The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity
Abstract
1. Introduction
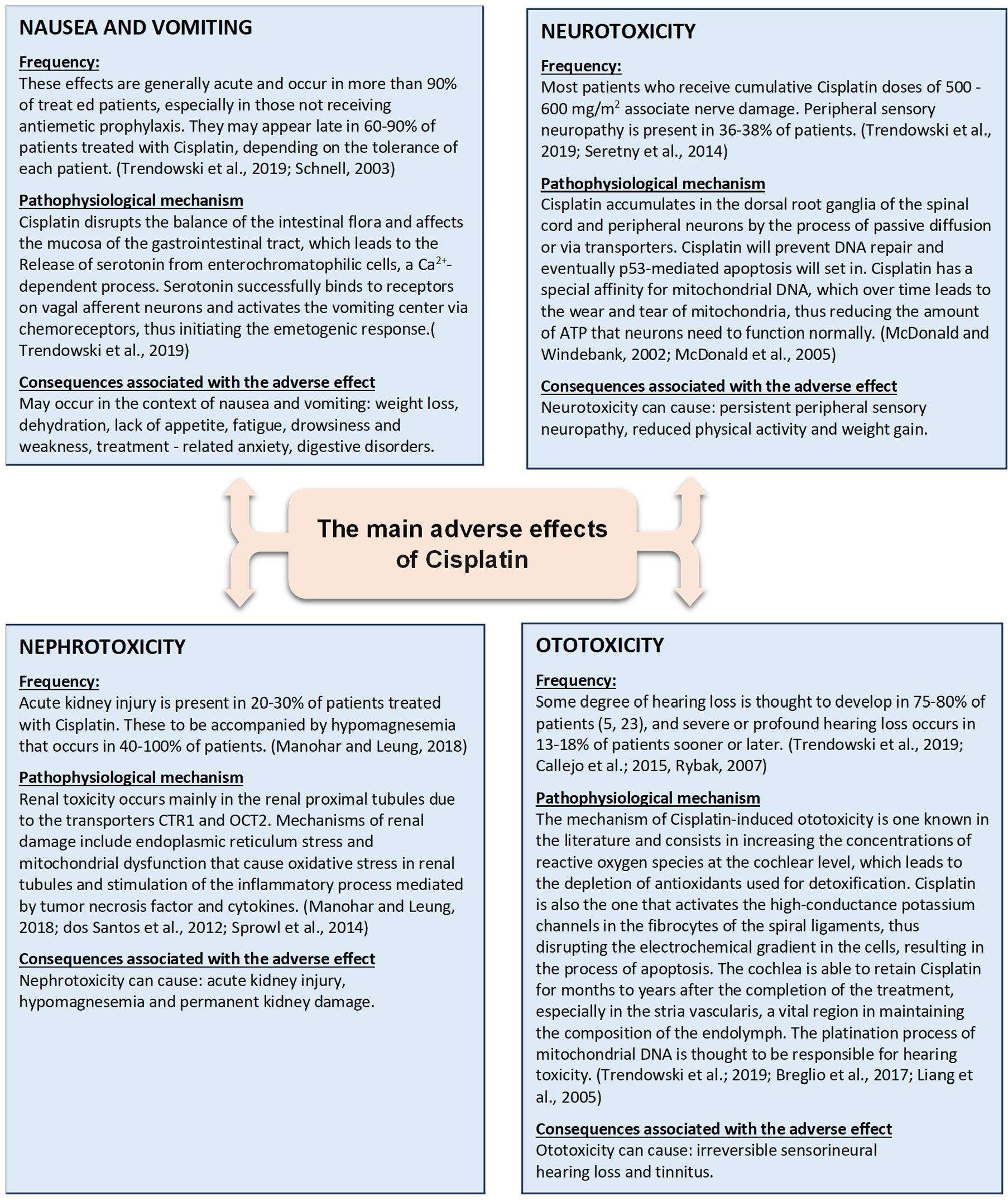
2. Main Side Effects of Cisplatin
3. Genetic and Non-Genetic Factors in Cisplatin Ototoxicity
3.1. Genetic Factors
| The Gene Involved | The Effect | The Study and the Authors Who Mention It | Remarks |
|---|---|---|---|
| The copper transporter protein 1 (CTR1) rs10981694 | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Lanvers-Kaminsky et al., 2015) [42] (Xu et al., 2012) [43] | One way in which cisplatin enters the cochlea is through the copper transporter 1 (CTR1). In the cochlea, CTR1 is in the outer hair cells, inner hair cells, the stria vascularis, and the neurons of the spiral ganglia, facilitating the drug’s entry at these sites and ultimately leading to cellular apoptosis. [42] This process could interfere with the synthesis of functional proteins and reduce the efficiency of gene translation. Any genotypic polymorphism may cause a structural modification of the CTR1 protein, thereby affecting its transporter function. Meanwhile, genetic mutations may also contribute to the regulation of CTR1 expression. The analyzed studies do not find a significant association with the risk of ototoxicity in patients treated with cisplatin. However, the study by Xu et al. [43] concludes that there may be an association with cisplatin-induced ototoxicity in Asian and Caucasian patients. Molecular and cellular research is needed to explore how CTR1 mutations affect the absorption, accumulation, efficacy, and response to Cisplatin treatment, as there are few studies available, and their conclusions are contradictory. |
| GSTM1 del | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Lui et al., 2018) [27] (Choeyprasert et al., 2013) [24] (Barahmani et al., 2009) [30] (Oldenburg et al., 2007) [28] | One of the mechanisms by which Cisplatin causes ototoxicity is the generation of reactive oxygen species, which induce oxidative stress in the cochlea. This process is counteracted by antioxidant enzymes, specifically glutathione S-transferases (GSTs), which prevent this effect. For GSTM1, all the studies we analyzed found an insignificant association with ototoxicity. The absence of both GSTM1 and GSTT1 may further increase the risk of ototoxicity. Some patients with a combined null GSTM1 + null GSTT1 genotype developed severe hearing loss compared to those who had at least one functional gene. |
| GSTT1 del | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Barahmani et al., 2009) [30] (Oldenburg et al., 2007) [28] | GSTT1 helps neutralize toxic compounds by conjugating them with glutathione. In the absence of GSTT1 (null GSTT1 genotype), cisplatin elimination is less efficient, which can lead to increased drug accumulation in the hair cells of the inner ear, thereby promoting cell death and hearing loss. Studies have shown that patients with a null GSTT1 genotype are more prone to severe ototoxicity following cisplatin treatment. In a murine model (CBA/CaJ mice), it was demonstrated that the simultaneous absence of GSTT1 and GSTM1 exacerbates cisplatin-induced hearing loss, suggesting a protective role of these genes against the drug’s toxic effects. Since the process of evolution had no opportunity to adapt to the toxic effects of chemical compounds introduced during industrialization and modern medications, genetically deleted GST isoenzymes may play a role in either the toxification or detoxification of synthetic chemicals or drugs. This explains the contradictory results of studies, in addition to differences in interpretation scales and the influence of various non-genetic factors (such as sex, age, cancer type, treatment duration, etc.) that can impact outcomes. |
| Partial ototoxic effect | (Lui et al., 2018) [27] (Talach et al., 2016) [31] | ||
| Potential otoprotective effect | (Choeyprasert et al., 2013) [24] | ||
| GSTP1 rs1695 | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Oldenburg et al., 2007) [28] | GSTP1 (glutathione S-transferase P1) is an enzyme that, like GSTM1 and GSTT1, helps detoxify toxic compounds, including cisplatin, by conjugating them with glutathione. Polymorphic variants of GSTP1, particularly rs1695 (which results in the substitution of the amino acid isoleucine with valine at position 105), can influence enzymatic activity and the efficiency of detoxification processes, which may affect susceptibility to cisplatin-induced ototoxicity [14, 27]. Oldenburg et al. [28] suggest that alterations in intracellular apoptosis pathways could be an additional explanation for the varying detoxification efficiencies: GSTP1 monomers bind to and thus inactivate stress-inducible Jun N-terminal kinase (JNK). Oxidative stress releases GSTP1 from JNK, which in turn activates the expression of GSTP1 and other genes involved in apoptosis and cytoprotection. Inhibition of JNK in guinea pigs treated with cisplatin increased ototoxicity. Hypothetically, protection against cisplatin-induced toxicities could be due to the less efficient inactivation of JNK by the GSTP1 enzyme [28]. The other studies analyzed reveal a clear association with cisplatin-induced ototoxicity. |
| Ototoxic effect | (Lui et al., 2018) [27] (Olgun et al., 2016) [14] | ||
| SLC16A5 rs4788863 | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Lui et al., 2018) [27] | SLC16A5 rs4788863 is a genetic polymorphism associated with the SLC16A5 gene, which is part of the monocarboxylate transporter family. Genes in this family are involved in the transport of important substances such as lactate, pyruvate, and other metabolites involved in cellular metabolism. Specifically, SLC16A5 is a transporter involved in the exchange of ions and metabolites, playing a crucial role in cellular metabolism, including in auditory cells [15]. Regarding hearing, SLC16A5 may be involved in regulating cellular metabolism in auditory cells of the inner ear, which are sensitive to oxidative stress and toxins, including cisplatin. Since SLC16A5 is involved in the transport of substances, a genetic variant that alters the activity of this transporter could influence the response of auditory cells to ototoxic drugs such as cisplatin. Studies exploring the link between SLC16A5 rs4788863 and ototoxicity are still ongoing, but it is hypothesized that genetic variants at this level may affect the susceptibility of auditory cells to cisplatin-induced damage. In general, polymorphisms that affect transport and cellular metabolism are often linked to cellular responses to oxidative stress and environmental toxins, with cisplatin being one such agent. It is important to note that studies on the link between SLC16A5 rs4788863 and ototoxicity are limited, and associations may vary depending on various factors. |
