Next-Level Prediction of Structural Progression in Knee Osteoarthritis: A Perspective
Abstract
1. Introduction
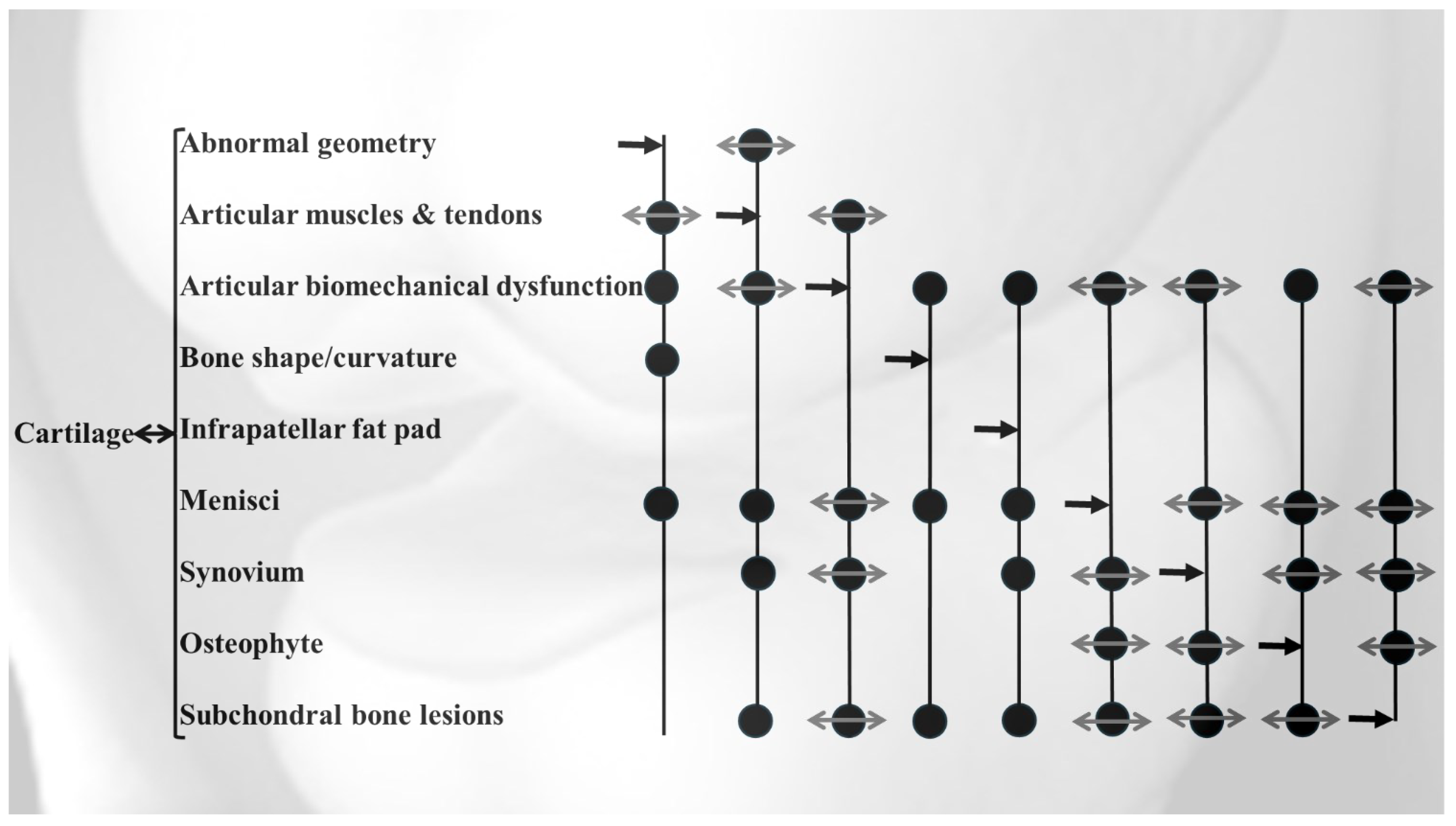
2. Features for OA Stratification
3. Machine and Deep Learning Approaches in OA Prognosis
4. Machine and Deep Learning OA Prognosis Models That Integrate Biochemical Markers and MRI Data
| Author/Year | Purpose of the Study | Cohort | Learning Algorithm for the Final Model | Best Predictive Input Features | Outcome (Progressors) Definition | Number of Participants | Best Prediction Performance for the Progressors | Validation with an External Cohort |
|---|---|---|---|---|---|---|---|---|
| Hafezi-Nejad et al./2017 [51] | To investigate the association between baseline lateral femoral cartilage volume and medial joint space loss progression | FNIH (subset of OAI) | Multi-layer-Perceptron (MLP) | 24–48-month changes in the lateral femoral plate cartilage volume | Medial joint space loss ˃ 0.7 mm progression 1. Baseline 2. 24-month change | Progressor: 297 Non-progressor: 303 | AUC 1. 0.63 2. 0.67 | No |
| Du et al./2018 [52] | To explore the hidden biomedical information from knee MRI for OA progression prediction | OAI | 1. Principal Component, Artificial Neural Network (ANN), Support Vector Machine (SVM), Random Forest (RF), Naïve Bayes (NB) | Features from 18 medial compartments showed better performances than the 18 lateral features. The total 36 features generated the best performance | Change over two years of: 1. Kellgren–Lawrence grade 2. JSN grade on medial compartment 3. JSN on lateral compartment grade | Progressor: 100 Non-progressor: 100 | AUC 1. 0.76 2. 0.79 3. 0.70 | No |
| MacKay et al./2018 [53] | To assess if a change in MRI subchondral bone texture is predictive of radiographic knee OA progression | OAI | Subchondral bone texture using radiomic approach | 12–18-month follow-up change in subchondral bone texture features when tibial and femoral data are combined | Decrease minimal JSW ≥0.7 mm 1. At 36 months (initial change) 2. Change between 36 and 72 months | Baseline Progressor: 61 Non-progressor: 61 12–18-month follow-up Progressor: 53 Non-progressor: 52 | AUC 1. 0.65 2. 0.68 | No |
| Nelson et al./2019 [23] | To define the progression of OA phenotypes potentially more responsive to interventions | FNIH (subset of OAI) | Distance Weighted Discrimination (DWD), Direction-Projection-Permutation (DPP), Clustering Methods | Baseline variables with the most significant contribution 1. To non-progression: WOMAC pain score, lateral meniscal extrusion, and serum N-terminal pro-peptide of collagen IIA 2. To progression: bone marrow lesions, osteophytes, medial meniscal extrusion, and urine C-terminal crosslinked telopeptide type II collagen | Medial JSN ≥0.7 mm and WOMAC total score increase ≥9 points at 48 months | Progressor: 192 Non-progressor: 200 | Z Score: 10.1 | No |
