of 2–30% of the body (trunk and/or limbs), with a PASI as a baseline of at least of 2 or 3 | Subjects were randomly allocated to one of three arms: - 551 for CAL/BDP PAD-cream; - 178 for cream vehicle; - 542 for active comparator [a marketed CAL/BDP gel/topical suspension. The number of subjects who completed the trial was, 526 (for CAL/BDP PAD cream), 149 (for cream vehicle), and 513 (for active comparator) respectively. | CAL/BDP PAD cream demonstrated superiority after 8 weeks compared to CAL/BDP TS for all efficacy endpoints, including PGA treatment success, mPASI, and PASI75. Superiority was also demonstrated for patient-reported DLQI and PTCS. |
| Heim et al. [147] | RCT | 4 weeks | 30 patients (18 male and 12 female subjects) with mild psoriasis defined by a PASI score below 10 and at least two symmetric lesions of 2 cm2 affecting the knees or the elbows. | Subjects were randomly allocated into three groups of ten. All patients applied calcipotriol/betamethasone foam to their elbows on one side of the body. Three groups of 10 patients applied either placebo foam (emollient), betamethasone foam or clobetasol propionate ointment to elbows on the opposite side of their body. The side of the treatment allocation was randomly assigned using central randomization. | All treatments, including placebo, provided a significant decrease in tPASI. The treatment with calcipotriol/betamethasone foam was the most effective in decreasing CD8+ cell infiltrate in both the dermis and epidermis after 4 weeks of treatment. They noticed that CD8+ IFNγ+ T cells decreased significantly after both betamethasone and combined calcipotriol/betamethasone treatment but also that calcipotriol/betamethasone significantly reduced the number of MPO+ neutrophils which were predominantly IL-17+ |
| Venegas-Iribarren et al. [150] | MT (eight SRs including 26 studies overall, of which 22 were RCTs) | N/A | 4238 adult patients (15 to 90 years) with plaque psoriasis in the trunk and limbs, not including the scalp and with low, moderate, or severe disease. | All trials used topical corticosteroids as intervention (fluocinonide 0.05% twice a day, betamethasone dipropionate 0.05% once or twice a day, betamethasone 17-valerate 0.1% once or twice a day, desoxymethasone 0.25% twice a day, clobetasol propionate 0.05% twice a day, and diflorasone diacetate 0.05% twice a day). As a comparison, topical treatment with calcipotriol 50 mcg/g once or twice a day, calcitriol 3 mcg/g twice a day, and tacalcitol 4 mcg/g once a day. | There might be little or no difference in PASI, IAGI, and PAGI scores between topical corticosteroids and topical vitamin D analogues. The certainty of the evidence is low. Topical corticosteroids lead to fewer local adverse events (skin irritation) and fewer withdrawals than topical vitamin D analogues. With a high certainty of the evidence. No studies were found that evaluated the impact of topical corticosteroids and topical vitamin D analogues in cutaneous atrophy. |
| VITAMIN D AND SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) | |||||
| Andreoli et al. [163] | RCT | 24 months | 34 patients (18/16) | Participants were randomly assigned to: - Group 1: Standard Regimen (SR) = 25,000 IU/month- Group 2: Intensive Regimen (IR) = 300,000 IU bolus + 50,000 IU/month for one year and then switched to the other regimen in the second year. | After 12 months, values above 30 ng/mL were found in 75% of Intensive Regimen patients, while only 28% were in the Standard Regimen. No significant differences in disease activity and SLE serology were found between SR and IR at any time. No changes in the mineral metabolism were observed. |
| Lima et al. [167] | RCT | 24 weeks | 40 patients with juvenile-onset SLE (20/20) | Participants were randomly assigned to: - Group 1: cholecalciferol 50,000 IU/week - Group 2: placebo | SLEDAI score and ECLAM significantly improved (p = 0.011, p = 0.006) Fatigue score and K-FSS significantly improved (p = 0.012, p = 0.008) No significant improvements in cutaneous and articular manifestations and proteinuria (p = 0.66, p = 0.18, p = 0.32). |
| Karimzadeh et al. [164] | RCT | 3 months | 90 patients (with levels of 25(OH)D < 30 ng/mL) (45/45) | Participants were randomly assigned to: - Interventional group patients: vitamin D3 soft gel capsules (50,000 IU/weekly for 12 weeks and then 50,000 IU/monthly for 3 months) - Placebo group: placebo | The mean of vitamin D levels significantly increased in the interventional group (p < 0.001) Not significantly different in SLEDAI score before and after vitamin D administration (p = 0.39). |
| Singgih Wahono et al. [165] | RCT | 14 months | 40 patients | Participants were randomly assigned to: - Group I: 3 × cholecalciferol 400 IU and 3 × 1 tablet placebo - Group II: 3 × cholecalciferol 400 IU and curcumin (Curcuma xanthorrhiza) 3 × 20 mg | Supplementation of cholecalciferol 1200 IU either with placebo (p = 0.000) or with curcumin (p = 0.003) significantly increased serum 25(OH)D levels. Serum vitamin D levels differed significantly, with higher levels in group I (p = 0.047). The delta of vitamin D levels (the difference between vitamin D levels after supplementation and before supplementation) did not differ significantly between the two groups (p = 0.166). SLEDAI scores significantly decreased in both groups (Group I: p = 0.001); Group II: p = 0.003) Serum IL-6 levels significantly decreased in both groups (Group I: p = 0.001); Group II: p = 0.013). |
| Pakchotanon et al. [166] | RCT | 24 weeks | 104 patients (52/52) | Participants were randomly assigned to: - Group A: ergocalciferol 100,000 IU weekly for 4 weeks, followed by ergocalciferol 40,000 IU weekly for 20 weeks, - Group B: placebo | The mean standard deviation of serum levels of vitamin D in group A was significantly higher than in group B (p < 0.001). There is no difference between groups A and B concerning SLEDAI-2K, flare events, ESR, CRP, and dosage of immunosuppressive drugs. |
| VITAMIN D AND AUTOIMMUNE THYROID DISEASES | |||||
| Zhang et al. [302] | MT (8 RCTs) | From 4 to 24 weeks | 652 patients with HT (332/320) | Participants were randomly assigned to: - Vitamin D (in different formulations and dosages) ± LT4 - LT4/Sunshine and diet/placebo | Vitamin D supplementation: - Significantly decrease TPO-Ab levels in the subgroups in whom the treatment duration > 3 months (p = 0.009) and in the subgroups treated with vitamin D3 (p = 0.006) - Was effective in reducing the Tg-Ab levels (p = 0.009) (due to limited data, no subgroup analysis and meta- regression analysis were performed). |
| Wang S. et al. [177] | MT (6 RCTs) | From 1 to 6 months | 344 patients with AIT (178/166) | Participants were randomly assigned to: - Vitamin D (in different formulations and dosages) ± LT4/elemental calcium 500 mg/day - LT4/elemental calcium 500 mg/day/placebo/no treatment | Vitamin D supplementation significantly decrease TPO-Ab levels at six months (3 RCT, p < 0.01) and significantly decrease Tg-Ab levels (4 RCT, p = 0.033; even if a significant heterogeneity was found) No significant effect on changes in TSH, FT3, or FT4 after vitamin D supplementation. |
| Tang et al. [178] | MT (12 RCTs) | From 12 to 24 weeks | 862 patients with HT (429/423) | Participants were randomly assigned to: - Vitamin D (in different formulations and dosages) ± LT4 - LT4/placebo/no treatment | Vitamin D supplementation: - Significantly decrease TPO-Ab levels (p = 0.03) - Significantly increase fT3 and fT4 levels (p < 0.001) at 12 weeks - Significantly decrease Tg-Ab levels (p < 0.001; even if a significant heterogeneity was found) -Significantly decreases TSH levels |
| VITAMIN D AND TYPE 1 AND TYPE 2 DIABETES MELLITUS | |||||
| Gregoriou et al. [303] | MT (7 RCTs) | From 4 to 52 weeks | 287 patients with T1DM | Participants were randomly assigned to: - Calcitriol 0.25 μg per day or on alternate days plus insulin - Alphacalcidol 0.5 μg daily plus insulin cholecalciferol 2000 IU per day plus insulin for 18 mo - Cholecalciferol 70 IU/kg body weight/day plus insulin | Vitamin D supplementation in the form of alphacalcidol and chole-calciferol appears to be beneficial in DID, FCP, SCP, and HbA1c. |
| Najjar et al. [304] | MT (10 studies: 3 cohort; 5 case–control; 2 matched case–control) | N/A | 39,884 patients with T1DM (16,370/23,514) | N/A | No large effect of a genetically determined reduction in 25(OH)D concentrations by selected polymorphisms on T1DM risk. |
| Nascimento et al. 2022 [305] | RS (10 studies) | From 6 to 52 weeks | Children and adolescents (0–19 years) with T1DM | - Cholecalciferol with dosages ranging from 1000 to 160,000 IU. - Just one study used vitamin D in the form of alfacalcidol at a dosage of 0.25 to 0.5 μg/day | This study did not provide evidence to support the effect of vitamin D supplementation on glycemic control to aid in the treatment of T1DM. |
| Yu et al. [306] | RS (13 studies: 9 RCTs; 2 open-label case–control, 1 open label, and 1 cohort | From 4 to 12 weeks | 527 patients with T1DM | The following therapeutic regimens were used: 1.25 D 0.25 μg twice daily; 25D 2000 IUdaily; 25D to achieve serum 25 D > 125 nmol/L; alfacalcidol 0.25 μg BD 25 D; 60,000 IU monthly; Ergocalciferol (D2) 2 m of 50,000 IU/w; 25D 2000 IU/d; 25D 3000 IU/d; Calciferol 2000 IU/d + etanercept + GAD-alum | The maintenance of optimal circulating 25 D levels may reduce the risk of T1DM, and that may have potential for benefits in delaying the development of absolute or near-absolute C-peptide deficiency. |
| Hu et al. [196] | MT (19 RCTs) | From 4 to 24 weeks | 1374 patients with T2DM (747/627) | - Up to 50,000 UI/weekly vitamin D3 - 300,000 UI single injection of vitamin D3 | Significant reduction in HbA1c, IR (marked by a decrease in HOMA-IR) and insulin levels in the short-term vitamin D supplementation group. |
| Krul-Poel et al. [219] | MT (23 RCTs) | From 4 to 52 weeks | 1797 patients with T2DM: for the effect on HbA1c, 1475 patients (755/720); for the effect on FBG 1180 patients (608/572) | - From 1000 IU/day vitamin D3 to 45,000 IU/week - Vitamina D3 or 1200 IU/day - Vitamin D3 for 2 weeks followed by 5600 IU/day for 10 weeks or from 100,000 to 300,000 IU - Vitamin D3 single dose | Significant effect on FBG in a subgroup of studies (n = 4); no significant effect on change in HbA1c. |