| Otoprotective effect | (Drögemöller et al., 2017) [44] | ||
| XPC rs2228001 | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Lui et al., 2018) [27] (Lopes-Aguiar et al., 2016) [45] (Caronia et al., 2009) [46] | The XPC genes are involved in DNA repair through the nucleotide excision repair (NER) mechanism, a process that helps repair damage caused by toxic chemicals such as cisplatin. XPC rs2228001 is a polymorphism in a non-coding region of the XPC gene [45]. XPC is crucial for detecting and repairing cisplatin-induced DNA damage. The rs2228001 variant may affect the efficiency of DNA repair, and individuals with certain variants of this polymorphism may have a reduced ability to repair DNA, thereby increasing the risk of toxic effects, including ototoxicity. Studies have suggested that polymorphisms in the XPC gene, including rs2228001, could be associated with an increased risk of ototoxicity following cisplatin treatment, as a less efficient repair system may allow DNA damage to accumulate in auditory cells, leading to their death and hearing loss [27]. However, the association is complex and may vary depending on other genetic or environmental factors. For example, some patients with the rs2228001 variant may not experience severe ototoxicity, while others with a different genetic profile may suffer more severe effects. |
| XPD rs1799793 | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Lopes-Aguiar et al., 2016) [45] | The XPD genes (also known as ERCC2) are also essential for DNA repair through the excision of lesions, being involved in the same nucleotide excision repair (NER) mechanism. XPD rs1799793 is a polymorphism that affects the function of the XPD gene, resulting in a change in the efficiency of the DNA repair process. XPD is involved in correcting DNA damage caused by toxic agents, including cisplatin. The rs1799793 variant (a polymorphism affecting the activity of the XPD enzyme) can modify the efficiency of this repair process. Individuals with certain variants of rs1799793 may have a slower or less efficient DNA repair system, which can lead to a greater accumulation of DNA damage in auditory cells, particularly after cisplatin treatment [45]. Studies have suggested that the rs1799793 variant may be associated with an increased risk of ototoxicity, as a reduced DNA repair capacity may promote the accumulation of damage in the inner ear. Additionally, the effects may vary depending on the specific type of polymorphism and other genetic or environmental factors. Some research has shown significant associations between the XPD rs1799793 polymorphism and hearing loss following cisplatin treatment. The study conducted by Lui et al. (2018) [27] found a significant association with ototoxicity in a study involving 106 children treated with platinum derivatives, using the Brock criteria. |
| Ototoxic effect | (Lui et al., 2018) [27] | ||
| LRP2 rs2075252 | Insignificant association with ototoxicity | (Choeyprasert et al., 2013) [24] | LRP2, also known as megalin, is a transmembrane receptor that plays an important role in the process of endocytosis and the transport of essential substances such as lipids, vitamins, hormones, and metabolites. Regarding LRP2 rs2075252, it is a polymorphism located in a non-coding region of the LRP2 gene. Typically, polymorphisms found in non-coding regions of genes can influence gene expression or regulate its function, even if they do not directly alter the structure of the encoded protein. Available studies and research suggest that the LRP2 rs2075252 variant may play a significant role in the process of ototoxicity by affecting the efficient elimination of toxic substances from the body [15,23]. Choeyprasert et al. [24] examined several genetic variants and their impact on sensitivity to drug-induced ototoxicity, such as cisplatin. Choeyprasert et al. [24] did not find a strong association between rs2075252 and ototoxicity, but they suggested that variants in LRP2, including rs2075252, might have an indirect role by influencing the capacity to eliminate toxic substances, thus affecting the risk of auditory damage. In this context, rs2075252 does not appear to be directly associated with an increased risk of ototoxicity, but there may be possible indirect implications, particularly concerning detoxification and the elimination of toxic substances from the body. |
| Ototoxic effect | (Tserga et al., 2019) [15] (Riedemann et al., 2008) [23] | ||
| LRP2 rs4668123 | Insignificant association with ototoxicity | (Choeyprasert et al., 2013) [24] | LRP2 rs4668123 is another polymorphism associated with the LRP2 gene, which may affect the functioning of this protein, indirectly influencing the body’s response to toxins, including cisplatin. Regarding LRP2 rs4668123, research shows a clearer association with ototoxic effects, particularly in how this polymorphism influences the transport and metabolism of toxic substances. This suggests that rs4668123 may have a more significant impact on the body’s response to toxins. Riedemann et al. [23] investigated polymorphisms in genes involved in detoxification processes, including LRP2, and found a stronger correlation between variants in this gene and cisplatin-induced ototoxicity. Their study indicated that rs4668123 might alter the efficiency of toxic substance transport and reduce the body’s ability to eliminate cisplatin, leading to increased accumulation in auditory cells and, consequently, a higher risk of hearing loss. According to this study, rs4668123 was associated with a greater risk of ototoxicity due to its effects on the elimination and transport of toxic substances, including cisplatin. Based on the studies by Choeyprasert et al. (2013) [24] and Riedemann et al. (2008) [23], LRP2 rs4668123 appears to be more ototoxic than LRP2 rs2075252, showing a stronger association with the risk of cisplatin-induced ototoxicity. |