| Jamshidi et al./2020 [54] | To identify the most important features of structural knee OA progressors | OAI | Six machine learning: Least Absolute Shrinkage and Selection Operator (LASSO), Elastic Net Regularization (ENR), Gradient Boosting Machine (GRM), Random Forest (RF), Information Gain (IF), Multi-Layer Perceptron (MLP) | Baseline medial minimum JSW, MRI-based mean cartilage thickness of peripheral, medial and central tibial plateau, and medial JSN as a score | 1. JSN ≥1 mm at 48 months 2. Medial plateau cartilage volume loss as evaluated by MRI at 96 months 3. Medial plateau cartilage volume loss as evaluated by MRI at 48 months 4. Kellgren-Laurence grade ≥2 | 1. Progressor: 620 Non-progressor: 200 2. Progressor: 795 Non-progressor: 803 3. Progressor: 514 Non-progressor: 530 4. Progressor: 811 Non-progressor: 657 | AUC 1. 0.92 2. 0.73 3. 0.70 4. 0.87 | No |
| Bonakdari et al./2021 [24] | To build a comprehensive gender-based machine learning model for early prediction of at-risk knee OA patient structural progressors using baseline serum levels of adipokines/related inflammatory factors and age and BMI | OAI | Support Vector Machine (SVM) | Age, BMI, C-reactive protein/monocyte chemoattractant protein-1 and leptin/C-reactive protein | Prediction of knee OA structural progressors as in [54], in which the inputs were baseline medial minimum JSW, MRI-based mean cartilage thickness of peripheral, medial and central tibial plateau, and medial JSN as a score | Progressor: 357 Non-progressor: 320 | Accuracy ≥ 0.81 | Cohort: Naproxen arm of the Licofelone clinical trial [55] Progressor: 30 Non-progressor: 14 Accuracy: ≥ 0.92 |
| Schiratti et al./2021 [56] | To develop a proof-of-concept predictive model for OA radiographic progression and knee pain | OAI | Multi-Layer-Perceptron (MLP) | 1. Medial joint space 2. Intraarticular space where effusion is observed | 1. OA progression defined as minimum JSN loss of at least 0.5 mm at 12 months 2. Pain prediction (WOMAC pain score ≥2 points) | Knees: 9280 | AUC 1. 0.63 2. 0.72 | No |
| Bonakdari et al./2022 [57] | To assess if baseline knee bone curvature could predict cartilage volume loss at one year. Development of a gender-based model | OAI | Adaptive Neuro-Fuzzy Inference System (ANFIS) | Baseline bone curvature regions of the lateral tibial plateau, medial central condyle, lateral posterior condyle, and lateral and medial trochlea | Twelve global or regional knee cartilage volume losses at one year (global knee, femur, condyle, tibial plateau; lateral compartment, femur, condyle, tibial plateau; medial compartment, femur condyle and tibial plateau) | Progressor as defined in [54], in which the inputs were baseline medial minimum JSW, MRI-based mean cartilage thickness of peripheral, medial and central tibial plateau, and medial JSN as a score | Accuracy 0.92–0.79 | Cohort: Naproxen arm of the Licofelone clinical trial [55] Progressor: 53 Accuracy: 0.96–0.79 except for medial tibial plateau for women |
| Bonakdari et al./2022 [32] | To evaluate if single nucleotide polymorphism genes and mitochondrial DNA haplogroups/clusters could predict early knee osteo-arthritis structural progressors | OAI | Support Vector Machine (SVM) | 1. Age, BMI, TP63, DUS4L, GDF5, FTO 2. Age, BMI, mitochondrial DNA haplogroup (H, J, T, Uk, and others), FTO, SUPT3H | Prediction of knee OA structural progressors as in [54], in which the inputs were baseline medial minimum JSW, MRI-based mean cartilage thickness of peripheral, medial and central tibial plateau, and medial JSN as a score | Progressor: 276 Non-progressor: 625 | Accuracy 1. 0.85 2. 0.83 | Cohort: TASOAC [58] Progressor: 71 Non-progressor: 158 Accuracy 1. 0.81 2. 0.86 |