| VITAMIN D AND INFECTIOUS DISEASES | |||||
| Bergman et al. [254] | RCT | 12 months | 140 patients with antibody deficiency disorder and increased susceptibility to RTIs (70/70) | Participants were randomly assigned to: - Group 1: cholecalciferol 4000 UI daily for 12 months - Group 2: placebo | The overall infectious score was significantly reduced for patients allocated to the vitamin D group compared with the placebo group. |
| Lehouck et al. [256] | RCT | 12 months | 182 patients with moderate to very severe COPD and a history of recent exacerbations (91/91) | Participants were randomly assigned to: - Group 1: 100,000 IU of vitamin D supplementation every 4 weeks for 1 year - Group 2: placebo | High-dose vitamin D supplementation in a sample of patients with COPD did not reduce the incidence of exacerbations. In participants with severe vitamin D deficiency at baseline, supplementation may reduce exacerbations. (rate ratio, 0.57 [CI, 0.33 to 0.98]; p = 0.042). |
| Slow et al. [255] | RCT | 3 years | 135 patients were admitted to the hospital with CAP (mean baseline plasma 25(OH)D concentration was 48.7 ± 21.6 (SD) nmol/L) (68/67) | Participants were randomly assigned to: - Group 1: An oral single dose of vitamin D 20,000 UI - Group 2: placebo | Vitamin D supplementation had a significantly greater effect on those with low baseline vitamin D (<50 nmol/L) in improving the radiological resolution of pneumonia. |
| Miroliaee et al. [260] | RCT | 10 months | 51 patients with VAP who were suffering from vitamin D deficiency (26/25) | Participants were randomly assigned to: - Group 1: received 300,000 units of intramuscular vitamin D. - Group 2: placebo | The levels of PCT were significantly decreased (p = 0.001) in the treatment group (–0.02 ± 0.59 ng/mL) compared to that of the placebo group (0.68 ± 1.03 ng/mL), no significant changes in SOFA and CPIS scores (p = 0.37 and p = 0.46, respectively). |
| Arpadi et al. [267] | RCT | 12 months | 56 children with HIV and vitamin D levels insufficiency (29/27) | Participants were randomly assigned to: - Group 1: HIV-infected children and adolescents who were aged 6 to 16 years receive vitamin D (100,000 IU bimonthly) and calcium - Group 2: double placebo | No group differences were seen in the change in CD4 count or CD4% or viral load. The overall mean monthly serum 25(OH)D concentrations were higher in the group that received vitamin D. |
| Hosseini et al. [261] | RCT | 16 weeks | 34 patients (healthcare workers as a primary prevention of SARS-CoV-2 infection) (19/15) | Participants were randomly assigned to: - Group 1: 100,000 IU vitamin D oral bolus at randomization followed by a weekly dose of 10,000 IU of vitamin D3 - Group 2: placebo | The groups which received vitamin D supplementation (95% CI) were in favor of supplementation; 77.8% of intervention, and 13.3% of control, patients were vitamin Dsufficient (OR:6.11, 95% CI:1.6, 22.9). |
| Anitua et al. [257] | MT (65 RCTs) | From 5 days to 3 years | 50,554 participants | All trials used vitamin D as intervention as dose ranging from 400 to 1000 IU/day | The incidence of RTIs in terms of count data (OR: 0.87; 95%CI [0.80–0.95]; p = 0.0028; I2 = 43%) and event rate (IRR: 0.81; 95%CI [0.70–0.95]; p = 0.010; I2 = 79%) was significantly reduced in the intervention group. However, no effect of vitamin D on duration or upper RTIs severity was observed. |
| Petrelli et al. [259] | MT (43 observational trials) | N/A | 612,601 patients | All trials used an intervention group with vitamin D supplementation (from 1000 UI daily to 80,000/100,000 IU every 2–3 months or 80,000 IU within a few hours of the diagnosis of COVID-19). | Risk of COVID-19 infection was higher compared to those with replete values (OR = 1.26; 95% CI, 1.19–1.34; p < 0.01). Vitamin D deficiency was also associated with worse severity and higher mortality than in non-deficient patients (OR = 2.6; 95% CI, 1.84–3.67; p < 0.01 and OR = 1.22; 95% CI, 1.04–1.43; p < 0.01, respectively). |
| Meng et al. [258] | MT (25 RCTs) | From 7 days to 1 year | 8128 participants | Preventing effect group - Group 1: 1949 participants received vitamin D supplementation, with daily dosages ranging from 800 IU to 5000 IU daily or weekly. - Group 2: 3703 participants received a placebo. Regarding the therapeutic effects - Group 1: 1270 participants received vitamin D supplementation - Group 2: 1206 participants did not receive vitamin D supplementation or received low dosage of vitamin D | The studies included in our analysis mainly administered a single-dose, high-dosage vitamin D supplementation (from 800 IU to 5000 IU) therapy for severe SARS-CoV-2 infection patients. Single-dose supplementation was found to reduce the rate of mechanical ventilation, while the multiple-dose therapy was found to reduce the rate of ICU admission and mortality. |
| VITAMIN D AND MULTIPLE SCLEROSIS (MS) | |||||
| McLaughlin et al. [290] | MT (12 interventional studies) | 6 to 22 months | 950 patients | Studies were divided into four groups because of heterogeneity in study design. Three studies only included patients with serum 25(OH)D levels below 50, 75, or 100 nmol/L The effective daily dosage of cholecalciferol (or equivalent ergocalciferol) used ranged from 2857–10,400 IU in the active or high-dose arms. The low-dose arms ranged from 800 to 1000 IU. | Non-significant trends in favor of vitamin D for all outcome measures: high-dose vitamin D has worse outomes in ARR (mean difference 0.15 [95% CI 0.01–0.30]) and non-significant trends of increased EDSS (mean difference −0.12 EDSS points [95% CI −0.78) to 0.01] and gadolinium-enhancing lesions for the higher-dose arms. |
| Jagannath et al. [287] | MT (12 RCTs) | From six months (24/26 weeks) to 12 months (48/52 weeks) | 933 participants | Participants were enrolled in two groups: 464 were randomized to the vitamin D group, and 469 to the comparator group. | Vitamin D has no effect on ARR: the mean in the intervention group was 0.05 lower (−0.17 lower to 0.07 higher); worsening of EDSS: the mean score in the intervention group was 0.25 lower (0.61 lower to 0.10 higher); or new MRI gadolinium-enhancing T1 lesions: in the intervention group was 0.02 higher (0.45 lower to 0.48 higher). |
| Berezowska et al. [291] | MT (10 RCTs) | From 2012 to 2018 | 627 participants | All studies evaluated the use of vitamin D supplementation (ranging from 10 to 98,000 IU), comparing it to a placebo or low-dose vitamin D. The duration of the intervention ranged from 12 to 96 weeks. | One trial demonstrated that vitamin D supplementation resulted in fewer new T2 lesions (a mean of 0.5 compared to a mean of 1.1 in the placebo group). Four of ten studies showed no significant differences between the vitamin D and control groups. One trial found that the EDSS score increased significantly (p < 0.01) in a placebo group from a mean of 1.7 to 1.94, with no significant difference in scores at the end of the trial between intervention and control groups (p > 0.05). |
| Etemadifar et al. [293] | RCT | 12 to 16 weeks of gestation till delivery | 15 participants | Participants were randomly allocated into two groups: - Group 1: 50,000 IU/week vitamin D - Group 2: routine care from 12 to 16 weeks of gestation till delivery | Average serum 25(OH)D level at the end of the trial in vitamin D supplemented group was higher than routine care group (33.7 ng/mL vs. 14.6 ng/mL, p < 0.050). In the vitamin D group, the mean EDSS did not change 6 months after delivery (p > 0.050), whereas in the routine care group, the mean EDSS increased from 1.3 (0.4) to 1.7 (0.6) (p < 0.070). |
| Thouvenot et al. [294] | RCT | From July 2013 to December 2020 (final follow-up on January 2023) | 316 participants | Patients were randomized 1:1 to receive oral cholecalciferol 100,000 IU every 2 weeks for 24 months: - Group 1: 163 participants - Group 2 placebo: 153 participants | In group 1 vs. the placebo group: MRI activity (89 patients [57.1%] vs. 96 patients [65.3%]; HR, 0.71 [95% CI, 0.53–0.95]; p = 0.02), new lesions (72 patients [46.2%] vs. 87 patients [59.2%]; HR, 0.61 [95% CI, 0.44–0.84]; p = 0.003), and contrast-enhancing lesions (29 patients [18.6%] vs. 50 patients [34.0%]; HR, 0.47 [95% CI, 0.30–0.75]; p = 0.001). The vitamin D oral supplementation significantly reduces the disease activity in CIS and relapsing-remitting MS. |
Funding
Conflicts of Interest
References
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Holick, M.F.; Mazzei, L.; García Menéndez, S.; Martín Giménez, V.M.; Al Anouti, F.; Manucha, W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 2023, 15, 767. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in Inflammatory Diseases. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Alswailmi, F.K.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular Mechanisms of Vitamin D-Mediated Immunomodulation. Galen Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Coussens, A.K. Immunomodulatory Actions of Vitamin D Metabolites and Their Potential Relevance to Human Lung Disease. Curr. Respir. Med. Rev. 2011, 7, 444–453. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Non-Musculoskeletal Benefits of Vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef]
- Maddaloni, E.; Cavallari, I.; Napoli, N.; Conte, C. Vitamin D and Diabetes Mellitus. In Frontiers of Hormone Research; Giustina, A., Bilezikian, J.P., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 50, pp. 161–176. ISBN 978-3-318-06338-7. [Google Scholar]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and Type 2 Diabetes: A Systematic Review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Yan, H.; Chen, J.; Wang, Y.; Mo, X. Association of Serum Total 25-Hydroxy-Vitamin D Concentration and Risk of All-Cause, Cardiovascular and Malignancies-Specific Mortality in Patients with Hyperlipidemia in the United States. Front. Nutr. 2022, 9, 971720. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between Serum 25-Hydroxyvitamin D3 Concentrations and Liver Histology in Patients with Non-Alcoholic Fatty Liver Disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef]