| Ototoxic effect | (Tserga et al., 2019) [15] (Riedemann et al., 2008) [23] | ||
| TPMT rs12201199 | Insignificant association with ototoxicity | (Thiesen et al., 2017) [17] (Hagleitner et al., 2014) [47] (Yang et al., 2013) [48] | Polymorphisms in the TPMT (thiopurine S-methyltransferase) gene have been studied for their possible association with cisplatin-induced ototoxicity, but the results remain mixed. TPMT is an enzyme involved in the metabolism of thiopurines, but it also plays a role in drug detoxification, including compounds derived from cisplatin. The effect of TPMT polymorphisms on ototoxicity is complex and context-dependent. Some studies do not find a clear association with the ototoxicity process, while others suggest an increased risk. These differences may be due to factors such as the studied population, cisplatin dosage, genetic interactions, and the analytical methods used. A broader approach, including meta-analytical studies and functional analyses, is needed to clarify the exact way in which TPMT influences ototoxicity. Currently, TPMT cannot be considered a definitive genetic marker for ototoxicity, but it remains a candidate for further research due to its potential role in predicting the risk of hearing loss in patients treated with cisplatin. TPMT rs12201199 appears to have the strongest association with ototoxicity according to studies. This polymorphism could serve as a useful genetic marker for identifying patients at higher risk. TPMT rs1142345 may influence ototoxicity, but the evidence is mixed. It is possible that this variant plays a more significant role when combined with other genetic factors. TPMT rs1800460 does not have a clear association with ototoxicity, and some studies even suggest a potential protective effect (Ross et al., 2009) [32]. Overall, TPMT variants likely influence the metabolism of cisplatin, but further studies are needed to confirm the exact impact of these polymorphisms on ototoxicity. |
| Ototoxic effect | (Tserga et al., 2019) [15] (Pussegoda et al., 2013) [49] (Ross et al., 2009) [32] | ||
| TPMT rs1142345 | Insignificant association with ototoxicity | (Thiesen et al., 2017) [17] (Hagleitner et al., 2014) [47] (Yang et al., 2013) [48] | |
| Ototoxic effect | (Tserga et al., 2019) [15] (Pussegoda et al., 2013) [49] (Ross et al., 2009) [32] | ||
| TPMT rs1800460 | Insignificant association with ototoxicity | (Thiesen et al., 2017) [17] (Hagleitner et al., 2014) [47] (Yang et al., 2013) [48] (Ross et al., 2009) [32] | |
| Ototoxic effect | (Tserga et al., 2019) [15] (Pussegoda et al., 2013) [49] | ||
| COMT rs9332377 | Insignificant association with ototoxicity | (Thiesen et al., 2017) [17] (Pussegoda et al., 2013) [49] (Yang et al., 2013) [48] | COMT (catechol-O-methyltransferase) is an enzyme involved in the metabolism of catecholaminergic neurotransmitters (dopamine, epinephrine, and norepinephrine) and cellular detoxification processes. COMT plays a role in regulating oxidative stress, and genetic variants of this gene may influence the sensitivity of auditory cells to damage induced by cisplatin. The rs9332377 polymorphism is in a regulatory region of the COMT gene and may affect the expression levels of the enzyme. The COMT rs9332377 polymorphism has been investigated in several studies on cisplatin-induced ototoxicity, but the results are mixed. Some studies suggest a possible association, while others do not confirm a significant effect. Hagleitner et al. [47] suggest that rs9332377 may have a protective effect against cisplatin-induced ototoxicity by reducing oxidative stress and protecting auditory cells. However, this finding has not been consistently confirmed by other studies, indicating that the protective effect may be influenced by additional factors such as population differences, genetic interactions, and the dosage of cisplatin. Overall, COMT rs9332377 remains an interesting polymorphism, but further studies are needed to confirm its role. |
| Ototoxic effect | (Tserga et al., 2019) [15] (Talach et al., 2016) [31] (Ross et al., 2009) [32] | ||
| Potential otoprotective effect | (Hagleitner et al., 2014) [47] | ||
| ACYP2 rs1872328 | Ototoxic effect | (Tserga et al., 2019) [15] (Drögemöller et al., 2018) [37] (Thiesen et al., 2017) [17] (Vos et al., 2016) [36] (Xu et al., 2015) [50] | The ACYP2 rs1872328 polymorphism has been investigated in multiple studies for its association with cisplatin-induced ototoxicity, and evidence suggests that it plays a significant role in genetic susceptibility to hearing loss. ACYP2 is a gene involved in energy metabolism and cellular signalling. Studies suggest that the rs1872328 polymorphism affects biochemical pathways related to the survival of hair cells in the inner ear, making them more vulnerable to damage caused by cisplatin. The study by Xu et al. was one of the first to identify ACYP2 rs1872328 as a genetic factor associated with cisplatin-induced ototoxicity. ACYP2 may influence metabolic pathways that affect the sensitivity of hair cells to oxidative stress, leading to cell death and ototoxicity. This study paved the way for further research, indicating that patients carrying the rs1872328 variant have a higher risk of ototoxicity. The study by Vos et al. [36] attempted to replicate Xu et al.’s findings [50] in a different cohort. Although they confirmed a certain association between rs1872328 and ototoxicity, the effect was weaker than in the previous study. The differences may be due to ethnic variations and the doses of cisplatin administered to patients. The study by Thiesen et al. [17] investigated multiple polymorphisms in the context of cisplatin-induced ototoxicity. They did not find a clear association between rs1872328 and ototoxicity but noted that the trend observed in other studies warrants further investigation. The study did not confirm a strong association but also did not rule out the possibility that rs1872328 plays a role. The study by Drögemöller et al. [37] analyzed genetic factors involved in ototoxicity in a large and diverse population. They identified rs1872328 as a significant marker of ototoxicity. The study by Tserga et al. [15] was a meta-analysis evaluating multiple genes associated with ototoxicity. It confirmed that ACYP2 rs1872328 is associated with an increased risk of ototoxicity, supporting the initial conclusions of Xu et al. (2015) [50]. The polymorphism may affect the cell cycle and metabolic pathways essential for the protection of auditory cells. Tserga et al. [15] suggest that rs1872328 could be used as a genetic marker to identify patients at higher risk of hearing loss before undergoing cisplatin treatment. Overall, rs1872328 appears to be one of the most well-studied biomarkers of cisplatin-induced ototoxicity, but its effect may depend on factors such as the cisplatin dose, genetic background, and interactions with other genetic and environmental factors. |