| Hu et al./2022 [59] | By using an adversarial evolving neural network to estimate longitudinal knee OA prediction | OAI | Adversarial evolving neural network (A-ENN) | Evolving traces of Kellgren–Lawrence grades determined with a discriminator for longitudinal grading | An increase >1 in the Kellgren–Lawrence grade compared to baseline | Knees: 3294 | Accuracy Overall: 0.63 Baseline: 0.65 12 months: 0.65 24 months: 0.64 36 months: 0.62 48 months: 0.60 | No |
| Panfilov et al./2022 [60] | To predict knee OA progression from structural MRI using deep learning | OAI | Convolutional Neural Network (CNN)-Transformer | Aggregation of features | Changes in Kellgren–Lawrence grade within 96 months with three classes: No progression within 96 months Slow progression (after 72 and within 96 months) Fast progression within 72 months | Knees: 4866 | AUC 0.78 | No |
| Costello et al./2023 [61] | To develop a machine learning model incorporating gait and physical activity to predictmedial tibiofemoral cartilage worsening over 2 years | MOST | An ensemble machine learning approach using: Bayesian Adaptive Regression Trees (BART), Generalized Linear Model (GLM), Least Absolute Shrinkage and Selection Operator (LASS0) Ridge Regression (RR), Elastic Net (E-Net), Random Forest (RF), and Extreme Gradient Boosting (XGBoost) | High lateral ground reaction force impulse, more time spent lying and low vertical ground reaction force unloading rate | Cartilage worsening over 2 years: area and/or dept in at least one of the five medial tibiofemoral subregions | Participants: 947 with Kellgren-Laurence ≤2, 133 experienced cartilage worsening over 2 years | AUC 0.73 | No |
| Hu et al./2023 [62] | To develop a deep-learning method for predicting the progression of knee OA based on MR images | FNIH (subset of OAI) | DenseNet169 | Patellofemoral joints, meniscus, infrapatellar fat pad, muscles posterior | Loss in medial minimum joint space width ≥0.7 mm from baseline to 24, 36, 48 months and pain progression: WOMAC pain subscale defined as a persistent increase from baseline to 24, 36, 48 months of ≥9 points on 1–100 score | Progressor: 182 Non-progressor 182 | AUC Baseline 0.66 12 months: 0.74 24 months: 0.78 | No |
| Jansen et al./2023 [63] | To predict 2-year structural progression | IMI-APPROACH (participants from five observational cohorts, namely, CHECK, HOSTAS, MUST, PROCOAC, and DIGICOD) | Random Forest (RF) | Minimum JSW decrease > 0.3 mm/year | Different parameters were used: Minimum JSW decrease > 0.3 mm/year, MRI data used the MOAKS scores (0–3) of the five medial or lateral tibiofemoral subregions were summarized to one score for each feature and included only if all subregions in the compartment could be scored. Progression was defined as an increase of at least one full score in the most