- Khademi, Z.; Hamedi-Shahraki, S.; Amirkhizi, F. Vitamin D Insufficiency Is Associated with Inflammation and Deregulation of Adipokines in Patients with Metabolic Syndrome. BMC Endocr. Disord. 2022, 22, 223. [Google Scholar] [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Teleni, L.; Baker, J.; Koczwara, B.; Kimlin, M.G.; Walpole, E.; Tsai, K.; Isenring, E.A. Clinical Outcomes of Vitamin D Deficiency and Supplementation in Cancer Patients. Nutr. Rev. 2013, 71, 611–621. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Lo Monaco, M.; Scibetta, S.; Natoli, G.; Cavezzi, A.; Troiani, E.; Corrao, S. An Overview of Systematic Reviews of the Role of Vitamin D on Inflammation in Patients with Diabetes and the Potentiality of Its Application on Diabetic Patients with COVID-19. Int. J. Mol. Sci. 2022, 23, 2873. [Google Scholar] [CrossRef]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mallaci Bocchio, R.; Natoli, G.; Scibetta, S.; Lo Monaco, M.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals 2023, 16, 130. [Google Scholar] [CrossRef]
- Yu, X.P.; Bellido, T.; Manolagas, S.C. Down-Regulation of NF-Kappa B Protein Levels in Activated Human Lymphocytes by 1,25-Dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1995, 92, 10990–10994. [Google Scholar] [CrossRef] [PubMed]
- Barragan, M.; Good, M.; Kolls, J. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. In Current Trends in Immunity and Respiratory Infections; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1108, pp. 13–23. ISBN 978-3-030-01634-0. [Google Scholar]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Corrao, S.; Colomba, D.; Arnone, S.; Argano, C.; Di Chiara, T.; Scaglione, R.; Licata, G. Improving Efficacy of PubMed Clinical Queries for Retrieving Scientifically Strong Studies on Treatment. J. Am. Med. Inform. Assoc. 2006, 13, 485–487. [Google Scholar] [CrossRef]
- Corrao, S.; Colomba, D.; Argano, C.; Calvo, L.; Scaglione, R.; Licata, G. Optimized Search Strategy for Detecting Scientifically Strong Studies on Treatment through PubMed. Intern. Emerg. Med. 2012, 7, 283–287. [Google Scholar] [CrossRef]
- Haynes, R.B.; McKibbon, K.A.; Wilczynski, N.L.; Walter, S.D.; Werre, S.R. Hedges Team Optimal Search Strategies for Retrieving Scientifically Strong Studies of Treatment from Medline: Analytical Survey. BMJ 2005, 330, 1179. [Google Scholar] [CrossRef]
- Al-Zohily, B.; Al-Menhali, A.; Gariballa, S.; Haq, A.; Shah, I. Epimers of Vitamin D: A Review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin D-Directed Rheostatic Regulation of Monocyte Antibacterial Responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef]
- Kroner, J.; Sommer, A.; Fabri, M. Vitamin D Every Day to Keep the Infection Away? Nutrients 2015, 7, 4170–4188. [Google Scholar] [CrossRef]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human Cathelicidin Antimicrobial Peptide (CAMP) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly up-Regulated in Myeloid Cells by 1,25-Dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.-M.; Jo, E.-K. Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages via Cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
- Dombrowski, Y.; Peric, M.; Koglin, S.; Ruzicka, T.; Schauber, J. Control of Cutaneous Antimicrobial Peptides by Vitamin D3. Arch. Dermatol. Res. 2010, 302, 401–408. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Salameh, L.; Mahmood, W.; Hamoudi, R.; Almazrouei, K.; Lochanan, M.; Seyhoglu, S.; Mahboub, B. The Role of Vitamin D Supplementation on Airway Remodeling in Asthma: A Systematic Review. Nutrients 2023, 15, 2477. [Google Scholar] [CrossRef]
- Iqbal, S.F.; Freishtat, R.J. Mechanism of Action of Vitamin D in the Asthmatic Lung. J. Investig. Med. 2011, 59, 1200–1202. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Mann, E.H.; Hornsby, E.; Chambers, E.S.; Chen, Y.-H.; Rice, L.; Hawrylowicz, C.M. Vitamin D Influences Asthmatic Pathology through Its Action on Diverse Immunological Pathways. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. S5), S314–S321. [Google Scholar] [CrossRef]
- Galowitz, S.; Chang, C. Immunobiology of Critical Pediatric Asthma. Clin. Rev. Allerg. Immunol. 2015, 48, 84–96. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Sundar, I.K.; Rahman, I. Vitamin D and Susceptibility of Chronic Lung Diseases: Role of Epigenetics. Front. Pharmacol. 2011, 2, 50. [Google Scholar] [CrossRef]
- Gois, P.; Ferreira, D.; Olenski, S.; Seguro, A. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An Update on the Association of Vitamin D Deficiency with Common Infectious Diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.-N.; Lee, Y.; Choe, Y.; Ahn, K. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A Helpful Immuno-Modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- He, L.; Zhou, X.; Mo, H.; Li, X.; Guo, S. The Association between Vitamin D Receptor Gene Polymorphisms and Asthma: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2022, 11, 574–587. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D(3) Inhibits Dendritic Cell Differentiation and Maturation In Vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Flayer, C.H.; Larson, E.D.; Haczku, A. Breaking Steroid Resistance: Effect of Vitamin D on IL-23. Am. J. Respir. Cell Mol. Biol. 2017, 57, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Shimizu, T.; Tada, Y.; Kamata, M.; Takeoka, S.; Shibata, S.; Mitsui, A.; Asano, Y.; Sugaya, M.; Kadono, T.; et al. The Vitamin D3 Analog, Maxacalcitol, Reduces Psoriasiform Skin Inflammation by Inducing Regulatory T Cells and Downregulating IL-23 and IL-17 Production. J. Dermatol. Sci. 2018, 92, 117–126. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Sun, D.; Luo, F.; Xing, J.; Zhang, F.; Xu, J.; Zhang, Z. 1,25(OH)2 D3 Inhibited Th17 Cells Differentiation via Regulating the NF-κB Activity and Expression of IL-17. Cell Prolif. 2018, 51, e12461. [Google Scholar] [CrossRef]
- Ikeda, U.; Wakita, D.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Iwakura, Y.; Nishimura, T. 1α,25-Dihydroxyvitamin D3 and All-Trans Retinoic Acid Synergistically Inhibit the Differentiation and Expansion of Th17 Cells. Immunol. Lett. 2010, 134, 7–16. [Google Scholar] [CrossRef]
- Joshi, S.; Pantalena, L.-C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-Dihydroxyvitamin D3 Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; Ferrantelli, E.; Meinster, E.; Pouw, S.M.; Kovačević, I.; De Menezes, R.X.; Niessen, H.W.; Beelen, R.H.J.; Hordijk, P.L.; Vervloet, M.G. Vitamin D Attenuates Endothelial Dysfunction in Uremic Rats and Maintains Human Endothelial Stability. J. Am. Heart Assoc. 2018, 7, e008776. [Google Scholar] [CrossRef]
- Nativel, B.; Ramin-Mangata, S.; Mevizou, R.; Figuester, A.; Andries, J.; Iwema, T.; Ikewaki, N.; Gasque, P.; Viranaïcken, W. CD 93 Is a Cell Surface Lectin Receptor Involved in the Control of the Inflammatory Response Stimulated by Exogenous DNA. Immunology 2019, 158, 85–93. [Google Scholar] [CrossRef]
- Jennewein, C.; Sowa, R.; Faber, A.C.; Dildey, M.; Von Knethen, A.; Meybohm, P.; Scheller, B.; Dröse, S.; Zacharowski, K. Contribution of Ninjurin1 to Toll-Like Receptor 4 Signaling and Systemic Inflammation. Am. J. Respir. Cell Mol. Biol. 2015, 53, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Kolseth, I.B.M.; Reine, T.M.; Vuong, T.T.; Meen, A.J.; Fan, Q.; Jenssen, T.G.; Grønning-Wang, L.M.; Kolset, S.O. Serglycin Is Part of the Secretory Repertoire of LPS-activated Monocytes. Immunity Inflam. Dis. 2015, 3, 23–31. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Grijincu, M.; Buzan, M.-R.; Zbîrcea, L.-E.; Păunescu, V.; Panaitescu, C. Prenatal Factors in the Development of Allergic Diseases. Int. J. Mol. Sci. 2024, 25, 6359. [Google Scholar] [CrossRef]
- Anogeianaki, A.; Castellani, M.L.; Tripodi, D.; Toniato, E.; De Lutiis, M.A.; Conti, F.; Felaco, P.; Fulcheri, M.; Theoharides, T.C.; Galzio, R.; et al. Vitamins and Mast Cells. Int. J. Immunopathol. Pharmacol. 2010, 23, 991–996. [Google Scholar] [CrossRef]
- Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. [Google Scholar] [CrossRef]
- Ladeira, J.M.C.D.; Zacas, O.; Ferreira, A.M.; Gomes Stegun, P.C.; Grotta, M.B.; Toro, A.A.D.C. The Role of Vitamin D in the Severity and Control of Asthma in Children and Adolescents: A Protocol for Systematic Review and Meta-Analysis. Medicine 2022, 101, e31457. [Google Scholar] [CrossRef]
- Sansoni, E.R.; Sautter, N.B.; Mace, J.C.; Smith, T.L.; Yawn, J.R.; Lawrence, L.A.; Schlosser, R.J.; Soler, Z.M.; Mulligan, J.K. Vitamin D3 as a Novel Regulator of Basic Fibroblast Growth Factor in Chronic Rhinosinusitis with Nasal Polyposis. Int. Forum Allergy Rhinol. 2015, 5, 191–196. [Google Scholar] [CrossRef]
- Confino-Cohen, R.; Brufman, I.; Goldberg, A.; Feldman, B.S. Vitamin D, Asthma Prevalence and Asthma Exacerbations: A Large Adult Population-Based Study. Allergy 2014, 69, 1673–1680. [Google Scholar] [CrossRef]
- Cassim, R.; Russell, M.A.; Lodge, C.J.; Lowe, A.J.; Koplin, J.J.; Dharmage, S.C. The Role of Circulating 25 Hydroxyvitamin D in Asthma: A Systematic Review. Allergy 2015, 70, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.A.; Abbas, A.; Viquar, W.; Munawar Hussain, A. Association of Vitamin D Levels and Asthma Exacerbations in Children and Adolescents: Experience from a Tertiary Care Center. Monaldi Arch. Chest Dis. 2022, 93. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, V.; Singh, J.; Jain, H.; Paras, P.; Kaur, N.; Sareen, A.K. Assessment of the Relation Between Asthma Severity and Serum Vitamin D Levels: A Cross-Sectional Study. Cureus 2023, 15, e46826. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Pawliczak, R. Relationship between Vitamin D and Asthma from Gestational to Adulthood Period: A Meta-Analysis of Randomized Clinical Trials. BMC Pulm. Med. 2023, 23, 212. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal Vitamin D Supplementation Reduces Risk of Asthma/Recurrent Wheeze in Early Childhood: A Combined Analysis of Two Randomized Controlled Trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Shi, D.; Wang, D.; Meng, Y.; Chen, J.; Mu, G.; Chen, W. Maternal Vitamin D Intake during Pregnancy and Risk of Asthma and Wheeze in Children: A Systematic Review and Meta-Analysis of Observational Studies. J. Matern.-Fetal Neonatal Med. 2021, 34, 653–659. [Google Scholar] [CrossRef]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In Utero Exposure to 25-Hydroxyvitamin D and Risk of Childhood Asthma, Wheeze, and Respiratory Tract Infections: A Meta-Analysis of Birth Cohort Studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Chen, L.; Zhang, H.; Yu, L.; Chi, Y.; Chen, M.; Cai, Y. Efficacy of Vitamin D Supplementation on COPD and Asthma Control: A Systematic Review and Meta-Analysis. J. Glob. Health 2022, 12, 04100. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, Q.; Zhu, W.; Chen, J. Vitamin D and Asthma Occurrence in Children: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2022, 62, e60–e68. [Google Scholar] [CrossRef]