| SOD 2(superoxide dismutase 2 mitochondrial) rs4880 | Insignificant association with ototoxicity | (Lui et al., 2018) [27] | The rs4880 polymorphism of the SOD2 gene has been investigated in the context of cisplatin-induced ototoxicity, particularly in pediatric patients with medulloblastoma. A significant study in this area is that of Brown et al., [38] which analyzed the association between this polymorphism and hearing loss in children treated with cisplatin. In this study, it was found that the C allele of the rs4880 polymorphism is associated with an increased risk of ototoxicity. Specifically, patients who inherited this allele were more likely to experience significant hearing loss following cisplatin treatment. The proposed mechanism suggests that the Ala variant (encoded by the C allele) increases the activity of the MnSOD enzyme, which may result in an excessive accumulation of hydrogen peroxide in cochlear cells, thereby contributing to oxidative stress and hearing damage. It is important to note that while this study provides evidence of an association between rs4880 and ototoxicity, further research is needed to confirm these findings and to fully understand the underlying mechanisms involved. |
| Ototoxic effect | (Tserga et al., 2019) [15] (Brown et al., 2015) [38] | ||
| GSTM3 rs1799735 | Insignificant association with ototoxicity | (Lui et al., 2018) [27] | In a study by Peters et al. [29], an association with otoprotection was observed for the GSTM3 polymorphism. It is indeed believed that variations in GSTM3 alter susceptibility to potential carcinogens and toxins. There is research suggesting that GSTM3 rs1799735 may be an otoprotective factor under certain conditions, but it has not been fully clarified by a single study. |
| Otoprotective effect | (Tserga et al., 2019) [15] (Peters et al., 2000) [29] | ||
| SLC22A2 rs316019 (drug efflux transporter) | Insignificant association with ototoxicity | (Tserga et al., 2019) [15] (Spracklen et al., 2017) [40] | The single-nucleotide polymorphism rs316019 in the SLC22A2 gene, also known as Ala270Ser, affects the function of the organic cation transporter 2 (hOCT2). This transporter is essential for the elimination of certain drugs and metabolites through the kidneys. Both the study by Lanvers-Kaminsky et al. [42] and that by Spracklen et al. [40] highlight the importance of the rs316019 polymorphism in modulating hOCT2 function. These studies provide valuable insights into how the rs316019 variant can influence drug response and the risk of side effects, paving the way for personalized therapeutic strategies based on the patient’s genetic profile. In theory, if rs316019 modulates the transport of cisplatin and thus reduces its accumulation in auditory tissues, this polymorphism could protect against ototoxicity. This would imply an otoprotective effect. However, direct evidence for such an effect is still lacking. |
| Otoprotective effect | (Lanvers-Kaminsky et al., 2015) [42] | ||
| ABCC3 rs1051640 | Insignificant association with ototoxicity | (Spracklen et al., 2017) [40] (Pussegoda et al., 2013) [49] | The ABCC3 rs1051640 polymorphism is part of the ABCC3 gene, which encodes an ATP-binding cassette (ABC) family membrane transporter called MRP3 (multidrug resistance protein 3). MRP3 is involved in the transport of various toxic substances, including drugs, across cell membranes, particularly in the liver, kidneys, and other tissues. Regarding ototoxicity and otoprotection, studies directly addressing ABCC3 and the rs1051640 polymorphism are limited. Based on its role in expelling toxic substances and various items of evidence suggesting that MRP3 transporters can reduce toxin accumulation, ABCC3 rs1051640 could be associated with an otoprotective effect if this variant leads to increased MRP3 transporter activity, promoting faster elimination of cisplatin from cochlear cells and thus reducing the risk of damage [40,49]. |
| Otoprotective effect | (Tserga et al., 2019) [15] |
3.2. Non-Genetic Factors
3.2.1. Age and Sex of the Patient
3.2.2. Concomitant Administration of Other Ototoxic Drugs
3.2.3. Cisplatin Doses
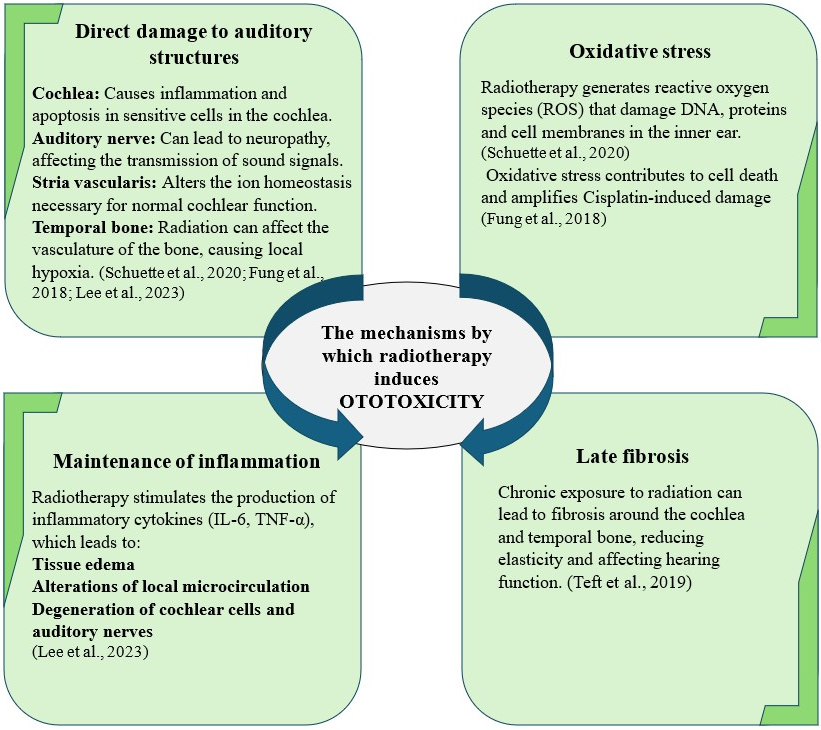
3.2.4. Head and Neck Radiotherapy
3.2.5. Renal Function
3.2.6. Nutritional Status
4. Limitations of Genetic and Non-Genetic Factors: Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACYP2 | Acylphosphatase 2 gene |
| ABCC3 | ATP-Binding Cassette Subfamily C member 3 |
| ABCB5 | ATP-Binding Cassette Subfamily B member 5 |
| CTR1 | Copper transporter 1 |
| COMT | Catechol-O-methyltransferase |
| ERCC | Excision Repair Cross-Complementing Group |
| EPXH1 | Epoxide hydrolase 1 |
| GST | Glutathione S-transferase |
| GSTM1 | Glutathion S-transferase Mu 1 |
| GSTM3 | Glutathion S-transferase Mu 3 |
| GSTT1 | Glutathion S-transferase theta 1 |
| GSTP1 | Glutathion S-transferase pi gene |
| LRP2 | Low-density lipoprotein receptor gene |
| OCT | Organic cation transporter |
| P53 | Tumor antigen p53 |
| ROS | Reactive oxygen species |
| SLC16A5 | Solute Carrier Family 16 Member 5 |
| SLC22A2 | Solute Carrier Family 22 Member 2 |
| SOD | Superoxide dismutase |
| SNP | Single-nucleotide polymorphism |
| TPMT | Thiopurine S-methyltransferase |
| TNF-α | Tumor necrosis factor-α |
| XPC | Xeroderma pigmentosum complementation group C |
| XPD | Xeroderma pigmentosum complementation group D |
References