affected compartment (MAC) | Participants: 237 Participants were progressors if at least one of two areas in the most affected compartment surpassed the progression cut-off (JSW predefined threshold). Accordingly, the number of progressors was 14–86, according to the cohort | s-score for progressor: It significantly predicts minimum JSW progression (p ≤ 0.03). It could not predict structural progression based on the predefined criterion or the smallest detectable change | No |
| Jamshidi et al./2024 [33] | To develop a miRNA prognosis model for identifying knee OA structural progressors using integrated machine/deep learning tools | OAI | Artificial Neural Network (ANN) | Age, has-miR-141-3p, has-miR-556-3p, has-miR-200a-5p, has-miR-3157-5p | Prediction of knee OA structural progressors as in [54], in which the inputs were baseline medial minimum JSW, MRI-based mean cartilage thickness of peripheral, medial and central tibial plateau, and medial JSN as a score | Progressor (n = 91) Non-progressor (n = 61) | AUC: 0.94 Accuracy: 0.84 | Independent OAI cohort Progressor (n = 14) Non-progressor (n = 16) AUC: 0.81 Accuracy: 0.83 |
| Lv et al./2025 [64] | Using a longitudinal MRI on 32 sub-structural texture-guided graph convolution networks to improve progression prediction of knee OA | FNIH (subset of OAI) | 1. Longitudinal MRI sub-structural texture-guided graph convolution network (LMSST-GCN) with clinical data (ceLMSST-GCN) 2. Support Vector Machine (SVM) 3. Random Forest (RF) 4. Extreme Gradient Boosting (XGBoost) | Loss of cartilage and sclerosis of subchondral bone at the tibial medial central region, the injury of lateral meniscus, and abnormal changes in the infrapatellar fat pad | Radiographic progression: combination of radiographic progression (JSW ≥0.7 mm) and WOMAC pain progression (increase of at least 9 points [0–100 scale] at 2 or more timepoints from baseline to 24, 36, 48 months) at 4-year follow-up compared to baseline (pain progression occurred at the third and fourth year of follow-up | Progressor: 194 Non-progressor: 406 | AUC (multi-timepoints) 1. 0.82 2. 0.73 3. 0.74 4. 0.75 | No |
5. Limitations of OA Prognosis Machine and Deep Learning Models
6. Path Toward Clinical Translation of Machine and Deep Learning Models
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, L.; Li, M.; Deng, F.; Wen, X.; Deng, H.; Xue, Z.; Wan, P.; Xiang, R.; Xie, Y.; He, H.; et al. Epidemiological trends of osteoarthritis at the global, regional, and national levels from 1990 to 2021 and projections to 2050. medRxiv 2024. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Stubbs, B.; Mamas, M.A.; Myint, P.K.; Smith, T.O. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 938–946. [Google Scholar] [CrossRef]
- Hawker, G.A.; Gignac, M.A.; Badley, E.; Davis, A.M.; French, M.R.; Li, Y.; Perruccio, A.V.; Power, J.D.; Sale, J.; Lou, W. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res. 2011, 63, 1382–1390. [Google Scholar] [CrossRef]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Juni, P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011, 342, c7086. [Google Scholar] [CrossRef]