- Song, H.; Yang, L.; Jia, C. Maternal Vitamin D Status during Pregnancy and Risk of Childhood Asthma: A Meta-analysis of Prospective Studies. Mol. Nutr. Food Res. 2017, 61, 1600657. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.-Q.; Yin, J.; Yao, J.; Shen, J.; Sheng, G.-J.; Li, K.; Lv, H.-F.; Fang, X.; Wu, W.-F. Meta-Analysis of Vitamin D and Lung Function in Patients with Asthma. Respir. Res. 2019, 20, 161. [Google Scholar] [CrossRef]
- Alnori, H.; Alassaf, F.A.; Alfahad, M.; Qazzaz, M.E.; Jasim, M.; Abed, M.N. Vitamin D and Immunoglobulin E Status in Allergic Rhinitis Patients Compared to Healthy People. J. Med. Life 2020, 13, 463–468. [Google Scholar] [CrossRef]
- Frieri, M. Asthma Linked with Rhinosinusitis: An Extensive Review. Allergy Rhinol. 2014, 5, ar.2014.5.0083. [Google Scholar] [CrossRef]
- Stokes, P.J.; Rimmer, J. The Relationship between Serum Vitamin D and Chronic Rhinosinusitis: A Systematic Review. Am. J. Rhinol. Allergy 2016, 30, 23–28. [Google Scholar] [CrossRef]
- Aryan, Z.; Rezaei, N.; Camargo, C.A. Vitamin D Status, Aeroallergen Sensitization, and Allergic Rhinitis: A Systematic Review and Meta-Analysis. Int. Rev. Immunol. 2017, 36, 41–53. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Berraïes, A.; Hamdi, B.; Kaabachi, W.; Ammar, J.; Hamzaoui, K. Vitamin D Reduces the Differentiation and Expansion of Th17 Cells in Young Asthmatic Children. Immunobiology 2014, 219, 873–879. [Google Scholar] [CrossRef]
- Fedora, K.; Setyoningrum, R.A.; Aina, Q.; Rosyidah, L.N.; Ni’mah, N.L.; Titiharja, F.F. Vitamin D Supplementation Decrease Asthma Exacerbations in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Med. 2024, 56, 2400313. [Google Scholar] [CrossRef]
- El Abd, A.; Dasari, H.; Dodin, P.; Trottier, H.; Ducharme, F.M. The Effects of Vitamin D Supplementation on Inflammatory Biomarkers in Patients with Asthma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Immunol. 2024, 15, 1335968. [Google Scholar] [CrossRef]
- Mahboub, B.; Al Heialy, S.; Hachim, M.Y.; Ramakrishnan, R.K.; Alzaabi, A.; Seliem, R.M.; Salameh, L.I.; Toor, S.M.; Shendi, F.S.; Al Ali, O.M.; et al. Vitamin D Regulates the Expression of Glucocorticoid Receptors in Blood of Severe Asthmatic Patients. J. Immunol. Res. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D Supplementation to Prevent Asthma Exacerbations: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of Vitamin D3 on Asthma Treatment Failures in Adults With Symptomatic Asthma and Lower Vitamin D Levels: The VIDA Randomized Clinical Trial. JAMA 2014, 311, 2083. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, D.; Ren, Q.; Dong, B.; Zhao, F.; Sun, Y. Association of Vitamin D Receptor Gene Polymorphisms with Susceptibility to Childhood Asthma: A Meta-analysis. Pediatr. Pulmonol. 2017, 52, 423–429. [Google Scholar] [CrossRef]
- Han, J.-C.; Du, J.; Zhang, Y.-J.; Qi, G.-B.; Li, H.-B.; Zhang, Y.J.; Yu, X. Vitamin D Receptor Polymorphisms May Contribute to Asthma Risk. J. Asthma 2016, 53, 790–800. [Google Scholar] [CrossRef]
- Makoui, M.H.; Imani, D.; Motallebnezhad, M.; Azimi, M.; Razi, B. Vitamin D Receptor Gene Polymorphism and Susceptibility to Asthma. Ann. Allergy Asthma Immunol. 2020, 124, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, B.; Singh, J. Efficacy of Vitamin D Supplementation in Allergic Rhinitis. Indian. J. Otolaryngol. Head. Neck Surg. 2021, 73, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Guo, M. Vitamin D Supplementation Improves the Therapeutic Effect of Mometasone on Allergic Rhinitis. Acta Biochim. Pol. 2023. [Google Scholar] [CrossRef]
- Bakhshaee, M.; Sharifian, M.; Esmatinia, F.; Rasoulian, B.; Mohebbi, M. Therapeutic Effect of Vitamin D Supplementation on Allergic Rhinitis. Eur. Arch. Otorhinolaryngol. 2019, 276, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Jerzynska, J.; Stelmach, W.; Rychlik, B.; Lechańska, J.; Podlecka, D.; Stelmach, I. The Clinical Effect of Vitamin D Supplementation Combined with Grass-Specific Sublingual Immunotherapy in Children with Allergic Rhinitis. Allergy Asthma Proc. 2016, 37, 105–114. [Google Scholar] [CrossRef]
- Fenner, J.; Silverberg, N.B. Oral Supplements in Atopic Dermatitis. Clin. Dermatol. 2018, 36, 653–658. [Google Scholar] [CrossRef]
- Hallau, J.; Hamann, L.; Schumann, R.; Worm, M.; Heine, G. A Promoter Polymorphism of the Vitamin D Metabolism Gene Cyp24a1 Is Associated with Severe Atopic Dermatitis in Adults. Acta Derm. Venerol. 2016, 96, 169–172. [Google Scholar] [CrossRef]
- Massoumi, R. The Central Role of Bcl-3 in Atopic Dermatitis. J. Investig. Dermatol. 2009, 129, 2088–2090. [Google Scholar] [CrossRef]
- Barlianto, W.; Wulandari, D.; Sari, T.; Firdayanti, V.; Avandi, M. Vitamin D, Cytokine Profiles, and Disease Severity in Infants with Atopic Dermatitis: A Single Centre, Cross-Sectional Study. Postępy Dermatol. Alergol. 2022, 39, 793–799. [Google Scholar] [CrossRef]
- Nahm, D.-H. Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives. Life 2023, 13, 1674. [Google Scholar] [CrossRef]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, A.N.; Sawitri, S.; Sari, D.W.; Prakoeswa, C.R.S.; Indramaya, D.M.; Damayanti, D.; Zulkarnain, I.; Citrashanty, I.; Widia, Y.; Anggraeni, S. Efficacy of Vitamin D Supplementation on the Severity of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis. F1000Research 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Lara-Corrales, I.; Pope, E. Effects of Vitamin D Levels and Supplementation on Atopic Dermatitis: A Systematic Review. Pediatr. Dermatol. 2018, 35, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, Y.; Huang, H.; Wang, D. Serum Vitamin D Level and Efficacy of Vitamin D Supplementation in Children with Atopic Dermatitis: A Systematic Review and Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 9407888. [Google Scholar] [CrossRef]
- Nielsen, A.Y.; Høj, S.; Thomsen, S.F.; Meteran, H. Vitamin D Supplementation for Treating Atopic Dermatitis in Children and Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4128. [Google Scholar] [CrossRef]
- Lim, J.J.; Liu, M.H.; Chew, F.T. Dietary Interventions in Atopic Dermatitis: A Comprehensive Scoping Review and Analysis. Int. Arch. Allergy Immunol. 2024, 185, 545–589. [Google Scholar] [CrossRef]
- Ishikawa, L.L.W.; Colavite, P.M.; Fraga-Silva, T.F.D.C.; Mimura, L.A.N.; França, T.G.D.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Marcolino, L.D.; Penitenti, M.; Ikoma, M.R.V.; et al. Vitamin D Deficiency and Rheumatoid Arthritis. Clinic Rev. Allerg. Immunol. 2017, 52, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; An, F.; Zhang, J.; Meng, X.; Liu, S.; Xia, R.; Wang, G.; Yan, C. The Mechanism of Dendritic Cell-T Cell Crosstalk in Rheumatoid Arthritis. Arthritis Res. Ther. 2023, 25, 193. [Google Scholar] [CrossRef]
- Hysa, E.; Gotelli, E.; Campitiello, R.; Paolino, S.; Pizzorni, C.; Casabella, A.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and Muscle Status in Inflammatory and Autoimmune Rheumatic Diseases: An Update. Nutrients 2024, 16, 2329. [Google Scholar] [CrossRef]
- Miler, M.; Nikolac Gabaj, N.; Grazio, S.; Vahtarić, A.; Vrtarić, A.; Grubišić, F.; Skala Kavanagh, H.; Doko Vajdić, I.; Vrkić, N. Lower Concentration of Vitamin D Is Associated with Lower DAS28 and VAS-Pain Scores in Patients with Inflammatory Rheumatic Diseases Treated with Infliximab: A Pilot Study. Rheumatol. Int. 2020, 40, 1455–1461. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum Vitamin D Level and Rheumatoid Arthritis Disease Activity: Review and Meta-Analysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.-C. Vitamin D Level in Rheumatoid Arthritis and Its Correlation with the Disease Activity: A Meta-Analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Vitamin D Endocrine System Involvement in Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2011, 11, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.K.; Winstead, M.; Kee, J.D.; Park, J.J.; Zhang, S.; Li, W.; Yi, A.-K.; Stuart, J.M.; Rosloniec, E.F.; Brand, D.D.; et al. 1,25-Dihydroxyvitamin D3 and 20-Hydroxyvitamin D3 Upregulate LAIR-1 and Attenuate Collagen Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 13342. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.H.; Jeffery, L.E.; Soskic, B.; Briggs, Z.; Hou, T.Z.; Raza, K.; Sansom, D.M. 1,25(OH)2D3 Promotes the Efficacy of CD28 Costimulation Blockade by Abatacept. J. Immunol. 2015, 195, 2657–2665. [Google Scholar] [CrossRef]
- Orgaz-Molina, J.; Buendía-Eisman, A.; Arrabal-Polo, M.A.; Ruiz, J.C.; Arias-Santiago, S. Deficiency of Serum Concentration of 25-Hydroxyvitamin D in Psoriatic Patients: A Case-Control Study. J. Am. Acad. Dermatol. 2012, 67, 931–938. [Google Scholar] [CrossRef]
- Bhat, G.H.; Guldin, S.; Khan, M.S.; Yasir, M.; Prasad, G. Vitamin D Status in Psoriasis: Impact and Clinical Correlations. BMC Nutr. 2022, 8, 115. [Google Scholar] [CrossRef]
- Van Hamburg, J.P.; Asmawidjaja, P.S.; Davelaar, N.; Mus, A.M.C.; Cornelissen, F.; Van Leeuwen, J.P.T.M.; Hazes, J.M.W.; Dolhain, R.J.E.M.; Bakx, P.A.G.M.; Colin, E.M.; et al. TNF Blockade Requires 1,25(OH)2D3 to Control Human Th17-Mediated Synovial Inflammation. Ann. Rheum. Dis. 2012, 71, 606–612. [Google Scholar] [CrossRef]
- Scharla, S.; Schmidt-Gayk, H.; Sattar, P. Exclusion of Autonomous 1,25(OH)2Vitamin D3 (Calcitriol) Formation as a Pathogenetic Principle of Inflammation-Mediated Osteopenia (IMO). Acta Endocrinol. 1984, 104, S7. [Google Scholar] [CrossRef]