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. A Review of Cisplatin-Associated Ototoxicity. Semin. Hear. 2019, 40, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Trendowski, M.R.; Charif, O.E.; Dinh, P.C., Jr.; Travis, L.B.; Dolan, M.E. Genetic and Modifiable Risk Factors Contributing to Cisplatin-Induced Toxicities. Clin. Cancer Res. 2019, 25, 1147–1155. [Google Scholar] [CrossRef]
- Schnell, F.M. Chemotherapy-induced nausea and vomiting: The importance of acute antiemetic control. Oncologist 2003, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Windebank, A.J. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol. Dis. 2002, 9, 220–233. [Google Scholar] [CrossRef]
- McDonald, E.S.; Randon, K.R.; Knight, A.; Windebank, A.J. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity. Neurobiol. Dis. 2005, 18, 305–313. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- dos Santos, N.A.G.; Rodrigues, M.A.C.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef]
- Sprowl, J.A.; Lancaster, C.S.; Pabla, N.; Hermann, E.; Kosloske, A.M.; Gibson, A.A.; Li, L.; Zeeh, D.; Schlatter, E.; Janke, L.J.; et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin. Cancer Res. 2014, 20, 4026–4035. [Google Scholar] [CrossRef]
- Callejo, A.; Sedó-Cabezón, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Rybak, L.P. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef]
- Liang, F.; Schulte, B.A.; Qu, C.; Hu, W.; Shen, Z. Inhibition of the calcium- and voltage-dependent big conductance potassium channel ameliorates cisplatin-induced apoptosis in spiral ligament fibrocytes of the cochlea. Neuroscience 2005, 135, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Olgun, Y.; Aktaş, S.; Altun, Z.; Kırkım, G.; Kızmazoğlu, D.Ç.; Erçetin, A.P.; Demir, B.; İnce, D.; Mutafoğlu, K.; Demirağ, B.; et al. Analysis of genetic and non genetic risk factors for cisplatin ototoxicity in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 64–69. [Google Scholar] [CrossRef]
- Tserga, E.; Nandwani, T.; Edvall, N.K.; Bulla, J.; Patel, P.; Canlon, B.; Cederroth, C.R.; Baguley, D.M. The genetic vulnerability to cisplatin ototoxicity: A systematic review. Sci. Rep. 2019, 9, 3455. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; Zehnhoff-Dinnesen, A.; Radtke, S.; Meitert, J.; Zolk, O. Understanding platinum-induced ototoxicity. Trends Pharmacol. Sci. 2013, 34, 458–469. [Google Scholar] [CrossRef]
- Thiesen, S.; Yin, P.; Jorgensen, A.L.; Zhang, J.E.; Manzo, V.; McEvoy, L.; Barton, C.; Picton, S.; Bailey, S.; Brock, P.; et al. TPMT, COMT and ACYP2 genetic variants in paediatric cancer patients with cisplatin-induced ototoxicity. Pharmacogenet. Genom. 2017, 27, 213–222. [Google Scholar] [CrossRef]
- Natalie, S.; Alida, N. An overview of pharmacotherapy-induced ototoxicity. S. Afr. Fam. Pract. 2013, 55, 357–365. [Google Scholar] [CrossRef]
- Yancey, A.; Harris, M.S.; Egbelakin, A.; Gilbert, J.; Pisoni, D.B.; Renbarger, J. Risk Factors for Cisplatin-Associated Ototoxicity in Pediatric Oncology Patients. Pediatr. Blood Cancer 2012, 59, 144–148. [Google Scholar] [CrossRef]
- Vona, B.; Nanda, I.; Shehata-Dieler, W.; Haaf, T. Genetics of Tinnitus: Still in its Infancy. Front. Neurosci. 2017, 11, 236. [Google Scholar] [CrossRef]
- Frisina, R.D.; Wheeler, H.E.; Fossa, S.D.; Kerns, S.L.; Fung, C.; Sesso, H.D.; Monahan, P.O.; Feldman, D.R.; Hamilton, R.; Vaughn, D.J.; et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J. Clin. Oncol. 2016, 34, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, C.; Liu, J.; Xu, Y.; Yang, H. Circulating IL-17 reduces the risk of cisplatin-induced hearing loss in children: A bidirectional two-sample Mendelian randomization study. Sci. Rep. 2023, 13, 18957. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, L.; Lanvers, C.; Deuster, D.; Peters, U.; Boos, J.; Jürgens, H.; Zehnhoff-Dinnesen, A.A. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenom. J. 2008, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Choeyprasert, W.; Sawangpanich, R.; Lertsukprasert, K.M.; Udomsubpayakul, U.M.; Songdej, D.; Unurathapan, U.; Pakakasama, S.; Hongeng, S. Cisplatin-induced ototoxicity in pediatric solid tumors: The role of glutathione S-transferases and megalin genetic polymorphisms. J. Pediatr. Hematol. Oncol. 2013, 35, e138–e143. [Google Scholar] [CrossRef]
- Marzolo, M.-P.; Farfán, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef]
- Rednam, S.; Scheurer, M.E.; Adesina, A.; Lau, C.C.; Okcu, M.F. Glutathione S-transferase P1 single nucleotide polymorphism predicts permanent ototoxicity in children with medulloblastoma. Pediatr. Blood Cancer 2013, 60, 593–598. [Google Scholar] [CrossRef]
- Lui, G.; Bouazza, N.; Denoyelle, F.; Moine, M.; Brugières, L.; Chastagner, P.; Corradini, N.; Entz-Werle, N.; Vérité, C.; Landmanparker, J.; et al. Association between genetic polymorphisms and platinum-induced ototoxicity in children. Oncotarget 2018, 9, 30883–30893. [Google Scholar] [CrossRef]
- Oldenburg, J.; Kraggerud, S.M.; Brydøy, M.; Cvancarova, M.; A Lothe, R.; Fossa, S.D. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J. Transl. Med. 2007, 5, 70. [Google Scholar] [CrossRef]
- Peters, U.; Preisler-Adams, S.; Hebeisen, A.; Hahn, M.; Seifert, E.; Lanvers, C.; Heinecke, A.; Horst, J.; Jürgens, H.; Lamprecht-Dinnesen, A. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anti-Cancer Drugs 2000, 11, 639–643. [Google Scholar] [CrossRef]
- Barahmani, N.; Carpentieri, S.; Li, X.-N.; Wang, T.; Cao, Y.; Howe, L.; Kilburn, L.; Chintagumpala, M.; Lau, C.; Okcu, M.F. Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro. Oncol. 2009, 11, 292–300. [Google Scholar] [CrossRef]
- Talach, T.; Rottenberg, J. Genetic risk factors of cisplatin induced ototoxicity in adult patients. Neoplasma 2016, 63, 263–268. [Google Scholar] [CrossRef]
- the CPNDS Consortium; Ross, C.J.D.; Katzov-Eckert, H.; Dubé, M.-P.; Brooks, B.; Rassekh, S.R.; Barhdadi, A.; Feroz-Zada, Y.; Visscher, H.; Brown, A.M.K.; et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat. Genet. 2009, 41, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Asadov, C.; Aliyeva, G.; Mustafayeva, K. Thiopurine S-Methyltransferase as a Pharmacogenetic Biomarker: Significance of Testing and Review of Major Methods. Cardiovasc. Hematol. Agents Med. Chem. 2017, 15, 23–30. [Google Scholar] [CrossRef]