- Parkinson, L.; Waters, D.L.; Franck, L. Systematic review of the impact of osteoarthritis on health outcomes for comorbid disease in older people. Osteoarthr. Cartil. 2017, 25, 1751–1770. [Google Scholar] [CrossRef]
- Cleveland, R.J.; Alvarez, C.; Schwartz, T.A.; Losina, E.; Renner, J.B.; Jordan, J.M.; Callahan, L.F. The impact of painful knee osteoarthritis on mortality: A community-based cohort study with over 24 years of follow-up. Osteoarthr. Cartil. 2019, 27, 593–602. [Google Scholar] [CrossRef]
- Driban, J.B.; Harkey, M.S.; Barbe, M.F.; Ward, R.J.; MacKay, J.W.; Davis, J.E.; Lu, B.; Price, L.L.; Eaton, C.B.; Lo, G.H.; et al. Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskelet. Disord. 2020, 21, 332. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Kloppenburg, M.; Schett, G.; Bijlsma, J.W. Osteoarthritis research priorities: A report from a EULAR ad hoc expert committee. Ann. Rheum. Dis. 2014, 73, 1442–1445. [Google Scholar] [CrossRef]
- van Helvoort, E.M.; van Spil, W.E.; Jansen, M.P.; Welsing, P.M.J.; Kloppenburg, M.; Loef, M.; Blanco, F.J.; Haugen, I.K.; Berenbaum, F.; Bacardit, J.; et al. Cohort profile: The Applied Public-Private Research enabling OsteoArthritis Clinical Headway (IMI-APPROACH) study: A 2-year, European, cohort study to describe, validate and predict phenotypes of osteoarthritis using clinical, imaging and biochemical markers. BMJ Open 2020, 10, e035101. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 2000, 27, 1513–1517. [Google Scholar] [PubMed]
- Eckstein, F.; Wirth, W.; Nevitt, M.C. Recent advances in osteoarthritis imaging-the osteoarthritis initiative. Nat. Rev. Rheumatol. 2012, 8, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, W.; Rachuene, P.A.; Dey, R.; Mzayiya, N.L.; Ramasuvha, B.E. The correlation between clinical and radiological severity of osteoarthritis of the knee. Sicot. J. 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Roemer, F.W.; Cicuttini, F.; MacKay, J.W.; Turmezei, T.; Link, T.M. Early knee OA definition-what do we know at this stage? An imaging perspective. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720x231158204. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Cooper, C.; Peterfy, C.; Reginster, J.Y.; Brandi, M.L.; Bruyere, O.; Chapurlat, R.; Cicuttini, F.; Conaghan, P.G.; Doherty, M.; et al. What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann. Rheum. Dis. 2013, 72, 1594–1604. [Google Scholar] [CrossRef]
- Roemer, F.W.; Wirth, W.; Demehri, S.; Kijowski, R.; Jarraya, M.; Hayashi, D.; Eckstein, F.; Guermazi, A. Imaging biomarkers of osteoarthritis. Semin. Musculoskelet. Radiol. 2024, 28, 14–25. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Song, G.; Li, Z.; Zhang, Y.; Fan, Y. A deep learning model integrating FCNNs and CRFs for brain tumor segmentation. Med. Image Anal. 2018, 43, 98–111. [Google Scholar] [CrossRef]
- van Ooijen, P.M.A.; Nagaraj, Y.; Olthof, A. Medical imaging informatics, more than ‘just’ deep learning. Eur. Radiol. 2020, 30, 5507–5509. [Google Scholar] [CrossRef]
