Effect of Inorganic Phosphorus Manipulation on the Growth and Progression of Prostate Cancer Cells In Vitro
Abstract
1. Introduction
2. Results
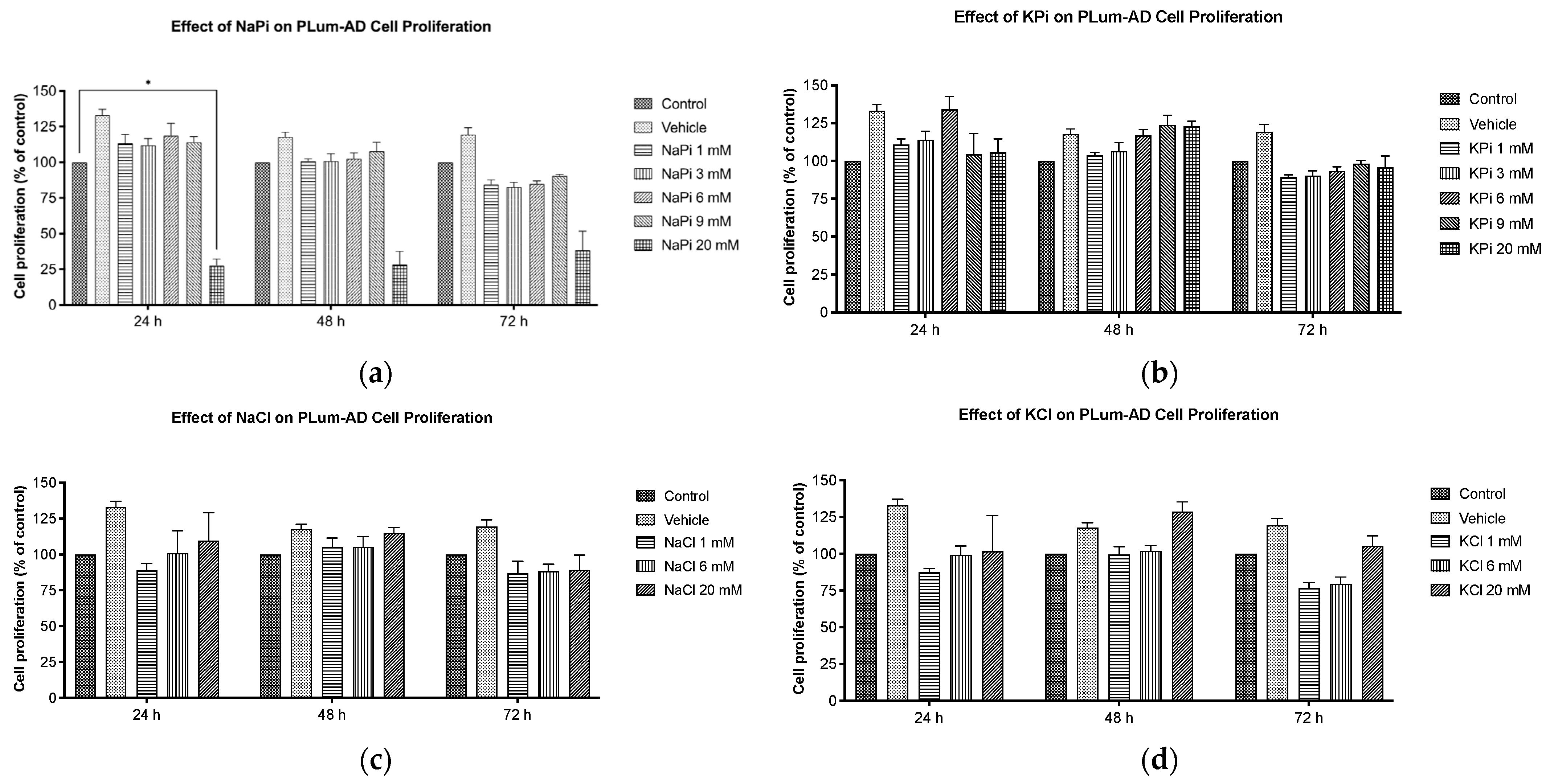
2.1. Effect of Pi on Cell Proliferation of PLum-AD Cells
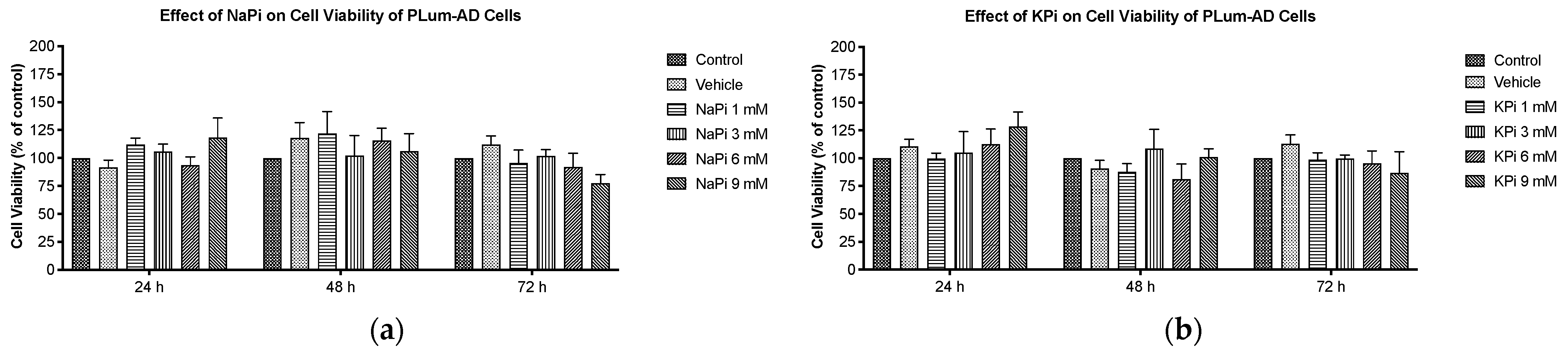
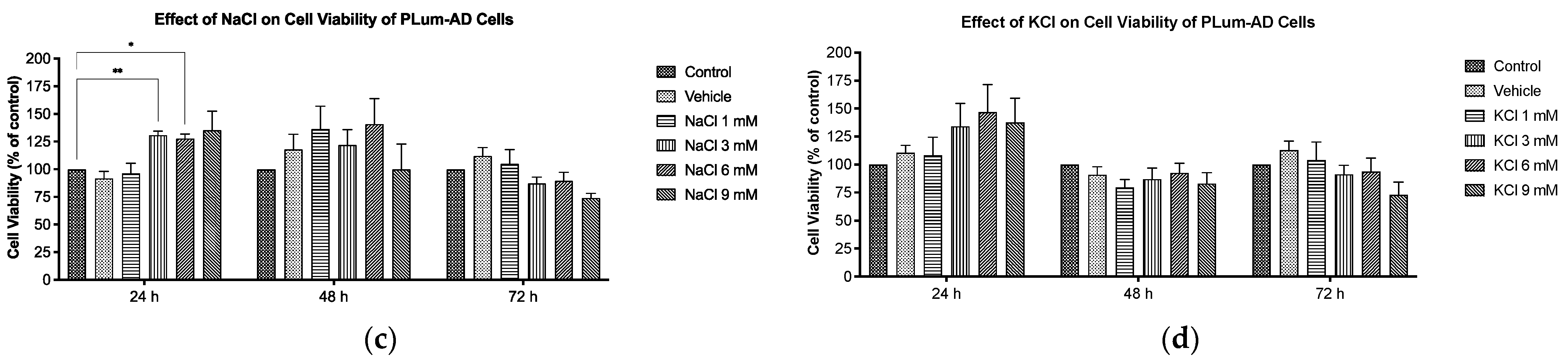
2.2. Effect of Pi on Cell Viability of PLum-AD Cells
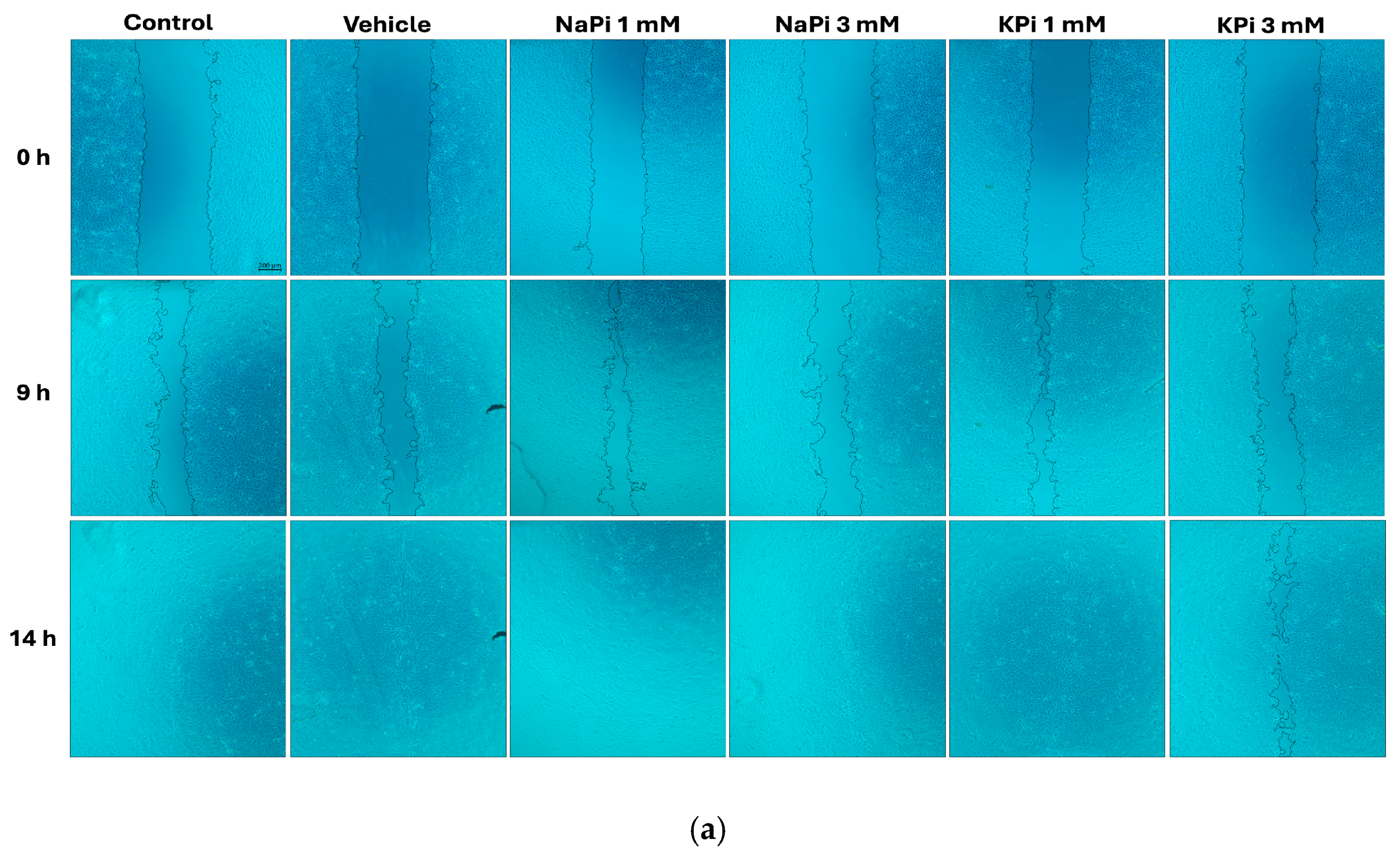
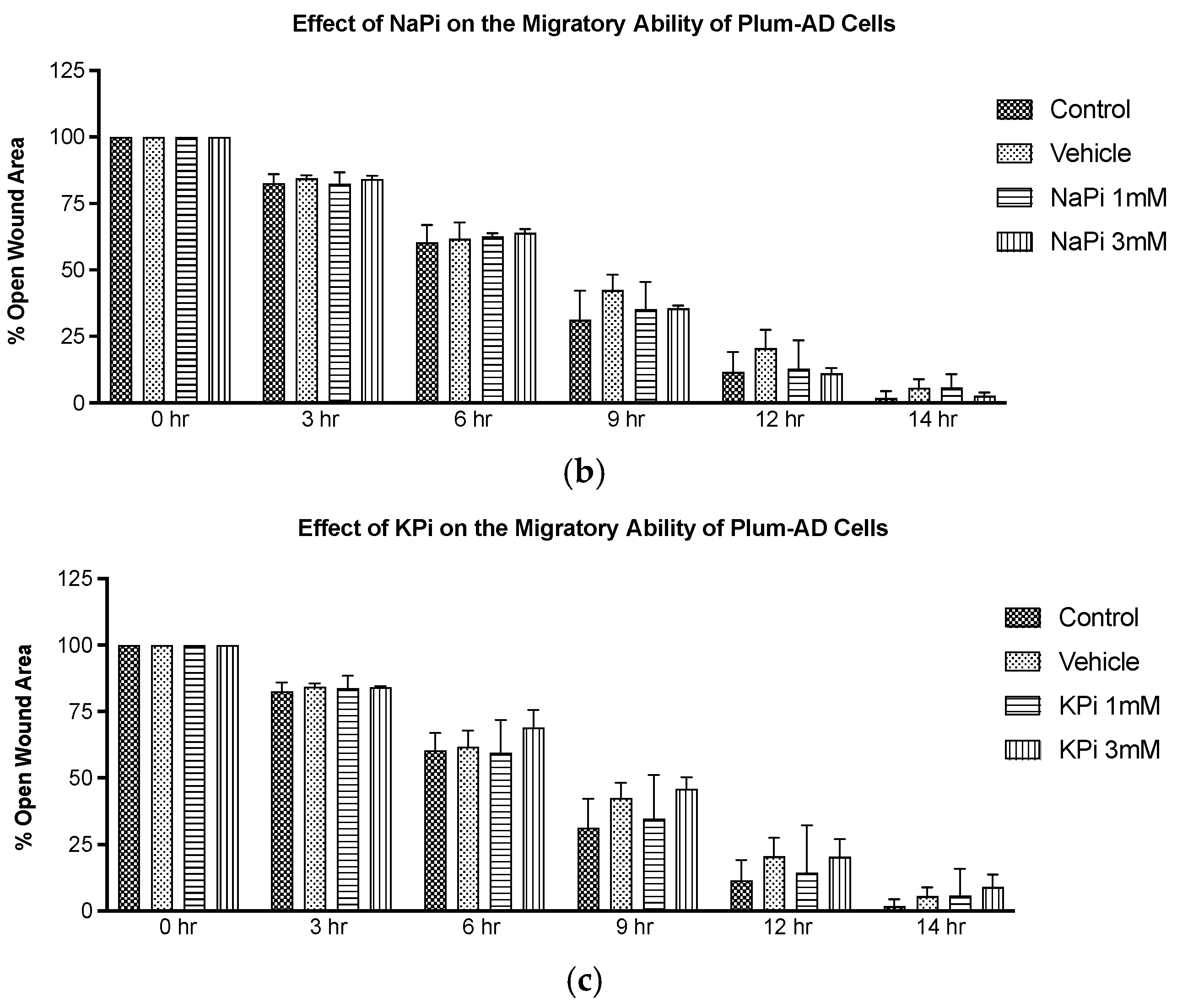
2.3. Effect of Pi on the Migratory Ability of PLum-AD Cells
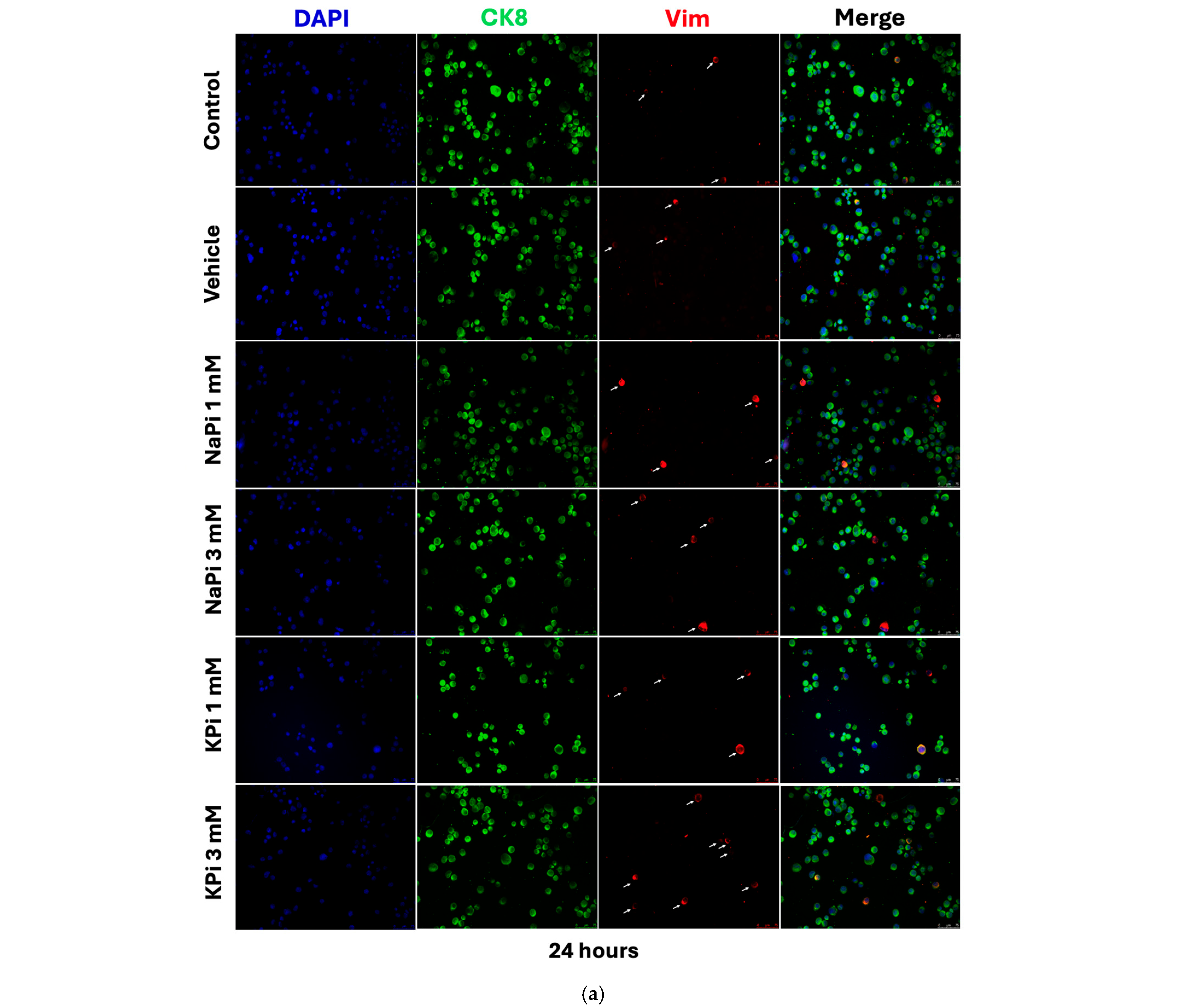
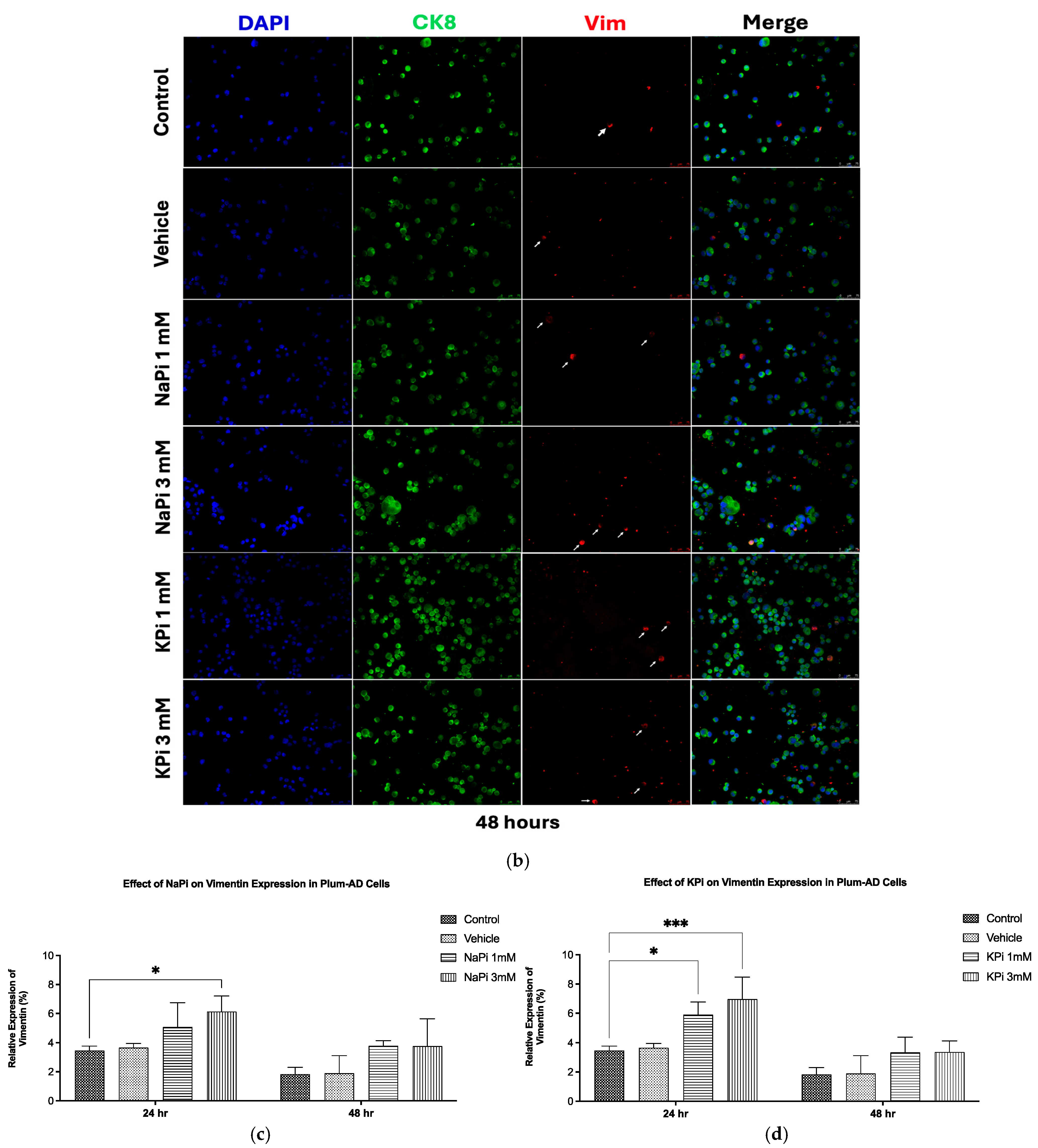
2.4. Effect of Pi on Vimentin Expression in PLum-AD Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line
4.2. Cell Culture
4.3. Treatment Protocols
4.4. Thiazolyl Blue Tetrazolium Bromide Cell Proliferation Assay
4.5. Trypan Blue Exclusion Cell Viability Assay
4.6. Wound Healing Cell Migration Assay
4.7. Immunofluorescence Staining and Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| CK18 | Cytokeratin 18 |
| CK8 | Cytokeratin 8 |
| CRPC | Castration-Resistant Prostate Cancer |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| ddH2O | Double-Distilled Water |
| EMT | Epithelial–Mesenchymal Transition |
| ERK1/2 | Extracellular Signal-Regulated Kinase ½ |
| FGF23 | Fibroblast Growth Factor 23 |
| HEK 293 | Human Embryonic Kidney 293 |
| HeLa | Cervical Cancer Cells |
| ICF | Intracellular Fluid |
| KCL | Potassium Chloride |
| KPi | Potassium Phosphate |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA-MB-231 | Triple-negative Human Breast Cancer Cells |
| MET | Mesenchymal–Epithelial Transition |
| MR | Mendelian Randomization |
| MTT | 3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide |
| NaCl | Sodium Chloride |
| NaPi | Sodium Phosphate |