- Forsblad d′Elia, H.; Carlsten, H. The Impact of Hormone Replacement Therapy on Humoral and Cell-mediated Immune Responses In Vivo in Post-menopausal Women with Rheumatoid Arthritis. Scand. J. Immunol. 2008, 68, 661–667. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging Role of Vitamin D in Autoimmune Diseases: An Update on Evidence and Therapeutic Implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, C.; Paolino, G.; Richetta, A.G.; Calvieri, S. Psoriasis, Vitamin D and the Importance of the Cutaneous Barrier’s Integrity: An Update. J. Dermatol. 2016, 43, 507–514. [Google Scholar] [CrossRef]
- Bittiner, B.; Bleehen, S.S.; MacNeil, S. 1 Alpha,25(OH)2 Vitamin D3 Increases Intracellular Calcium in Human Keratinocytes. Br. J. Dermatol. 1991, 124, 230–235. [Google Scholar] [CrossRef]
- Reichrath, J.; Reichrath, S.; Heyne, K.; Vogt, T.; Roemer, K. Tumor Suppression in Skin and Other Tissues via Cross-Talk between Vitamin D- and P53-Signaling. Front. Physiol. 2014, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Saternus, R.; Vogt, T. Challenge and Perspective: The Relevance of Ultraviolet (UV) Radiation and the Vitamin D Endocrine System (VDES) for Psoriasis and Other Inflammatory Skin Diseases. Photochem. Photobiol. Sci. 2017, 16, 433–444. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Fukuo, K.; Shiraishi, T.; Koh, E.; Imanaka, S.; Kitano, S.; Ogihara, T. Inverse Relation between Severity of Psoriasis and Serum 1,25-Dihydroxyvitamin D Level. J. Dermatol. Sci. 1990, 1, 277–282. [Google Scholar] [CrossRef]
- Tajjour, R.; Baddour, R.; Redwan, F.; Hassan, F. The Relationship between Psoriasis and Serum Levels of Vitamin D. J. Adv. Med. Med. Res. 2018, 26, 1–12. [Google Scholar] [CrossRef]
- Bergler-Czop, B.; Brzezińska-Wcisło, L. Serum Vitamin D Level—The Effect on the Clinical Course of Psoriasis. Postępy Dermatol. Alergol. 2016, 6, 445–449. [Google Scholar] [CrossRef]
- Ricceri, F.; Pescitelli, L.; Tripo, L.; Prignano, F. Deficiency of Serum Concentration of 25-Hydroxyvitamin D Correlates with Severity of Disease in Chronic Plaque Psoriasis. J. Am. Acad. Dermatol. 2013, 68, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Hung, T.; Soung, J. The Role of Vitamin D in Psoriasis: A Review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Association between Circulating 25-Hydroxyvitamin D Levels and Psoriasis, and Correlation with Disease Severity: A Meta-Analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef]
- Formisano, E.; Proietti, E.; Borgarelli, C.; Pisciotta, L. Psoriasis and Vitamin D: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3387. [Google Scholar] [CrossRef]
- Gamonal, S.B.L.; Gamonal, A.C.C.; Marques, N.C.V.; Brandão, M.A.F.; Raposo, N.R.B. Is Vitamin D Status Relevant to Psoriasis and Psoriatic Arthritis? A Retrospective Cross-Sectional Study. Sao Paulo Med. J. 2022, 141, e2022216. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, L.; Kumari, G.R.K.; Rajappa, M.; Revathy, G.; Munisamy, M.; Thappa, D.M. 25-Hydroxy Vitamin D and Ischaemia-Modified Albumin Levels in Psoriasis and Their Association with Disease Severity. Br. J. Biomed. Sci. 2015, 72, 56–60. [Google Scholar] [CrossRef]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the Vitamin D Endocrine System (VDES) for the Management of Inflammatory and Malignant Skin Diseases: An Historical View and Outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and Its Role in Psoriasis: An Overview of the Dermatologist and Nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef]
- van de Kerkhof, P.C. Biological Activity of Vitamin D Analogues in the Skin, with Special Reference to Antipsoriatic Mechanisms. Br. J. Dermatol. 1995, 132, 675–682. [Google Scholar] [CrossRef]
- Oxholm, A.; Oxholm, P.; Staberg, B.; Bendtzen, K. Expression of Interleukin-6-like Molecules and Tumour Necrosis Factor after Topical Treatment of Psoriasis with a New Vitamin D Analogue (MC 903). Acta Derm. Venereol. 1989, 69, 385–390. [Google Scholar] [PubMed]
- Van De Kerkhof, P.C.M.; Green, C.; Hamberg, K.J.; Hutchinson, P.E.; Jensen, J.K.; Kidson, P.; Kragballe, K.; Larsen, F.G.; Munro, C.S.; Tillman, D.M. Safety and Efficacy of Combined High-Dose Treatment with Calcipotriol Ointment and Solution in Patients with Psoriasis. Dermatology 2002, 204, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H. Topical Calcipotriene 0.005% and Betamethasone Dipropionate 0.064% Maintains Efficacy of Etanercept after Step-down Dose in Patients with Moderate-to-Severe Plaque Psoriasis: Results of an Open Label Trial. J. Drugs Dermatol. 2011, 10, 878–882. [Google Scholar]
- Pinter, A.; Green, L.J.; Selmer, J.; Praestegaard, M.; Gold, L.S.; Augustin, M.; trial investigator group. A Pooled Analysis of Randomized, Controlled, Phase 3 Trials Investigating the Efficacy and Safety of a Novel, Fixed Dose Calcipotriene and Betamethasone Dipropionate Cream for the Topical Treatment of Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 228–236. [Google Scholar] [CrossRef]
- Heim, M.; Irondelle, M.; Duteil, L.; Cardot-Leccia, N.; Rocchi, S.; Passeron, T.; Tulic, M.K. Impact of Topical Emollient, Steroids Alone or Combined with Calcipotriol, on the Immune Infiltrate and Clinical Outcome in Psoriasis. Exp. Dermatol. 2022, 31, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, P.C.M.; Berth-Jones, J.; Griffiths, C.E.M.; Harrison, P.V.; Hönigsmann, H.; Marks, R.; Roelandts, R.; Schöpf, E.; Trompke, C. Long-Term Efficacy and Safety of Tacalcitol Ointment in Patients with Chronic Plaque Psoriasis. Br. J. Dermatol. 2002, 146, 414–422. [Google Scholar] [CrossRef]
- Miyachi, Y.; Ohkawara, A.; Ohkido, M.; Harada, S.; Tamaki, K.; Nakagawa, H.; Hori, Y.; Nishiyama, S. Long-Term Safety and Efficacy of High-Concentration (20 Microg/g) Tacalcitol Ointment in Psoriasis Vulgaris. Eur. J. Dermatol. 2002, 12, 463–468. [Google Scholar]
- Venegas-Iribarren, S.; Andino, R. Topical corticosteroids or vitamin D analogues for plaque psoriasis? Medwave 2017, 17, e6981. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.R.; Siegel, M.; Bagel, J.; Cordoro, K.M.; Garg, A.; Gottlieb, A.; Green, L.J.; Gudjonsson, J.E.; Koo, J.; Lebwohl, M.; et al. Dietary Recommendations for Adults With Psoriasis or Psoriatic Arthritis From the Medical Board of the National Psoriasis Foundation: A Systematic Review. JAMA Dermatol. 2018, 154, 934–950. [Google Scholar] [CrossRef]
- Zold, E.; Barta, Z.; Bodolay, E. Vitamin D Deficiency and Connective Tissue Disease. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 261–286. ISBN 978-0-12-386960-9. [Google Scholar]
- Wright, T.B.; Shults, J.; Leonard, M.B.; Zemel, B.S.; Burnham, J.M. Hypovitaminosis D Is Associated with Greater Body Mass Index and Disease Activity in Pediatric Systemic Lupus Erythematosus. J. Pediatr. 2009, 155, 260–265. [Google Scholar] [CrossRef]
- Baxter, A.G.; Smyth, M.J. The Role of NK Cells in Autoimmune Disease. Autoimmunity 2002, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grammer, A.C.; Lipsky, P.E. B Cell Abnormalities in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2003, 5 (Suppl. S4), S22-27. [Google Scholar] [CrossRef]
- Zhou, T.-B.; Jiang, Z.-P.; Lin, Z.-J.; Su, N. Association of Vitamin D Receptor Gene Polymorphism with the Risk of Systemic Lupus Erythematosus. J. Recept. Signal Transduct. 2015, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Magro, R.; Saliba, C.; Camilleri, L.; Scerri, C.; Borg, A.A. Vitamin D Supplementation in Systemic Lupus Erythematosus: Relationship to Disease Activity, Fatigue and the Interferon Signature Gene Expression. BMC Rheumatol. 2021, 5, 53. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Blank, M.; Kiss, E.; Tarr, T.; Amital, H.; Shoenfeld, Y. Anti-Vitamin D, Vitamin D in SLE: Preliminary Results. Ann. New York Acad. Sci. 2007, 1109, 550–557. [Google Scholar] [CrossRef]
- Koizumi, T.; Nakao, Y.; Matsui, T.; Nakagawa, T.; Matsuda, S.; Komoriya, K.; Kanai, Y.; Fujita, T. Effects of Corticosteroid and 1,24R-Dihydroxy-Vitamin D3 Administration on Lymphoproliferation and Autoimmune Disease in MRL/MP-Lpr/Lpr Mice. Int. Arch. Allergy Immunol. 1985, 77, 396–404. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, S.; Chesney, R.W.; Hamstra, A.; Eisman, J.A.; O’Gorman, A.M.; Deluca, H.F. Reduced Serum 1,25-(OH)2 Vitamin D3 Levels in Prednisone-Treated Adolescents with Systemic Lupus Erythematosus. Acta Paediatr. Scand. 1979, 68, 109–111. [Google Scholar] [CrossRef]
- Müller, K.; Kriegbaum, N.J.; Baslund, B.; Sørensen, O.H.; Thymann, M.; Bentzen, K. Vitamin D3 Metabolism in Patients with Rheumatic Diseases: Low Serum Levels of 25-Hydroxyvitamin D3 in Patients with Systemic Lupus Erythematosus. Clin. Rheumatol. 1995, 14, 397–400. [Google Scholar] [CrossRef]
- Sarİ, F.; Saraç, D.; Bayram, S.; Baglan Yentur, S.; Tore, N.G.; Oskay, D.; Tufan, A. Pos1583-Hpr The Effect of Vitamin D Deficiency on Exercise Capacity, Respiratory Muscle Strength, and Peripheral Muscle Strength in Patients with Connective Tissue Diseases. Ann. Rheum. Dis. 2023, 82, 1168. [Google Scholar] [CrossRef]
- Andreoli, L.; Dall’Ara, F.; Piantoni, S.; Zanola, A.; Piva, N.; Cutolo, M.; Tincani, A. A 24-Month Prospective Study on the Efficacy and Safety of Two Different Monthly Regimens of Vitamin D Supplementation in Pre-Menopausal Women with Systemic Lupus Erythematosus. Lupus 2015, 24, 499–506. [Google Scholar] [CrossRef]
- Karimzadeh, H.; Shirzadi, M.; Karimifar, M. The Effect of Vitamin D Supplementation in Disease Activity of Systemic Lupus Erythematosus Patients with Vitamin D Deficiency: A Randomized Clinical Trial. J. Res. Med. Sci. 2017, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Singgih Wahono, C.; Diah Setyorini, C.; Kalim, H.; Nurdiana, N.; Handono, K. Effect of Curcuma xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients with Hypovitamin D Which Were Given Vitamin D3 towards Disease Activity (SLEDAI), IL-6, and TGF- β 1 Serum. Int. J. Rheumatol. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Pakchotanon, R.; Lomarat, W.; Narongroeknawin, P.; Chaiamnuay, S.; Asavatanabodee, P. Randomized Double-Blind Controlled Trial to Evaluate Efficacy of Vitamin D Supplementation Among Patients with Systemic Lupus Erythematosus. J. Southeast. Asian Med. Res. 2020, 4, 24–32. [Google Scholar] [CrossRef]