- Bhavsar, A.P.; Gunaretnam, E.P.; Li, Y.; Hasbullah, J.S.; Carleton, B.C.; Ross, C.J.D. Pharmacogenetic variants in TPMT alter cellular responses to cisplatin in inner ear cell lines. PLoS ONE 2017, 12, e0175711. [Google Scholar] [CrossRef]
- Toro, C.; Trapani, J.G.; Pacentine, I.; Maeda, R.; Sheets, L.; Mo, W.; Nicolson, T. Dopamine Modulates the Activity of Sensory Hair Cells. J. Neurosci. 2015, 35, 16494–16503. [Google Scholar] [CrossRef] [PubMed]
- Vos, H.I.; Guchelaar, H.-J.; Gelderblom, H.; de Bont, E.S.; Kremer, L.C.; Naber, A.M.; Hakobjan, M.H.; van der Graaf, W.T.; Coenen, M.J.; Loo, D.M.W.T. Replication of a genetic variant in ACYP2 associated with cisplatin-induced hearing loss in patients with osteosarcoma. Pharmacogenet. Genom. 2016, 26, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Drögemöller, B.I.; Brooks, B.; Critchley, C.; Monzon, J.G.; Wright, G.E.; Liu, G.; Renouf, D.J.; Kollmannsberger, C.K.; Bedard, P.L.; Hayden, M.R.; et al. Further Investigation of the Role of ACYP2 and WFS1 Pharmacogenomic Variants in the Development of Cisplatin-Induced Ototoxicity in Testicular Cancer Patients. Clin. Cancer Res. 2018, 24, 1866–1871. [Google Scholar] [CrossRef]
- Brown, A.L.; Lupo, P.J.; Okcu, M.F.; Lau, C.C.; Rednam, S.; Scheurer, M.E. SOD2 genetic variant associated with treatment-related ototoxicity in cisplatin-treated pediatric medulloblastoma. Cancer Med. 2015, 4, 1679–1686. [Google Scholar] [CrossRef]
- Khokhrin, D.V.; Khrunin, A.V.; Ivanova, F.G.; Moisseev, A.A.; Gorbunova, V.A.; Limborska, S.A. Pharmacogenomics of cisplatin-based chemotherapy in ovarian cancer patients from Yakutia. Mol. Genet. Microbial. Vitrosol. 2013, 28, 137–140. [Google Scholar] [CrossRef]
- Spracklen, T.F.; Vorster, A.A.; Ramma, L.; Dalvie, S.; Ramesar, R.S. Promoter region variation in NFE2L2 influences susceptibility to ototoxicity in patients exposed to high cumulative doses of cisplatin. Pharmacogenom. J. 2017, 17, 515–520. [Google Scholar] [CrossRef]
- Grondin, Y.; Bortoni, M.E.; Sepulveda, R.; Ghelfi, E.; Bartos, A.; Cotanche, D.; Clifford, R.E.; Rogers, R.A. Genetic Polymorphisms Associated with Hearing Threshold Shift in Subjects during First Encounter with Occupational Impulse Noise. PLoS ONE 2015, 10, e0130827. [Google Scholar] [CrossRef] [PubMed]
- Lanvers-Kaminsky, C.; Sprowl, J.A.; Malath, I.; Deuster, D.; Eveslage, M.; Schlatter, E.; Mathijssen, R.H.; Boos, J.; Jürgens, H.; Zehnhoff-Dinnesen, A.G.A.; et al. Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics 2015, 16, 323–332. [Google Scholar] [CrossRef]
- Xu, X.; Ren, H.; Zhou, B.; Zhao, Y.; Yuan, R.; Ma, R.; Zhou, H.; Liu, Z. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer 2012, 77, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Drögemöller, B.I.; Monzon, J.G.; Bhavsar, A.P.; Borrie, A.E.; Brooks, B.; Wright, G.E.B.; Liu, G.; Renouf, D.J.; Kollmannsberger, C.K.; Bedard, P.L.; et al. Association Between SLC16A5 Genetic Variation and Cisplatin-Induced Ototoxic Effects in Adult Patients with Testicular Cancer. JAMA Oncol. 2017, 3, 1558–1562. [Google Scholar] [CrossRef][Green Version]
- Lopes-Aguiar, L.; Costa, E.F.D.; Nogueira, G.A.S.; Lima, T.R.P.; Visacri, M.B.; Pincinato, E.C.; Calonga, L.; Mariano, F.V.; Altemani, A.M.d.A.M.; Altemani, J.M.C.; et al. XPD c.934G>A polymorphism of nucleotide excision repair pathway in outcome of head and neck squamous cell carcinoma patients treated with cisplatin chemoradiation. Oncotarget 2016, 8, 16190–16201. [Google Scholar] [CrossRef] [PubMed]
- Caronia, D.; Patiño-García, A.; Milne, R.L.; Zalacain-Díez, M.; Pita, G.; Alonso, M.R.; Moreno, L.T.; Sierrasesumaga-Ariznabarreta, L.; Benítez, J.; González-Neira, A. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009, 9, 347–353. [Google Scholar] [CrossRef]
- Hagleitner, M.M.; Coenen, M.J.H.; Patino-Garcia, A.; de Bont, E.S.J.M.; Gonzalez-Neira, A.; Vos, H.I.; van Leeuwen, F.N.; Gelderblom, H.; Hoogerbrugge, P.M.; Guchelaar, H.-J.; et al. Influence of Genetic Variants in TPMT and COMT Associated with Cisplatin Induced Hearing Loss in Patients with Cancer: Two New Cohorts and a Meta-Analysis Reveal Significant Heterogeneity between Cohorts. PLoS ONE 2014, 9, e115869. [Google Scholar] [CrossRef]
- Yang, J.J.; Lim, J.Y.-S.; Huang, J.; Bass, J.; Wu, J.; Wang, C.; Fang, J.; Stewart, E.; Harstead, E.H.; Robinson, G.W.; et al. The Role of Inherited TPMT and COMT Genetic Variation in Cisplatin-induced Ototoxicity in Children with Cancer. Clin. Pharmacol. Ther. 2013, 94, 252–259. [Google Scholar] [CrossRef]
- Pussegoda, K.; Ross, C.; Visscher, H.; Yazdanpanah, M.; Brooks, B.; Rassekh, S.R.; Zada, Y.F.; Dubé, M.-P.; Carleton, B.C.; Hayden, M.R. Replication of TPMT and ABCC3 Genetic Variants Highly Associated with Cisplatin-Induced Hearing Loss in Children. Clin. Pharmacol. Ther. 2013, 94, 243–251. [Google Scholar] [CrossRef]
- Xu, H.; Robinson, G.W.; Huang, J.; Lim, J.Y.-S.; Zhang, H.; Bass, J.K.; Broniscer, A.; Chintagumpala, M.; Bartels, U.; Gururangan, S.; et al. Common Variants in ACYP2 Influence Susceptibility to Cisplatin-induced Hearing Loss. Nat. Genet. 2015, 47, 263–266. [Google Scholar] [CrossRef]
- Knight, K.R.G.; Kraemer, D.F.; Neuwelt, E.A. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005, 23, 8588–8596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Womer, R.B.; Silber, J. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur. J. Cancer 2004, 40, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Skalleberg, J.; Småstuen, M.C.; Oldenburg, J.; Osnes, T.; Fosså, S.D.; Bunne, M. The Relationship Between Cisplatin-related and Age-related Hearing Loss During an Extended Follow-up. Laryngoscope 2020, 130, E515–E521. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Wu, C.-C.; Hsieh, M.-C.; Rau, K.-M.; Chiang, P.-H.; Sung, M.-T.; Luo, H.-L.; Huang, C.-C.; Huang, C.-H.; Liu, J.-M.; et al. Comparative Study of the Safety and Efficacy of First-Line Cisplatin and Carboplatin Chemotherapy in Elderly Patients with Metastatic Urothelial Carcinoma. Oncology 2020, 98, 146–153. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, X.; Chai, R.; Fan, J. Progress on mechanisms of age-related hearing loss. Front. Neurosci. 2023, 17, 1253574. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Berger, C.C.; Hartmann, J.; Kollmannsberger, C.; Schmoll, H.; Kuczyk, M.; Kanz, L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer. 1998, 77, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Delhez, A.; Lefebvre, P.; Péqueux, C.; Malgrange, B.; Delacroix, L. Auditory function and dysfunction: Estrogen makes a difference. Cell Mol. Life Sci. 2020, 77, 619–635. [Google Scholar] [CrossRef]