| NaPi2b | Sodium-Dependent Phosphate Transport Protein 2B |
| PBS | Phosphate-Buffered Saline |
| PC | Prostate Cancer |
| Pi | Inorganic Phosphate |
| PTH | Parathyroid Hormone |
| TME | Tumor Microenvironment |
| Vim | Vimentin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Pan, J.; Huang, S.; Peng, X.; Zou, X.; Luo, Y.; Ren, D.; Zhang, X.; Li, R.; He, P.; et al. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 160. [Google Scholar] [CrossRef] [PubMed]
- Kensler, K.H.; Rebbeck, T.R. Cancer Progress and Priorities: Prostate Cancer. Cancer Epidemiol. Biomark. Prev 2020, 29, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Skelton, W.P. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Daher, M.; Telvizian, T.; Dagher, C.; Abdul-Sater, Z.; Massih, S.A.; Chediak, A.E.; Charafeddine, M.; Shahait, M.; Alameddine, R.; Temraz, S.; et al. High rates of advanced prostate cancer in the Middle East: Analysis from a tertiary care center. Urol. Ann. 2021, 13, 418–423. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Coleman, W. Molecular Pathogenesis of Prostate Cancer. In Molecular Pathology; Academic Press: Cambridge, MA, USA, 2018; pp. 555–568. [Google Scholar]
- Børretzen, A.; Gravdal, K.; Haukaas, S.A.; Mannelqvist, M.; Beisland, C.; Akslen, L.A.; Halvorsen, O.J. The epithelial–mesenchymal transition regulators Twist, Slug, and Snail are associated with aggressive tumour features and poor outcome in prostate cancer patients. J. Pathol. Clin. Res. 2021, 7, 253–270. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. In Cell & Molecular Biology of Prostate Cancer: Updates, Insights and New Frontiers; Schatten, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 101–110. [Google Scholar]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer-Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M.E. Prostate cancer and the influence of dietary factors and supplements: A systematic review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef]
- Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients 2024, 16, 527. [Google Scholar] [CrossRef]
- Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Cobalt Serum Level as a Biomarker of Cause-Specific Survival among Prostate Cancer Patients. Cancers 2024, 16, 2618. [Google Scholar] [CrossRef] [PubMed]
- Tshoni, U.A.; Mbonane, T.P.; Rathebe, P.C. The Role of Trace Metals in the Development and Progression of Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 10725. [Google Scholar] [CrossRef]
- Lv, L.; Ye, D.; Chen, J.; Qian, Y.; Fu, A.N.; Song, J.; Yang, H.; Liu, B.; Sun, X.; Du, L.; et al. Circulating phosphorus concentration and risk of prostate cancer: A Mendelian randomization study. Am. J. Clin. Nutr. 2022, 115, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Kalantar-Zadeh, K.; Mehrotra, R. Clinical Aspects of Natural and Added Phosphorus in Foods; Springer: New York, NY, USA, 2017. [Google Scholar]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Leone, K.A. 166-Calcium, Magnesium, and Phosphorus. In Emergency Medicine, 2nd ed.; Adams, J.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 1405–1417.e1401. [Google Scholar]
- Berndt, T.J.; Schiavi, S.; Kumar, R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am. J. Physiol. Renal Physiol. 2005, 289, F1170–F1182. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Uribarri, J. Phosphorus and the kidney: What is known and what is needed. Adv. Nutr. 2014, 5, 98–103. [Google Scholar] [CrossRef]