- Lima, G.L.; Paupitz, J.; Aikawa, N.E.; Takayama, L.; Bonfa, E.; Pereira, R.M.R. Vitamin D Supplementation in Adolescents and Young Adults With Juvenile Systemic Lupus Erythematosus for Improvement in Disease Activity and Fatigue Scores: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2016, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Smith, V.; Paolino, S.; Gotelli, E. Involvement of the Secosteroid Vitamin D in Autoimmune Rheumatic Diseases and COVID-19. Nat. Rev. Rheumatol. 2023, 19, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Boucher, B.J.; Berry, D.J.; Power, C. 25-Hydroxyvitamin D, IGF-1, and Metabolic Syndrome at 45 Years of Age: A Cross-Sectional Study in the 1958 British Birth Cohort. Diabetes 2008, 57, 298–305. [Google Scholar] [CrossRef]
- Przybelski, R.J.; Binkley, N.C. Is Vitamin D Important for Preserving Cognition? A Positive Correlation of Serum 25-Hydroxyvitamin D Concentration with Cognitive Function. Arch. Biochem. Biophys. 2007, 460, 202–205. [Google Scholar] [CrossRef]
- Sousa, J.R.; Rosa, É.P.C.; Nunes, I.F.D.O.C.; Carvalho, C.M.R.G.D. Effect of Vitamin D Supplementation on Patients with Systemic Lupus Erythematosus: A Systematic Review. Rev. Bras. Reumatol. 2017, 57, 466–471. [Google Scholar] [CrossRef]
- Heine, G.; Drozdenko, G.; Lahl, A.; Unterwalder, N.; Mei, H.; Volk, H.-D.; Dörner, T.; Radbruch, A.; Worm, M. Efficient Tetanus Toxoid Immunization on Vitamin D Supplementation. Eur. J. Clin. Nutr. 2011, 65, 329–334. [Google Scholar] [CrossRef]
- Salman-Monte, T.C.; Torrente-Segarra, V.; Vega-Vidal, A.L.; Corzo, P.; Castro-Dominguez, F.; Ojeda, F.; Carbonell-Abelló, J. Bone Mineral Density and Vitamin D Status in Systemic Lupus Erythematosus (SLE): A Systematic Review. Autoimmun. Rev. 2017, 16, 1155–1159. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Ma, Y.; Zhu, J. Vitamin D Receptor Gene FokI but Not TaqI, ApaI, BsmI Polymorphism Is Associated with Hashimoto’s Thyroiditis: A Meta-Analysis. Sci. Rep. 2017, 7, 41540. [Google Scholar] [CrossRef]
- Bhakat, B.; Pal, J.; Das, S.; Charaborty, S.K.; SircarMedical, N.R.; Kolkata; RGKar; Bengal, N.; Siliguri. A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto’s Thyroiditis. J. Assoc. Physicians India 2023, 71, 1. [Google Scholar] [PubMed]
- Simsek, Y.; Cakir, I.; Yetmis, M.; Dizdar, O.; Baspinar, O.; Gokay, F. Effects of Vitamin D Treatment on Thyroid Autoimmunity. J. Res. Med. Sci. 2016, 21, 85. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Zuo, Z.; Zhao, Y.; Wang, K. The Effect of Vitamin D Supplementation on Thyroid Autoantibody Levels in the Treatment of Autoimmune Thyroiditis: A Systematic Review and a Meta-Analysis. Endocrine 2018, 59, 499–505. [Google Scholar] [CrossRef]
- Tang, J.; Shan, S.; Li, F.; Yun, P. Effects of Vitamin D Supplementation on Autoantibodies and Thyroid Function in Patients with Hashimoto’s Thyroiditis: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e36759. [Google Scholar] [CrossRef] [PubMed]
- Borgogni, E.; Sarchielli, E.; Sottili, M.; Santarlasci, V.; Cosmi, L.; Gelmini, S.; Lombardi, A.; Cantini, G.; Perigli, G.; Luconi, M.; et al. Elocalcitol Inhibits Inflammatory Responses in Human Thyroid Cells and T Cells. Endocrinology 2008, 149, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [Google Scholar] [CrossRef]
- Luong, K.V.Q.; Nguyen, L.T.H.; Pham Nguyen, D.N. The Role of Vitamin D in Protecting Type 1 Diabetes Mellitus. Diabetes Metabolism Res. 2005, 21, 338–346. [Google Scholar] [CrossRef]
- Danescu, L.G.; Levy, S.; Levy, J. Vitamin D and Diabetes Mellitus. Endocr 2009, 35, 11–17. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Leung, P.S.C.; Adamopoulos, I.E.; Gershwin, M.E. The Implication of Vitamin D and Autoimmunity: A Comprehensive Review. Clinic Rev. Allerg. Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 Diabetes—Early Life Origins and Changing Epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef]
- Zella, J.B.; McCary, L.C.; DeLuca, H.F. Oral Administration of 1,25-Dihydroxyvitamin D3 Completely Protects NOD Mice from Insulin-Dependent Diabetes Mellitus. Arch. Biochem. Biophys. 2003, 417, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of Autoimmune Diabetes in NOD Mice by 1,25 Dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Casteels, K.M.; Mathieu, C.; Waer, M.; Valckx, D.; Overbergh, L.; Laureys, J.M.; Bouillon, R. Prevention of Type I Diabetes in Nonobese Diabetic Mice by Late Intervention with Nonhypercalcemic Analogs of 1,25-Dihydroxyvitamin D3 in Combination with a Short Induction Course of Cyclosporin A. Endocrinology 1998, 139, 95–102. [Google Scholar] [CrossRef]
- Fronczak, C.M.; Barón, A.E.; Chase, H.P.; Ross, C.; Brady, H.L.; Hoffman, M.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. In Utero Dietary Exposures and Risk of Islet Autoimmunity in Children. Diabetes Care 2003, 26, 3237–3242. [Google Scholar] [CrossRef]
- Hou, Y.; Song, A.; Jin, Y.; Xia, Q.; Song, G.; Xing, X. A Dose–Response Meta-Analysis between Serum Concentration of 25-Hydroxy Vitamin D and Risk of Type 1 Diabetes Mellitus. Eur. J. Clin. Nutr. 2021, 75, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Storm, T.L.; Sørensen, O.H.; Lund, B.; Lund, B.; Christiansen, J.S.; Andersen, A.R.; Lumholtz, I.B.; Parving, H.-H. Vitamin D Metabolism in Insulin-Dependent Diabetes Mellitus. Metab. Bone Dis. Relat. Res. 1983, 5, 107–110. [Google Scholar] [CrossRef]
- Mathieu, C.; Badenhoop, K. Vitamin D and Type 1 Diabetes Mellitus: State of the Art. Trends Endocrinol. Metab. 2005, 16, 261–266. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Zhang, W.; Chen, J.; Zhang, Z.-L.; Han, S.-F.; Qin, L.-Q. Vitamin D Intake and Risk of Type 1 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef]
- Norris, J.M.; Lee, H.-S.; Frederiksen, B.; Erlund, I.; Uusitalo, U.; Yang, J.; Lernmark, Å.; Simell, O.; Toppari, J.; Rewers, M.; et al. Plasma 25-Hydroxyvitamin D Concentration and Risk of Islet Autoimmunity. Diabetes 2018, 67, 146–154. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.-R.; Virtanen, S.M. Intake of Vitamin D and Risk of Type 1 Diabetes: A Birth-Cohort Study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- The EURODIAB Substudy 2 Study Group. Vitamin D Supplement in Early Childhood and Risk for Type I (Insulin-Dependent) Diabetes Mellitus. Diabetologia 1999, 42, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Sun, X.; Wang, L.; Wang, A. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Meta-Analysis of Interventional Studies. Medicine 2019, 98, e14970. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, J.; Wang, H.; Shang, H.; Zhang, D.; Liao, L. Efficacy and Safety of Vitamin D3 in Patients with Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Chin. Med. J. 2014, 127, 2837–2843. [Google Scholar]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Corrao, S.; Gervasi, F.; Di Bernardo, F.; Natoli, G.; Raspanti, M.; Catalano, N.; Argano, C. Immunological Characteristics of Non-Intensive Care Hospitalized COVID-19 Patients: A Preliminary Report. J. Clin. Med. 2021, 10, 849. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host Cell Death and Inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L. The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; De Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Corrao, S.; Raspanti, M.; Agugliaro, F.; Gervasi, F.; Di Bernardo, F.; Natoli, G.; Argano, C. Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study. J. Clin. Med. 2024, 13, 3987. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Sun, N.; Li, J.; Ma, Z.; Rao, Z.; Sun, X.; Zeng, Q.; Wu, Y.; Li, J.; et al. Vitamin D Receptor Enhances NLRC4 Inflammasome Activation by Promoting NAIPs–NLRC4 Association. EMBO Rep. 2022, 23, e54611. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes After 5 Years. Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of Vitamin D and Calcium Supplementation on Pancreatic β Cell Function, Insulin Sensitivity, and Glycemia in Adults at High Risk of Diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef]
- Nazarian, S.; St Peter, J.V.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 Supplementation Improves Insulin Sensitivity in Subjects with Impaired Fasting Glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E. Vitamin D Deficiency in the Aetiology of Obesity-related Insulin Resistance. Diabetes Metab. Res. 2019, 35, e3146. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2010, 2010, 1–18. [Google Scholar] [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and Pancreas: The Role of Sunshine Vitamin in the Pathogenesis of Diabetes Mellitus and Pancreatic Cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Vitamin D3 Improves Impaired Glucose Tolerance and Insulin Secretion in the Vitamin D-Deficient Rat in Vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired Insulin Secretory Capacity in Mice Lacking a Functional Vitamin D Receptor. FASEB J. 2003, 17, 1–14. [Google Scholar] [CrossRef]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.F.; Squires, P.E.; Hewison, M. Expression of 25-Hydroxyvitamin D3-1α-Hydroxylase in Pancreatic Islets. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 121–125. [Google Scholar] [CrossRef]
- Reusch, J.E.-B.; Begum, N.; Sussman, K.E.; Draznin, B. Regulation of GLUT-4 Phosphorylation by Intracellular Calcium in Adipocytes. Endocrinology 1991, 129, 3269–3273. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and Tissue Sites of Non-Insulin- and Insulin-Mediated Glucose Uptake in Humans. Am. J. Physiol.-Endocrinol. Metab. 1988, 255, E769–E774. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Jefferson, G.E.; Schnell, D.M.; Thomas, D.T.; Bollinger, L.M. Calcitriol Concomitantly Enhances Insulin Sensitivity and Alters Myocellular Lipid Partitioning in High Fat-Treated Skeletal Muscle Cells. J. Physiol. Biochem. 2017, 73, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Krul-Poel, Y.H.M.; Ter Wee, M.M.; Lips, P.; Simsek, S. Management of Endocrine Disease: The Effect of Vitamin D Supplementation on Glycaemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2017, 176, R1–R14. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The Link between Obesity and Low Circulating 25-Hydroxyvitamin D Concentrations: Considerations and Implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Bajaj, S.; Singh, R.; Dwivedi, N.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D Levels and Microvascular Complications in Type 2 Diabetes. Indian. J. Endocr. Metab. 2014, 18, 537. [Google Scholar] [CrossRef]