- Kilicdag, E.B.; Yavuz, H.; Bagis, T.; Tarim, E.; Erkan, A.N.; Kazanci, F. Effects of estrogen therapy on hearing in postmenopausal women. Am. J. Obstet. Gynecol. 2004, 190, 77–82. [Google Scholar] [CrossRef]
- Kirkim, G.; Olgun, Y.; Aktas, S.; Kiray, M.; Kolatan, E.; Altun, Z.; Erçetin, P.; Bagriyanik, A.; Yilmaz, O.; Ellidokuz, H. Is there a gender-related susceptibility for cisplatin ototoxicity? Eur. Arch. Otorhinolaryngol. 2015, 272, 2755–2763. [Google Scholar] [CrossRef]
- Marcu, L.G. Gender and Sex-Related Differences in Normal Tissue Effects Induced by Platinum Compounds. Pharmaceuticals 2022, 15, 255. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007, 226, 157–167. [Google Scholar] [CrossRef]
- Bertolini, P.; Lassalle, M.; Mercier, G.; Raquin, M.A.; Izzi, G.; Corradini, N.; Hartmann, O. Platinum compound-related ototoxicity in children: Long-term follow-up reveals continuous worsening of hearing loss. J. Pediatr. Hematol. Oncol. 2004, 26, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Allman, B.L.; Salvi, R. Review: Ototoxic characteristics of platinum antitumor drugs. Anat. Rec. 2012, 295, 1851–1867. [Google Scholar] [CrossRef]
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a033548. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, D.; Jiang, H.; Fu, Y.; Salvi, R. Co-administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurotox. Res. 2011, 20, 307–319. [Google Scholar] [CrossRef]
- Xia, L.; Chen, Z.; Su, K.; Yin, S.; Wang, J. Comparison of cochlear cell death caused by cisplatin, alone and in combination with furosemide. Toxicol. Pathol. 2014, 42, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.K.; Fullerton, A.M.; McMahon, C.M. Ototoxic hearing loss from antimalarials: A systematic narrative review. Travel Med. Infect. Dis. 2021, 43, 102117. [Google Scholar] [CrossRef]
- Dean, J.B.; Hayashi, S.S.; Albert, C.M.; King, A.A.; Karzon, R.; Hayashi, R.J. Hearing loss in pediatric oncology patients receiving carboplatin-containing regimens. J. Pediatr. Hematol. Oncol. 2008, 30, 130–134. [Google Scholar] [CrossRef]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Schuette, A.; Lander, D.P.; Kallogjeri, D.; Collopy, C.; Goddu, S.; Wildes, T.M.; Daly, M.; Piccirillo, J.F. Predicting Hearing Loss After Radiotherapy and Cisplatin Chemotherapy in Patients with Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 106–112. [Google Scholar] [CrossRef]
- Fung, C.; Dinh, P.; Ardeshir-Rouhani-Fard, S.; Schaffer, K.; Fossa, S.D.; Travis, L.B. Toxicities Associated with Cisplatin-Based Chemotherapy and Radiotherapy in Long-Term Testicular Cancer Survivors. Adv. Urol. 2018, 2018, 8671832. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Travis, E.Y.; Wong, S.K.; Collopy, C.; McClannahan, K.S.; Ortmann, A.J.; Rich, J.T.; Pipkorn, P.; Puram, S.V.; Jackson, R.S.; et al. Audiologic Follow-up in Patients with Head and Neck Cancer Treated With Cisplatin and Radiation. Laryngoscope 2023, 133, 3161–3168. [Google Scholar] [CrossRef]
- Teft, W.A.; Winquist, E.; Nichols, A.C.; Kuruvilla, S.; Richter, S.; Parker, C.; Francis, P.; Trinnear, M.; Lukovic, J.; Bukhari, N.; et al. Predictors of cisplatin-induced ototoxicity and survival in chemoradiation treated head and neck cancer patients. Oral Oncol. 2019, 89, 72–78. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.-W.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Zuur, C.L.; Simis, Y.J.; Lansdaal, P.E.; Hart, A.A.; Rasch, C.R.; Schornagel, J.H.; Dreschler, W.A.; Balm, A.J. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: A multivariate analysis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1320–1325. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, S.H.; Yeo, S.G. Association of Nutritional Factors with Hearing Loss. Nutrients 2019, 11, 307. [Google Scholar] [CrossRef]
- Olusanya, B.O. Is undernutrition a risk factor for sensorineural hearing loss in early infancy? Br. J. Nutr. 2010, 103, 1296–1301. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Miller, J.M.; Tucker, K.L.; Hu, H.; Park, S.K. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am. J. Clin. Nutr. 2014, 99, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, N.; Taheri, S. A narrative review of obesity and hearing loss. Int. J. Obes. 2017, 41, 1066–1073. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Curhan, S.G.; Eavey, R.; Curhan, G.C. A prospective study of vitamin intake and the risk of hearing loss in men. Otolaryngol. Head Neck Surg. 2010, 142, 231–236. [Google Scholar] [CrossRef]
- Gopinath, B.; Flood, V.M. Consumption of omega-3 fatty acids and fish and risk of age-related hearing loss. Am. J. Clin. Nutr. 2010, 92, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Clemens, E.; Brooks, B.; de Vries, A.C.H.; van Grotel, M.; Heuvel-Eibrink, M.M.v.D.; Carleton, B. A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy. PLoS ONE 2019, 14, e0210646. [Google Scholar] [CrossRef]
- Chang, K.W.; Chinosornvatana, N. Practical grading system for evaluating cisplatin ototoxicity in children. J. Clin. Oncol. 2010, 28, 1788–1795. [Google Scholar] [CrossRef]
- Brock, P.R.; Knight, K.R.; Freyer, D.R.; Campbell, K.C.; Steyger, P.S.; Blakley, B.W.; Rassekh, S.R.; Chang, K.W.; Fligor, B.J.; Rajput, K.; et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012, 30, 2408–2417. [Google Scholar] [CrossRef]
- Schmidt, C.-M.; Bartholomäus, E.; Deuster, D.; Heinecke, A.; Dinnesen, A.G. The “Muenster classification” of high frequency hearing loss following cisplatin chemotherapy. HNO 2007, 55, 299–306. [Google Scholar] [CrossRef] [PubMed]
- ASHA. Ototoxic Medications (Medication Effects). Available online: https://www.asha.org/public/hearing/ototoxic-medications/?srsltid=AfmBOoqDgdKFqt7TSqnTEa7ifRiKgwoZinUgB-99SG45RwFjIQbwU5cj&utm_source=chatgpt.com (accessed on 12 December 2024).