- De Boer, I.H.; Rue, T.C.; Kestenbaum, B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 2009, 53, 399–407. [Google Scholar] [CrossRef]
- Portale, A.A.; Halloran, B.P.; Morris, R.C., Jr. Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J. Clin. Investig. 1989, 83, 1494–1499. [Google Scholar] [CrossRef]
- Nishida, Y.; Taketani, Y.; Yamanaka-Okumura, H.; Imamura, F.; Taniguchi, A.; Sato, T.; Shuto, E.; Nashiki, K.; Arai, H.; Yamamoto, H.; et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006, 70, 2141–2147. [Google Scholar] [CrossRef]
- Khattab, M.; Abi-Rashed, C.; Ghattas, H.; Hlais, S.; Obeid, O. Phosphorus ingestion improves oral glucose tolerance of healthy male subjects: A crossover experiment. Nutr. J. 2015, 14, 112. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Kalantar-Zadeh, K.; Chen, J. Focusing on Phosphorus Loads: From Healthy People to Chronic Kidney Disease. Nutrients 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Shyian, M.; Gryshkova, V.; Kostianets, O.; Gorshkov, V.; Gogolev, Y.; Goncharuk, I.; Nespryadko, S.; Vorobjova, L.; Filonenko, V.; Kiyamova, R. Quantitative analysis of SLC34A2 expression in different types of ovarian tumors. Exp. Oncol. 2011, 33, 94–98. [Google Scholar] [PubMed]
- Kiyamova, R.; Shyian, M.; Lyzogubov, V.V.; Usenko, V.S.; Gout, T.; Filonenko, V. Immunohistochemical analysis of NaPi2b protein (MX35 antigen) expression and subcellular localization in human normal and cancer tissues. Exp. Oncol. 2011, 33, 157–161. [Google Scholar] [PubMed]
- Gryshkova, V.; Goncharuk, I.; Gurtovyy, V.; Khozhayenko, Y.; Nespryadko, S.; Vorobjova, L.; Usenko, V.; Gout, I.; Filonenko, V.; Kiyamova, R. The study of phosphate transporter NAPI2B expression in different histological types of epithelial ovarian cancer. Exp. Oncol. 2009, 31, 37–42. [Google Scholar]