- Gu, Y.; Chi, J.; Liu, J.; Yang, L.; Zhang, B.; Yu, D.; Zhao, Y.; Lu, X. A survey of computer-aided diagnosis of lung nodules from CT scans using deep learning. Comput. Biol. Med. 2021, 137, 104806. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Liu, S.L.; Sun, P.; Li, Y.; Liu, G.W.; Liu, S.S.; Hu, J.L.; Niu, T.Y.; Lu, Y. Establishment and clinical application value of an automatic diagnosis platform for rectal cancer T-staging based on a deep neural network. Chin. Med. J. 2021, 134, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, J.; Yuan, C.; Zhu, X. Interpretable learning-based dynamic graph convolutional networks for Alzheimer’s disease analysis. Inf. Fusion 2022, 77, 53–61. [Google Scholar] [CrossRef]
- Nelson, A.E.; Fang, F.; Arbeeva, L.; Cleveland, R.J.; Schwartz, T.A.; Callahan, L.F.; Marron, J.S.; Loeser, R.F. A machine learning approach to knee osteoarthritis phenotyping: Data from the FNIH Biomarkers Consortium. Osteoarthr. Cartil. 2019, 27, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Bonakdari, H.; Jamshidi, A.; Pelletier, J.P.; Abram, F.; Tardif, G.; Martel-Pelletier, J. A warning machine learning algorithm for early knee osteoarthritis structural progressor patient screening. Ther. Adv. Musculoskel. Dis. 2021, 13, 1759720X21993254. [Google Scholar] [CrossRef]
- Smith, J.W.; Martins, T.B.; Gopez, E.; Johnson, T.; Hill, H.R.; Rosenberg, T.D. Significance of C-reactive protein in osteoarthritis and total knee arthroplasty outcomes. Ther. Adv. Musculoskelet. Dis. 2012, 4, 315–325. [Google Scholar] [CrossRef]
- Xu, Y.K.; Ke, Y.; Wang, B.; Lin, J.H. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol. Res. 2015, 48, 64. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Raynauld, J.P.; Dorais, M.; Abram, F.; Pelletier, J.P. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: A post hoc analysis. Rheumatology 2016, 55, 680–688. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Tardif, G.; Rousseau Trepanier, J.; Abram, F.; Dorais, M.; Raynauld, J.P.; Pelletier, J.P. The ratio adipsin/MCP-1 is strongly associated with structural changes and CRP/MCP-1 with symptoms in obese knee osteoarthritis subjects: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2019, 28, 1163–1173. [Google Scholar] [CrossRef]
- Pearle, A.D.; Scanzello, C.R.; George, S.; Mandl, L.A.; DiCarlo, E.F.; Peterson, M.; Sculco, T.P.; Crow, M.K. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 2007, 15, 516–523. [Google Scholar] [CrossRef]
- Luo, H.; Li, L.; Han, S.; Liu, T. The role of monocyte/macrophage chemokines in pathogenesis of osteoarthritis: A review. Int. J. Immunogenet. 2024, 51, 130–142. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, Z.H.; Yu, H.T.; Liu, B. Significance of increased leptin expression in osteoarthritis patients. PLoS ONE 2015, 10, e0123224. [Google Scholar] [CrossRef]
- Bonakdari, H.; Pelletier, J.P.; Blanco, F.; Rego-Perez, I.; Duran-Sotuela, A.; Jamshidi, A.; Abram, F.; Martel-Pelletier, J. Single nucleotide polymorphism genes and mitochondrial DNA haplogroups as biomarkers for early prediction of knee osteoarthritis structural progressors: Use of supervised machine learning classifiers. BMC Med. 2022, 20, 316. [Google Scholar] [CrossRef]