- De Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M.; et al. Relationship between Vitamin D and Inflammatory Markers in Older Individuals. AGE 2014, 36, 9694. [Google Scholar] [CrossRef]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D Deficiency Is Associated With Inflammation in Older Irish Adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef]
- Kruit, A.; Zanen, P. The Association between Vitamin D and C-Reactive Protein Levels in Patients with Inflammatory and Non-Inflammatory Diseases. Clin. Biochem. 2016, 49, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Vargas Vargas, R.A.; Varela Millán, J.M.; Fajardo Bonilla, E. Renin–Angiotensin System: Basic and Clinical Aspects—A General Perspective. Endocrinol. Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.; Chambers, S.; Bhatia, M. ACE and ACE2 in Inflammation: A Tale of Two Enzymes. Inflamm. Allergy-Drug Targets-Inflamm. Allergy 2014, 13, 224–234. [Google Scholar] [CrossRef]
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 Spike Pseudotyped Virus by Recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070. [Google Scholar] [CrossRef]
- Fedson, D.S. Treating the Host Response to Emerging Virus Diseases: Lessons Learned from Sepsis, Pneumonia, Influenza and Ebola. Ann. Transl. Med. 2016, 4, 421. [Google Scholar] [CrossRef]
- Wee, C.L.; Azemi, A.K.; Mokhtar, S.S.; Yahaya, S.; Yaacob, N.S.; Rasool, A.H.G. Vitamin D Deficiency Enhances Vascular Oxidative Stress, Inflammation, and Angiotensin II Levels in the Microcirculation of Diabetic Patients. Microvasc. Res. 2023, 150, 104574. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR Attenuates Acute Lung Injury by Blocking Ang-2-Tie-2 Pathway and Renin-Angiotensin System. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef]
- Marshall, R.P.; McANULTY, R.J.; Laurent, G.J. Angiotensin II Is Mitogenic for Human Lung Fibroblasts via Activation of the Type 1 Receptor. Am. J. Respir. Crit. Care Med. 2000, 161, 1999–2004. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Wang, Y.; Wang, Z.; Liang, Y.; Huang, T.; Zhang, H.; Sun, W.; Wang, Y. COVID-19 Patients’ Clinical Characteristics, Discharge Rate, and Fatality Rate of Meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Glowacka, I.; Müller, M.A.; Lavender, H.; Gnirss, K.; Nehlmeier, I.; Niemeyer, D.; He, Y.; Simmons, G.; Drosten, C.; et al. Cleavage and Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by Human Airway Trypsin-Like Protease. J. Virol. 2011, 85, 13363–13372. [Google Scholar] [CrossRef]
- Ali, R.M.; Al-Shorbagy, M.Y.; Helmy, M.W.; El-Abhar, H.S. Role of Wnt4/β-Catenin, Ang II/TGFβ, ACE2, NF-κB, and IL-18 in Attenuating Renal Ischemia/Reperfusion-Induced Injury in Rats Treated with Vit D and Pioglitazone. Eur. J. Pharmacol. 2018, 831, 68–76. [Google Scholar] [CrossRef]
- Cui, C.; Xu, P.; Li, G.; Qiao, Y.; Han, W.; Geng, C.; Liao, D.; Yang, M.; Chen, D.; Jiang, P. Vitamin D Receptor Activation Regulates Microglia Polarization and Oxidative Stress in Spontaneously Hypertensive Rats and Angiotensin II-Exposed Microglial Cells: Role of Renin-Angiotensin System. Redox Biol. 2019, 26, 101295. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Regulation of the Renin-Angiotensin System. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, F.; Cao, K.; Xu, D.; Goltzman, D.; Miao, D. Calcium-Independent and 1,25(OH)2D3-Dependent Regulation of the Renin-Angiotensin System in 1α-Hydroxylase Knockout Mice. Kidney Int. 2008, 74, 170–179. [Google Scholar] [CrossRef]
- Tiwari, V.; Singh, J.; Tiwari, P.; Chaturvedi, S.; Gupta, S.; Mishra, A.; Singh, S.; Wahajuddin, M.; Hanif, K.; Shukla, S. ACE2/ANG-(1-7)/Mas Receptor Axis Activation Prevents Inflammation and Improves Cognitive Functions in Streptozotocin Induced Rat Model of Alzheimer’s Disease-like Phenotypes. Eur. J. Pharmacol. 2023, 946, 175623. [Google Scholar] [CrossRef]
- Cui, D.; Liu, Y.; Jiang, X.; Ding, C.; Poon, L.C.; Wang, H.; Yang, H. Single-cell RNA Expression Profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in Human Trophectoderm and Placenta. Ultrasound Obs. Gyne 2021, 57, 248–256. [Google Scholar] [CrossRef]
- Lin, S.; Pan, H.; Wu, H.; Ren, D.; Lu, J. Role of the ACE2-Ang-(1-7)-Mas Axis in Blood Pressure Regulation and Its Potential as an Antihypertensive in Functional Foods. Mol. Med. Rep. 2017, 16, 4403–4412. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Tizabi, Y. Vitamin D and COVID-19: Role of ACE2, Age, Gender, and Ethnicity. J. Med. Virol. 2021, 93, 5285–5294. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; García-Giménez, J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Mukhyaprana Prabhu, M.; Prabhu, K.; Changani, M.; Patel, N.; Belle, V.S. Vitamin D as a Biomarker in Predicting Sepsis Outcome at a Tertiary Care Hospital. Asian J. Med. Sci. 2023, 14, 89–94. [Google Scholar] [CrossRef]
- Corrao, S.; Gervasi, F.; Di Bernardo, F.; Argano, C. Immune Response Failure in Paucisymptomatic Long-Standing SARS-CoV-2 Spreaders. Clin. Pract. 2021, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D Up-Regulates the Vitamin D Receptor by Protecting It from Proteasomal Degradation in Human CD4+ T Cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.A.; El-Braky, A.A.-A.; Ghazal, A.A.E.-R.; Shamseya, M.M. Autophagy, Apoptosis, Vitamin D, and Vitamin D Receptor in Hepatocellular Carcinoma Associated with Hepatitis C Virus. Medicine 2018, 97, e0172. [Google Scholar] [CrossRef] [PubMed]
- Gunville, C.; Mourani, P.; Ginde, A. The Role of Vitamin D in Prevention and Treatment of Infection. Inflamm. Allergy-Drug Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Equils, O.; Naiki, Y.; Shapiro, A.M.; Michelsen, K.; Lu, D.; Adams, J.; Jordan, S. 1,25-Dihydroxyvitamin D3 Inhibits Lipopolysaccharide-Induced Immune Activation in Human Endothelial Cells. Clin. Exp. Immunol. 2005, 143, 58–64. [Google Scholar] [CrossRef]
- Ginde, A.A.; Camargo, C.A.; Shapiro, N.I. Vitamin D Insufficiency and Sepsis Severity in Emergency Department Patients With Suspected Infection. Acad. Emerg. Med. 2011, 18, 551–554. [Google Scholar] [CrossRef]
- Bergman, P.; Norlin, A.-C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J.D.; Andersson, J. Vitamin D3 Supplementation in Patients with Frequent Respiratory Tract Infections: A Randomised and Double-Blind Intervention Study. BMJ Open 2012, 2, e001663. [Google Scholar] [CrossRef]
- Slow, S.; Epton, M.; Storer, M.; Thiessen, R.; Lim, S.; Wong, J.; Chin, P.; Tovaranonte, P.; Pearson, J.; Chambers, S.T.; et al. Effect of Adjunctive Single High-Dose Vitamin D3 on Outcome of Community-Acquired Pneumonia in Hospitalised Adults: The VIDCAPS Randomised Controlled Trial. Sci. Rep. 2018, 8, 13829. [Google Scholar] [CrossRef] [PubMed]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High Doses of Vitamin D to Reduce Exacerbations in Chronic Obstructive Pulmonary Disease: A Randomized Trial. Ann. Intern. Med. 2012, 156, 105. [Google Scholar] [CrossRef]
- Anitua, E.; Tierno, R.; Alkhraisat, M.H. Current Opinion on the Role of Vitamin D Supplementation in Respiratory Infections and Asthma/COPD Exacerbations: A Need to Establish Publication Guidelines for Overcoming the Unpublished Data. Clin. Nutr. 2022, 41, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The Role of Vitamin D in the Prevention and Treatment of SARS-CoV-2 Infection: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2023, 42, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Luciani, A.; Perego, G.; Dognini, G.; Colombelli, P.L.; Ghidini, A. Therapeutic and Prognostic Role of Vitamin D for COVID-19 Infection: A Systematic Review and Meta-Analysis of 43 Observational Studies. J. Steroid Biochem. Mol. Biol. 2021, 211, 105883. [Google Scholar] [CrossRef]
- Miroliaee, A.E.; Salamzadeh, J.; Shokouhi, S.; Fatemi, A.; Ardehali, S.H.; Hajiesmaeili, M.R.; Sahraei, Z. Effect of Vitamin D Supplementation on Procalcitonin as Prognostic Biomarker in Patients with Ventilator Associated Pneumonia Complicated with Vitamin D Deficiency. Iran. J. Pharm. Res. 2017, 16, 1254–1263. [Google Scholar]
- Hosseini, B.; Tremblay, C.L.; Longo, C.; Glochi, S.; White, J.H.; Quach, C.; Ste-Marie, L.-G.; Platt, R.W.; Ducharme, F.M. Oral Vitamin D Supplemental Therapy to Attain a Desired Serum 25-Hydroxyvitamin D Concentration in Essential Healthcare Teams. Trials 2022, 23, 1019. [Google Scholar] [CrossRef]
- Theodorou, M.; Sersté, T.; Van Gossum, M.; Dewit, S. Factors Associated with Vitamin D Deficiency in a Population of 2044 HIV-Infected Patients. Clin. Nutr. 2014, 33, 274–279. [Google Scholar] [CrossRef]
- Coelho, L.; Cardoso, S.W.; Luz, P.M.; Hoffman, R.M.; Mendonça, L.; Veloso, V.G.; Currier, J.S.; Grinsztejn, B.; Lake, J.E. Vitamin D3 Supplementation in HIV Infection: Effectiveness and Associations with Antiretroviral Therapy. Nutr. J. 2015, 14, 81. [Google Scholar] [CrossRef]
- Shepherd, L.; Souberbielle, J.-C.; Bastard, J.-P.; Fellahi, S.; Capeau, J.; Reekie, J.; Reiss, P.; Blaxhult, A.; Bickel, M.; Leen, C.; et al. Prognostic Value of Vitamin D Level for All-Cause Mortality, and Association With Inflammatory Markers, in HIV-Infected Persons. J. Infect. Dis. 2014, 210, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Viard, J.-P.; Souberbielle, J.-C.; Kirk, O.; Reekie, J.; Knysz, B.; Losso, M.; Gatell, J.; Pedersen, C.; Bogner, J.R.; Lundgren, J.D.; et al. Vitamin D and Clinical Disease Progression in HIV Infection: Results from the EuroSIDA Study. AIDS 2011, 25, 1305–1315. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Hormonally Active Vitamin D3 (1α,25-Dihydroxycholecalciferol) Triggers Autophagy in Human Macrophages That Inhibits HIV-1 Infection. J. Biol. Chem. 2011, 286, 18890–18902. [Google Scholar] [CrossRef]