- Hong, S.H.; Minai-Tehrani, A.; Chang, S.H.; Jiang, H.L.; Lee, S.; Lee, A.Y.; Seo, H.W.; Chae, C.; Beck, G.R., Jr.; Cho, M.H. Knockdown of the sodium-dependent phosphate co-transporter 2b (NPT2b) suppresses lung tumorigenesis. PLoS ONE 2013, 8, e77121. [Google Scholar] [CrossRef]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci. Rep. 2017, 7, 41233. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Meyer-Fernandes, J.R. The Roles of Sodium-Independent Inorganic Phosphate Transporters in Inorganic Phosphate Homeostasis and in Cancer and Other Diseases. Int. J. Mol. Sci. 2020, 21, 9298. [Google Scholar] [CrossRef]
- Lewis, E.; Seltun, F.; Razzaque, M.S.; He, P. Phosphate Toxicity and Epithelial to Mesenchymal Transition. Adv. Exp. Med. Biol. 2022, 1362, 73–84. [Google Scholar] [CrossRef]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 2, e1028. [Google Scholar] [CrossRef]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R., Jr. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef]
- Chang, S.H.; Yu, K.N.; Lee, Y.S.; An, G.H.; Beck, G.R., Jr.; Colburn, N.H.; Lee, K.H.; Cho, M.H. Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Fensom, G.K.; Andrews, C.; Watts, E.L.; Allen, N.E.; Martin, R.M.; Van Hemelrijck, M.; Key, T.J.; Travis, R.C. Examination of potential novel biochemical factors in relation to prostate cancer incidence and mortality in UK Biobank. Br. J. Cancer 2020, 123, 1808–1817. [Google Scholar] [CrossRef]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef]
- Brändstedt, J.; Almquist, M.; Manjer, J.; Malm, J. Vitamin D, PTH, and calcium in relation to survival following prostate cancer. Cancer Causes Control 2016, 27, 669–677. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J.; Zhang, Y.; Creighton, C.J.; Ittmann, M. FGF23 promotes prostate cancer progression. Oncotarget 2015, 6, 17291–17301. [Google Scholar] [CrossRef]