- Gilbert, A.; Piccinin, C.; Velikova, G.; Groenvold, M.; Kuliś, D.; Blazeby, J.M.; Bottomley, A. Linking the European Organisation for Research and Treatment of Cancer Item Library to the Common Terminology Criteria for Adverse Events. J. Clin. Oncol. 2022, 40, 3770–3780. [Google Scholar] [CrossRef] [PubMed]

| Foods That Can Amplify the Process of Ototoxicity [76,78,79,80,81] | |
|---|---|
| FOOD | SOURCE |
| Foods Rich in Salt Excessive salt consumption can affect the ion balance in the inner ear, aggravating the damage caused by cisplatin. | Meats Processed food Snacks Cheese |
| Saturated and trans fats Diets high in saturated and trans fats may promote systemic inflammation and reduce antioxidant activity | Saturated fats Butter Fat cheeses Cream Fatty meat (pork, lamb) Bacon Meats Coconut/palm oil Trans fats Pastry products Fried products (fries, chips, fast food) Margarine Processed products |
| Artificial additives Some food additives, such as monosodium glutamate (MSG), can amplify oxidative stress in the inner ear. | Processed products Canned foods Packaged products |
| Alcohol Alcohol consumption may increase oxidative stress and inflammation, sensitizing the cochlea to the effects of cisplatin. | |
| Foods that can prevent the process of ototoxicity [76,78,79,80,81] | |
| FOOD | SOURCE |
| Natural antioxidants Vitamin C It neutralizes ROS and reduces oxidative stress in the cochlea. It increases the synthesis of glutathione, a powerful endogenous antioxidant. Vitamin E Protects cell membranes from lipid peroxidation. Carotenoids Beta-carotene and lycopene can reduce inflammation and oxidative stress. | Vitamin C Citrus fruits, tropical fruits, strawberries, kiwi, berries, sea buckthorn, melon, guava Bell pepper, tomatoes, zucchini, rocket, parsley, spinach, broccoli, cauliflower, cabbage Vitamin E Almonds, walnuts, peanuts Olive oil, sunflower oil, pumpkin seeds, sunflower seeds, grains Avocados, spinach, kale, parsley, mango, kiwi, salmon, trout, shrimp, Soy, peanut butter Carotenoids Carrots, spinach, tomatoes, pumpkin, sweet potato, red pepper, cabbage, lettuce, broccoli, parsley, apricot, papaya |
| Minerals with an antioxidant role Selenium This cofactor of glutathione peroxidase reduces ROS accumulation. Zinc Supports the functioning of antioxidant enzymes and cell regeneration. | Selenium Nuts, fish, seafood, Eggs, beef liver, turkey, pork, chicken Whole grains (brown rice, wheat, oats, rye) Yogurt Beans, lentils, chickpeas, mushrooms, garlic, onions Zinc Seafood Lamb, pork, beef, eggs (especially the yolk) Pumpkin seeds, sesame seeds, cashews, walnuts, almonds Lentils, beans, chickpeas, peas Whole grains Milk, yogurt |
| Omega-3 fatty acids They have anti-inflammatory properties that may reduce cisplatin-induced inflammation in the cochlea. | Omega-3 fatty acids Fatty fish, seafood, cod liver oil, fish oil Flax seeds, chia seeds, hemp seeds, walnuts, flax oil, hemp oil Soy, edamame, cabbage, spinach |
| Polyphenols Resveratrol Powerful antioxidant that can prevent cell death. Curcumin It reduces inflammation and protects against oxidative stress. | Resveratrol Grapes, blueberries, dark chocolate |
| Sulfur-rich foods They can increase the synthesis of glutathione and other antioxidant enzymes. | Garlic, onion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iațentiuc, A.; Iațentiuc, I.M.; Frăsinariu, O.E.; Cozma, S.R.; Bitere-Popa, O.R.; Olariu, R.; Rădulescu, L.M.; Ioniuc, I.; Cuciureanu, M.; Alecsa, M.; et al. The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity. Int. J. Mol. Sci. 2025, 26, 4787. https://doi.org/10.3390/ijms26104787
Iațentiuc A, Iațentiuc IM, Frăsinariu OE, Cozma SR, Bitere-Popa OR, Olariu R, Rădulescu LM, Ioniuc I, Cuciureanu M, Alecsa M, et al. The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity. International Journal of Molecular Sciences. 2025; 26(10):4787. https://doi.org/10.3390/ijms26104787
Chicago/Turabian StyleIațentiuc, Andreea, Iustin Mihai Iațentiuc, Otilia Elena Frăsinariu, Sebastian Romică Cozma, Oana Roxana Bitere-Popa, Raluca Olariu, Luminița Mihaela Rădulescu, Ileana Ioniuc, Magdalena Cuciureanu, Mirabela Alecsa, and et al. 2025. "The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity" International Journal of Molecular Sciences 26, no. 10: 4787. https://doi.org/10.3390/ijms26104787
APA StyleIațentiuc, A., Iațentiuc, I. M., Frăsinariu, O. E., Cozma, S. R., Bitere-Popa, O. R., Olariu, R., Rădulescu, L. M., Ioniuc, I., Cuciureanu, M., Alecsa, M., Guma, C., & Miron, I. C. (2025). The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity. International Journal of Molecular Sciences, 26(10), 4787. https://doi.org/10.3390/ijms26104787



_Kim.png)