- Jamshidi, A.; Espin-Garcia, O.; Wilson, T.G.; Loveless, I.; Pelletier, J.-P.; Martel-Pelletier, J.; Ali, S.A. MicroRNA signature for early prediction of knee osteoarthritis structural progression using integrated machine and deep learning approaches. Osteoarthr. Cartil. 2025, 33, 330–340. [Google Scholar] [CrossRef]
- arcOgenConsortium; arcOgenCollaborators; Zeggini, E.; Panoutsopoulou, K.; Southam, L.; Rayner, N.W.; Day-Williams, A.G.; Lopes, M.C.; Boraska, V.; Esko, T.; et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): A genome-wide association study. Lancet 2012, 380, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, Y.; Li, H.; Li, H.; He, Q.; Xue, Y.; Shen, C.; Zhang, C.; Xiang, J.; Ding, J.; et al. Single nucleotide polymorphisms and osteoarthritis: An overview and a meta-analysis. Medicine 2016, 95, e2811. [Google Scholar] [CrossRef] [PubMed]
- Zengini, E.; Hatzikotoulas, K.; Tachmazidou, I.; Steinberg, J.; Hartwig, F.P.; Southam, L.; Hackinger, S.; Boer, C.G.; Styrkarsdottir, U.; Gilly, A.; et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat. Genet. 2018, 50, 549–558. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Metrustry, S.; Doherty, S.A.; Laslett, L.L.; Maciewicz, R.A.; Hart, D.J.; Zhang, W.; Muir, K.R.; Wheeler, M.; Cooper, C.; et al. The effect of FTO variation on increased osteoarthritis risk is mediated through body mass index: A Mendelian randomisation study. Ann. Rheum. Dis. 2014, 73, 2082–2086. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, J.; Yang, J.; Lv, Q.; Zhong, C. Overexpression of FTO alleviates osteoarthritis by regulating the processing of miR-515-5p and the TLR4/MyD88/NF-κB axis. Int. Immunopharmacol. 2023, 114, 109524. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Soto-Hermida, A.; Vazquez-Mosquera, M.E.; Cortes-Pereira, E.; Pertega, S.; Relano, S.; Oreiro-Villar, N.; Fernandez-Lopez, C.; Blanco, F.J.; Rego-Perez, I. A replication study and meta-analysis of mitochondrial DNA variants in the radiographic progression of knee osteoarthritis. Rheumatology 2017, 56, 263–270. [Google Scholar] [CrossRef]
- Fernandez-Moreno, M.; Soto-Hermida, A.; Vazquez-Mosquera, M.E.; Cortes-Pereira, E.; Relano, S.; Hermida-Gomez, T.; Pertega, S.; Oreiro-Villar, N.; Fernandez-Lopez, C.; Garesse, R.; et al. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in OAI and CHECK cohorts. A meta-analysis and functional study. Ann. Rheum. Dis. 2017, 76, 1114–1122. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Jin, Y.; Liao, W.; Zhao, Z.; Fang, J. Mitochondrial DNA haplogroups participate in osteoarthritis: Current evidence based on a meta-analysis. Clin. Rheumatol. 2020, 39, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Mirzamohammadi, F.; Papaioannou, G.; Kobayashi, T. MicroRNAs in cartilage development, homeostasis, and disease. Curr. Osteoporos. Rep. 2014, 12, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, P.; Clegg, P.D.; Fang, Y.; Cremers, A.; Smagul, A.; Welting, T.J.; Peffers, M.J. MicroRNA signatures in cartilage ageing and osteoarthritis. Biomedicines 2023, 11, 1189. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Zhou, Y.; Fu, W.; Wang, J.; Wei, Q. A LINC00341-mediated regulatory pathway supports chondrocyte survival and may prevent osteoarthritis progression. J. Cell Biochem. 2019, 120, 10812–10820. [Google Scholar] [CrossRef]