- Kim, W.T.; Bang, W.J.; Seo, S.P.; Kang, H.W.; Byun, Y.J.; Piao, X.M.; Jeong, P.; Shin, K.S.; Choi, S.Y.; Lee, O.J.; et al. Parathyroid hormone is associated with prostate cancer. Prostate Int. 2020, 8, 116–120. [Google Scholar] [CrossRef]
- Arnst, J.L.; Beck, G.R., Jr. Modulating phosphate consumption, a novel therapeutic approach for the control of cancer cell proliferation and tumorigenesis. Biochem. Pharmacol. 2021, 183, 114305. [Google Scholar] [CrossRef]
- McCarty, M.F. Plant-based diets relatively low in bioavailable phosphate and calcium may aid prevention and control of prostate cancer by lessening production of fibroblast growth factor 23. Med. Hypotheses 2017, 99, 68–72. [Google Scholar] [CrossRef]
- Schwartz, G.G. Prostate Cancer, Serum Parathyroid Hormone, and the Progression of Skeletal Metastases. Cancer Epidemiol. Biomark. Prev. 2008, 17, 478–483. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Mann-Collura, O.; Fling, J.; Edara, N.; Hetz, R.; Razzaque, M.S. High phosphate actively induces cytotoxicity by rewiring pro-survival and pro-apoptotic signaling networks in HEK293 and HeLa cells. FASEB J. 2021, 35, e20997. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, G.S.; Hausberg, M.; Hillebrand, U.; Rustemeyer, P.; Wittkowski, W.; Lang, D.; Pavenstädt, H. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Renal Physiol. 2008, 294, F1381–F1387. [Google Scholar] [CrossRef] [PubMed]
- Shanti, A.; Al Adem, K.; Stefanini, C.; Lee, S. Hydrogen phosphate selectively induces MDA MB 231 triple negative breast cancer cell death in vitro. Sci. Rep. 2022, 12, 5333. [Google Scholar] [CrossRef]