- Arpadi, S.M.; McMahon, D.; Abrams, E.J.; Bamji, M.; Purswani, M.; Engelson, E.S.; Horlick, M.; Shane, E. Effect of Bimonthly Supplementation With Oral Cholecalciferol on Serum 25-Hydroxyvitamin D Concentrations in HIV-Infected Children and Adolescents. Pediatrics 2009, 123, e121–e126. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Zhang, Y.; Zhang, M.; Wu, H.; Zheng, L.; Ji, J.; Li, Z.; Wang, W.; Zhang, T. Association of Vitamin D with HIV Infected Individuals, TB Infected Individuals, and HIV-TB Co-Infected Individuals: A Systematic Review and Meta-Analysis. Front. Public Health 2024, 12, 1344024. [Google Scholar] [CrossRef]
- Rezamand, G.; Estêvão, M.D.; Morvaridzadeh, M.; Akbari, A.; Tabaeian, S.P.; Pizarro, A.B.; Malekahmadi, M.; Hasani, M.; Roffey, D.M.; Mirzaei, A.; et al. Effects of Vitamin D Supplementation on Bone Health and Bone-Related Parameters in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Ther. 2022, 44, e11-25.e8. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.-C. Vitamin D and Multiple Sclerosis: An Update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple Sclerosis: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- The Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) Genome-Wide Association Study Identifies New Multiple Sclerosis Susceptibility Loci on Chromosomes 12 and 20. Nat. Genet. 2009, 41, 824–828. [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.-C. Contribution of Vitamin D Insufficiency to the Pathogenesis of Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 81–116. [Google Scholar] [CrossRef]
- Vieth, R.; Fraser, D. Vitamin D Insufficiency: No Recommended Dietary Allowance Exists for This Nutrient. CMAJ 2002, 166, 1541–1542. [Google Scholar]
- Mowry, E.M.; Waubant, E.; McCulloch, C.E.; Okuda, D.T.; Evangelista, A.A.; Lincoln, R.R.; Gourraud, P.; Brenneman, D.; Owen, M.C.; Qualley, P.; et al. Vitamin D Status Predicts New Brain Magnetic Resonance Imaging Activity in Multiple Sclerosis. Ann. Neurol. 2012, 72, 234–240. [Google Scholar] [CrossRef]
- Dickinson, J.; Perera, D.; Van Der Mei, A.; Ponsonby, A.-L.; Polanowski, A.; Thomson, R.; Taylor, B.; McKay, J.; Stankovich, J.; Dwyer, T. Past Environmental Sun Exposure and Risk of Multiple Sclerosis: A Role for the Cdx-2 Vitamin D Receptor Variant in This Interaction. Mult. Scler. 2009, 15, 563–570. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, Q.; Ma, W.; Liu, H.; Ji, J.; Li, X. Associations of Vitamin D Deficiency and Vitamin D Receptor (Cdx-2, Fok I, Bsm I and Taq I) Polymorphisms with the Risk of Primary Open-Angle Glaucoma. BMC Ophthalmol. 2016, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Jurutka, P.W.; Remus, L.S.; Whitfield, G.K.; Thompson, P.D.; Hsieh, J.-C.; Zitzer, H.; Tavakkoli, P.; Galligan, M.A.; Dang, H.T.L.; Haussler, C.A.; et al. The Polymorphic N Terminus in Human Vitamin D Receptor Isoforms Influences Transcriptional Activity by Modulating Interaction with Transcription Factor IIB. Mol. Endocrinol. 2000, 14, 401–420. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.J.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental Risk Factors for Multiple Sclerosis: A Review with a Focus on Molecular Mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Sandve, G.K.; Disanto, G.; Berlanga-Taylor, A.J.; Gallone, G.; Hanwell, H.; Drabløs, F.; Giovannoni, G.; Ebers, G.C.; Ramagopalan, S.V. Vitamin D Receptor ChIP-Seq in Primary CD4+ Cells: Relationship to Serum 25-Hydroxyvitamin D Levels and Autoimmune Disease. BMC Med. 2013, 11, 163. [Google Scholar] [CrossRef]
- Tizaoui, K.; Hamzaoui, K. Association between VDR Polymorphisms and Rheumatoid Arthritis Disease: Systematic Review and Updated Meta-Analysis of Case-Control Studies. Immunobiology 2015, 220, 807–816. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Cohen Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. The Relevance of Vitamin D Receptor Gene Polymorphisms for Vitamin D Research in Multiple Sclerosis. Autoimmun. Rev. 2009, 8, 621–626. [Google Scholar] [CrossRef]
- Simon, K.C.; Munger, K.L.; Yang, X.; Ascherio, A. Polymorphisms in Vitamin D Metabolism Related Genes and Risk of Multiple Sclerosis. Mult. Scler. 2010, 16, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Boltjes, R.; Knippenberg, S.; Gerlach, O.; Hupperts, R.; Damoiseaux, J. Vitamin D Supplementation in Multiple Sclerosis: An Expert Opinion Based on the Review of Current Evidence. Expert Rev. Neurother. 2021, 21, 715–725. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, L.; Jiang, C.; Yang, X.; Huang, J. The Effect of Different Administration Time and Dosage of Vitamin D Supplementation in Patients with Multiple Sclerosis: A Meta-Analysis of Randomized Controlled Trials. Neuroimmunomodulation 2021, 28, 118–128. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Borges Do Nascimento, I.J.; Di Pietrantonj, C.; Robak, E.W.; Whamond, L. Vitamin D for the Management of Multiple Sclerosis. Cochrane Database Syst. Rev. 2018, 2023. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Munger, K.L.; Koch-Henriksen, N.; Hougaard, D.M.; Magyari, M.; Jørgensen, K.T.; Lundqvist, M.; Simonsen, J.; Jess, T.; Cohen, A.; et al. Neonatal Vitamin D Status and Risk of Multiple Sclerosis: A Population-Based Case-Control Study. Neurology 2017, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mei, I.A.F.; Ponsonby, A.-L.; Dwyer, T.; Blizzard, L.; Taylor, B.V.; Kilpatrick, T.; Butzkueven, H.; McMichael, A.J. Vitamin D Levels in People with Multiple Sclerosis and Community Controls in Tasmania, Australia. J. Neurol. 2007, 254, 581–590. [Google Scholar] [CrossRef]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the Treatment of Multiple Sclerosis: A Meta-Analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef]
- Berezowska, M.; Coe, S.; Dawes, H. Effectiveness of Vitamin D Supplementation in the Management of Multiple Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 1301. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.; Mei, J.; Zhu, L.; Kong, H. Vitamin D Can Safely Reduce Asthma Exacerbations among Corticosteroid-Using Children and Adults with Asthma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Res. 2021, 92, 49–61. [Google Scholar] [CrossRef]
- Etemadifar, M.; Janghorbani, M. Efficacy of High-Dose Vitamin D3 Supplementation in Vitamin D Deficient Pregnant Women with Multiple Sclerosis: Preliminary Findings of a Randomized-Controlled Trial. Iran. J. Neurol. 2015, 14, 67–73. [Google Scholar]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial. JAMA 2025, 333, 1413. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D Supplementation in Children May Prevent Asthma Exacerbation Triggered by Acute Respiratory Infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Nanzer, A.M.; Chambers, E.S.; Ryanna, K.; Freeman, A.T.; Colligan, G.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; et al. The Effects of Calcitriol Treatment in Glucocorticoid-Resistant Asthma. J. Allergy Clin. Immunol. 2014, 133, 1755–1757.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between Vitamin D Status and Asthma Control: A Meta-Analysis of Randomized Trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef]
- Pojsupap, S.; Iliriani, K.; Sampaio, T.Z.A.L.; O’Hearn, K.; Kovesi, T.; Menon, K.; McNally, J.D. Efficacy of High-Dose Vitamin D in Pediatric Asthma: A Systematic Review and Meta-Analysis. J. Asthma 2015, 52, 382–390. [Google Scholar] [CrossRef]
- Niu, H.; He, H.; Zhao, Z.; Lu, X.; Zhao, G. Asthmatic Patients with Vitamin D Deficiency: Can Vitamin D Supplementation Make a Difference. Technol. Health Care 2024, 32, 3985–4008. [Google Scholar] [CrossRef]
- Riverin, B.D.; Maguire, J.L.; Li, P. Vitamin D Supplementation for Childhood Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0136841. [Google Scholar] [CrossRef]
- Andjelkovic, Z.; Vojinovic, J.; Pejnovic, N.; Popovic, M.; Dujic, A.; Mitrovic, D.; Pavlica, L.; Stefanovic, D. Disease Modifying and Immunomodulatory Effects of High Dose 1 Alpha (OH) D3 in Rheumatoid Arthritis Patients. Clin. Exp. Rheumatol. 1999, 17, 453–456. [Google Scholar]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of Vitamin D on Thyroid Autoimmunity Markers in Hashimoto’s Thyroiditis: Systematic Review and Meta-Analysis. J. Int. Med. Res. 2021, 49, 03000605211060675. [Google Scholar] [CrossRef]
- Gregoriou, E.; Mamais, I.; Tzanetakou, I.; Lavranos, G.; Chrysostomou, S. The Effects of Vitamin D Supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials. Rev. Diabet. Stud. 2017, 14, 260–268. [Google Scholar] [CrossRef]
- Najjar, L.; Sutherland, J.; Zhou, A.; Hyppönen, E. Vitamin D and Type 1 Diabetes Risk: A Systematic Review and Meta-Analysis of Genetic Evidence. Nutrients 2021, 13, 4260. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.F.; Moreira, C.F.F.; Da Fonseca, E.R.; Fedeszen, P.M.K.; De Paula, T.P.; De Sena, A.S.S.; De Almeida, N.F.A.; Bandeira Filho, O.C.D.S.; Curval, D.R.; Padilha, P.D.C. Effects of Vitamin D Supplementation on Glycemic Control of Children and Adolescents with Type 1 Diabetes Mellitus: A Systematic Review. J. Pediatr. Endocrinol. Metab. 2022, 35, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef] [PubMed]
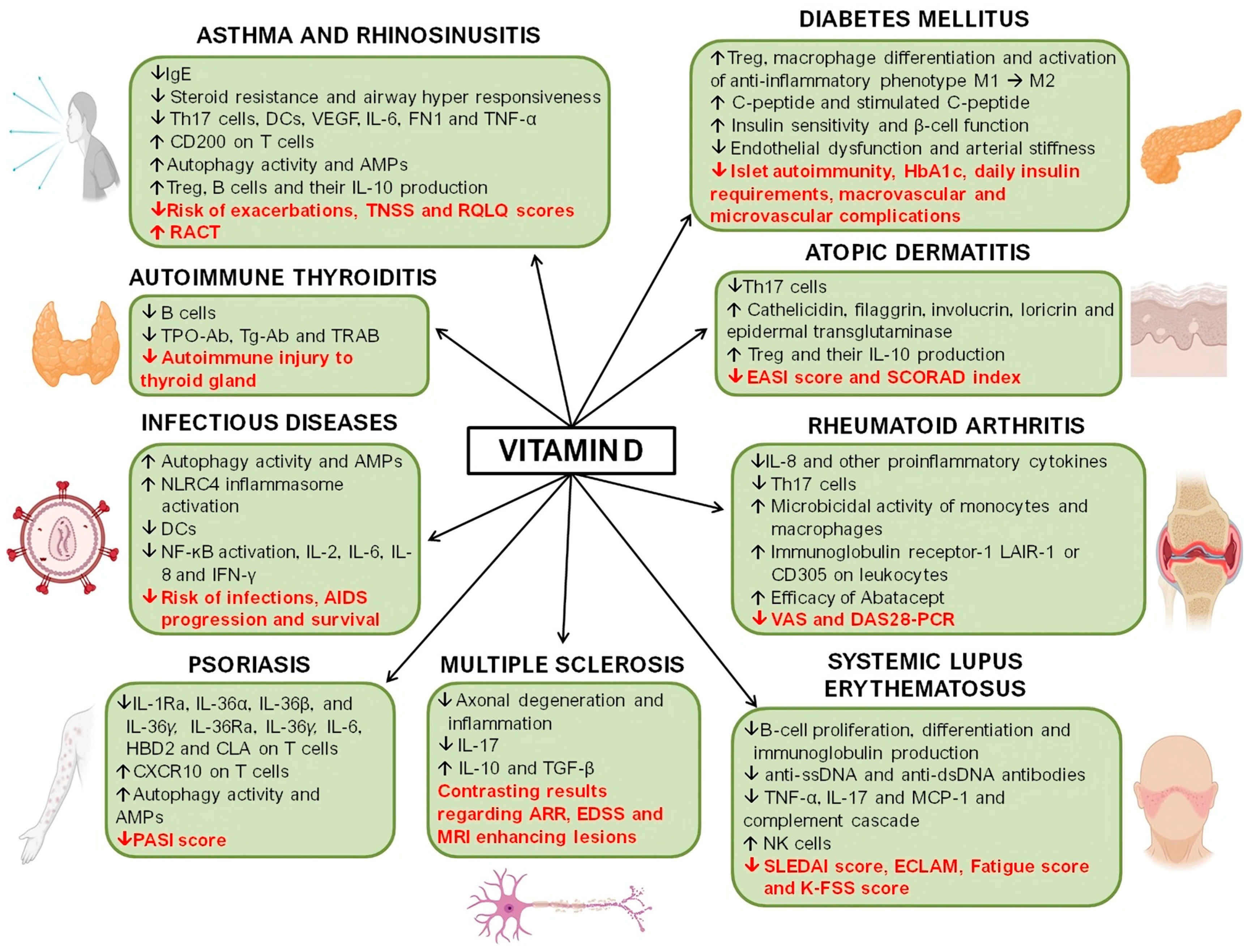

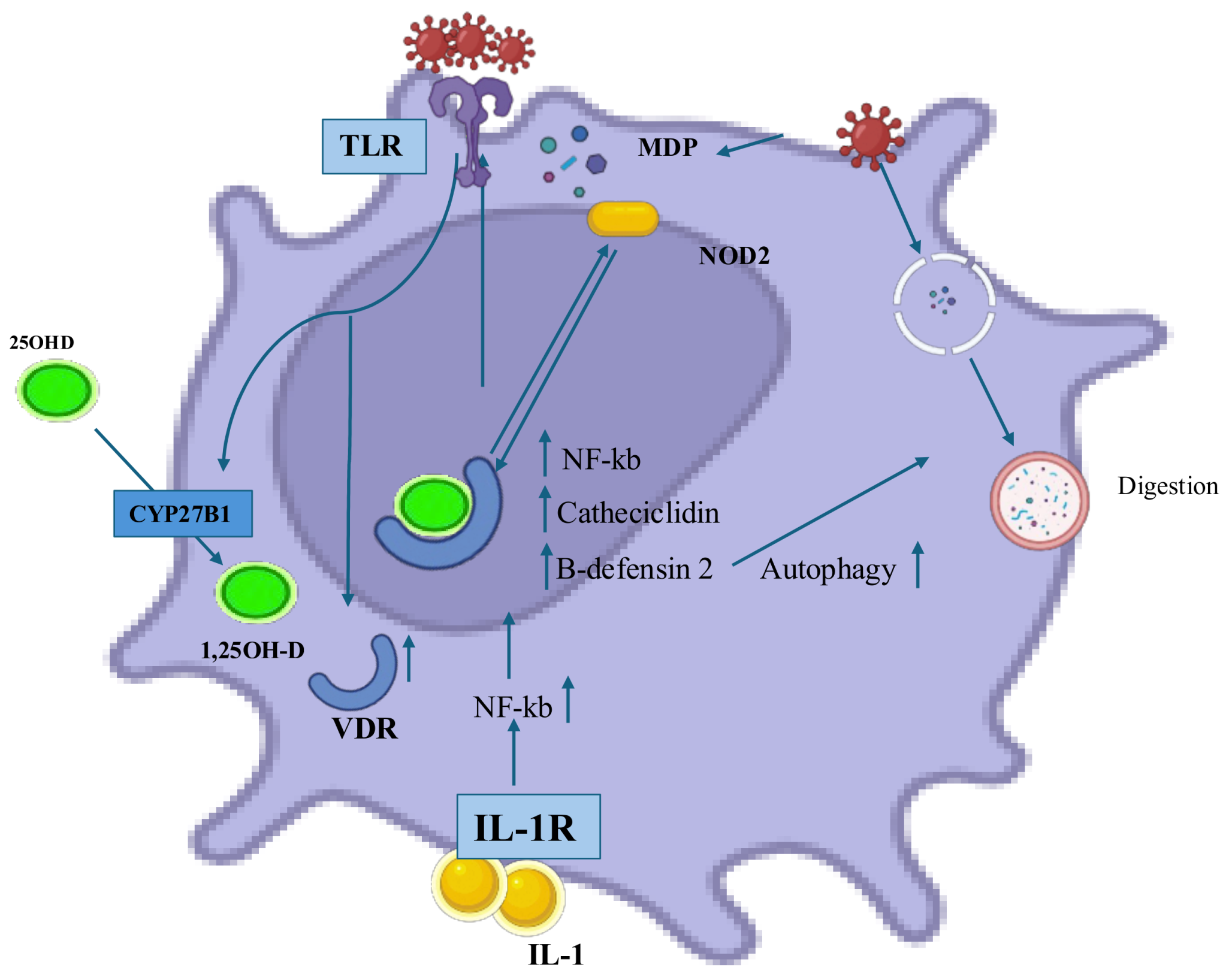
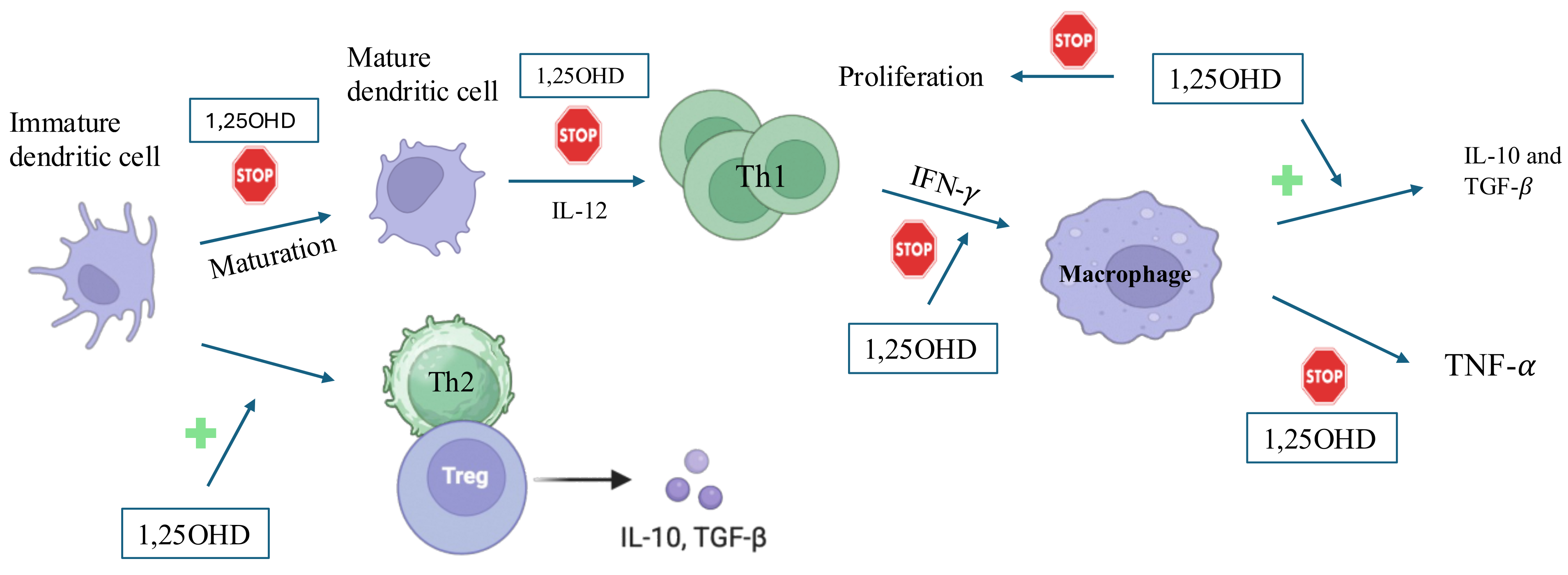
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argano, C.; Torres, A.; Orlando, V.; Cangialosi, V.; Maggio, D.; Pollicino, C.; Corrao, S. Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2025, 26, 4798. https://doi.org/10.3390/ijms26104798
Argano C, Torres A, Orlando V, Cangialosi V, Maggio D, Pollicino C, Corrao S. Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases. International Journal of Molecular Sciences. 2025; 26(10):4798. https://doi.org/10.3390/ijms26104798
Chicago/Turabian StyleArgano, Christiano, Alessandra Torres, Valentina Orlando, Virginia Cangialosi, Dalila Maggio, Chiara Pollicino, and Salvatore Corrao. 2025. "Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases" International Journal of Molecular Sciences 26, no. 10: 4798. https://doi.org/10.3390/ijms26104798
APA StyleArgano, C., Torres, A., Orlando, V., Cangialosi, V., Maggio, D., Pollicino, C., & Corrao, S. (2025). Molecular Insight into the Role of Vitamin D in Immune-Mediated Inflammatory Diseases. International Journal of Molecular Sciences, 26(10), 4798. https://doi.org/10.3390/ijms26104798