- Spina, A.; Sapio, L.; Esposito, A.; Di Maiolo, F.; Sorvillo, L.; Naviglio, S. Inorganic Phosphate as a Novel Signaling Molecule with Antiproliferative Action in MDA-MB-231 Breast Cancer Cells. Biores Open Access 2013, 2, 47–54. [Google Scholar] [CrossRef]
- Russo-Abrahão, T.; Lacerda-Abreu, M.A.; Gomes, T.; Cosentino-Gomes, D.; Carvalho-de-Araújo, A.D.; Rodrigues, M.F.; Oliveira, A.C.L.d.; Rumjanek, F.D.; Monteiro, R.d.Q.; Meyer-Fernandes, J.R. Characterization of inorganic phosphate transport in the triple-negative breast cancer cell line, MDA-MB-231. PLoS ONE 2018, 13, e0191270. [Google Scholar] [CrossRef]
- Nurgalieva, A.K.; Popov, V.E.; Skripova, V.S.; Bulatova, L.F.; Savenkova, D.V.; Vlasenkova, R.A.; Safina, S.Z.; Shakirova, E.Z.; Filonenko, V.V.; Bogdanov, M.V.; et al. Sodium-dependent phosphate transporter NaPi2b as a potential predictive marker for targeted therapy of ovarian cancer. Biochem. Biophys. Rep. 2021, 28, 101104. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Rocco-Machado, N.; Cosentino-Gomes, D.; Dick, C.F.; Carvalho-Kelly, L.F.; Cunha Nascimento, M.T.; Rocha-Vieira, T.C.; Meyer-Fernandes, J.R. Hydrogen Peroxide Generation as an Underlying Response to High Extracellular Inorganic Phosphate (Pi) in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10096. [Google Scholar] [CrossRef]
- Trump, D.L. Calcitriol and cancer therapy: A missed opportunity. Bone Rep. 2018, 9, 110–119. [Google Scholar] [CrossRef]
- Abou-Kheir, W.; Hynes, P.G.; Martin, P.; Yin, J.J.; Liu, Y.N.; Seng, V.; Lake, R.; Spurrier, J.; Kelly, K. Self-renewing Pten-/- TP53-/- protospheres produce metastatic adenocarcinoma cell lines with multipotent progenitor activity. PLoS ONE 2011, 6, e26112. [Google Scholar] [CrossRef]
- Daoud, G.; Monzer, A.; Bahmad, H.; Chamaa, F.; Hamdar, L.; Mouhieddine, T.H.; Shayya, S.; Eid, A.; Kobeissy, F.; Liu, Y.N.; et al. Primary versus castration-resistant prostate cancer: Modeling through novel murine prostate cancer cell lines. Oncotarget 2016, 7, 28961–28975. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, Y.; Lei, Q.; Lin, D.; Chen, J.; Wu, C. Effect of inorganic phosphate on migration and osteogenic differentiation of bone marrow mesenchymal stem cells. BMC Dev. Biol. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourie, C.M.; Ghamlouche, F.; Hachem, S.; Yehya, A.; Jaafar, L.; El-Mallah, C.; Abou-Kheir, W.; Obeid, O. Effect of Inorganic Phosphorus Manipulation on the Growth and Progression of Prostate Cancer Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 4762. https://doi.org/10.3390/ijms26104762
Kourie CM, Ghamlouche F, Hachem S, Yehya A, Jaafar L, El-Mallah C, Abou-Kheir W, Obeid O. Effect of Inorganic Phosphorus Manipulation on the Growth and Progression of Prostate Cancer Cells In Vitro. International Journal of Molecular Sciences. 2025; 26(10):4762. https://doi.org/10.3390/ijms26104762
Chicago/Turabian StyleKourie, Christina Mary, Fatima Ghamlouche, Sana Hachem, Amani Yehya, Layal Jaafar, Carla El-Mallah, Wassim Abou-Kheir, and Omar Obeid. 2025. "Effect of Inorganic Phosphorus Manipulation on the Growth and Progression of Prostate Cancer Cells In Vitro" International Journal of Molecular Sciences 26, no. 10: 4762. https://doi.org/10.3390/ijms26104762
APA StyleKourie, C. M., Ghamlouche, F., Hachem, S., Yehya, A., Jaafar, L., El-Mallah, C., Abou-Kheir, W., & Obeid, O. (2025). Effect of Inorganic Phosphorus Manipulation on the Growth and Progression of Prostate Cancer Cells In Vitro. International Journal of Molecular Sciences, 26(10), 4762. https://doi.org/10.3390/ijms26104762