- Ali, S.A.; Gandhi, R.; Potla, P.; Keshavarzi, S.; Espin-Garcia, O.; Shestopaloff, K.; Pastrello, C.; Bethune-Waddell, D.; Lively, S.; Perruccio, A.V.; et al. Sequencing identifies a distinct signature of circulating microRNAs in early radiographic knee osteoarthritis. Osteoarthr. Cartil. 2020, 28, 1471–1481. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Li, Y.; Zhou, Q.; Zhao, H.; Zeng, K.; Zhao, B.; Lu, Z. A comprehensive overview of PPM1B: From biological functions to diseases. Eur. J. Pharmacol. 2023, 947, 175633. [Google Scholar] [CrossRef]
- Qiu, Y.; Yao, J.; Li, L.; Xiao, M.; Meng, J.; Huang, X.; Cai, Y.; Wen, Z.; Huang, J.; Zhu, M.; et al. Machine learning identifies ferroptosis-related genes as potential diagnostic biomarkers for osteoarthritis. Front. Endocrinol. 2023, 14, 1198763. [Google Scholar] [CrossRef]
- Elkhawaga, S.Y.; Ismail, A.; Elsakka, E.G.E.; Doghish, A.S.; Elkady, M.A.; El-Mahdy, H.A. miRNAs as cornerstones in adipogenesis and obesity. Life Sci. 2023, 315, 121382. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). The Osteoarthritis Initiative. Available online: https://nda.nih.gov/oai/ (accessed on 30 April 2025).
- Fernandes, G.S.; Bhattacharya, A.; McWilliams, D.F.; Ingham, S.L.; Doherty, M.; Zhang, W. Risk prediction model for knee pain in the Nottingham community: A Bayesian modelling approach. Arthritis Res. Ther. 2017, 19, 59. [Google Scholar] [CrossRef]
- Hafezi-Nejad, N.; Guermazi, A.; Roemer, F.W.; Hunter, D.J.; Dam, E.B.; Zikria, B.; Kwoh, C.K.; Demehri, S. Prediction of medial tibiofemoral compartment joint space loss progression using volumetric cartilage measurements: Data from the FNIH OA biomarkers consortium. Eur. Radiol. 2017, 27, 464–473. [Google Scholar] [CrossRef]
- Du, Y.; Almajalid, R.; Shan, J.; Zhang, M. A novel method to predict knee osteoarthritis progression on MRI using machine learning methods. IEEE Trans. Nanobioscience 2018, 17, 228–236. [Google Scholar] [CrossRef] [PubMed]
- MacKay, J.W.; Kapoor, G.; Driban, J.B.; Lo, G.H.; McAlindon, T.E.; Toms, A.P.; McCaskie, A.W.; Gilbert, F.J. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: Data from the Osteoarthritis Initiative Bone Ancillary Study. Eur. Radiol. 2018, 28, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Leclercq, M.; Labbe, A.; Pelletier, J.P.; Abram, F.; Droit, A.; Martel-Pelletier, J. Identification of the most important features of knee osteoarthritis structural progressors using machine learning methods. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20933468. [Google Scholar] [CrossRef] [PubMed]
- Raynauld, J.P.; Martel-Pelletier, J.; Bias, P.; Laufer, S.; Haraoui, B.; Choquette, D.; Beaulieu, A.D.; Abram, F.; Dorais, M.; Vignon, E.; et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: A first multicentre clinical trial using quantitative MRI. Ann. Rheum. Dis. 2009, 68, 938–947. [Google Scholar] [CrossRef]
- Schiratti, J.-B.; Dubois, R.; Herent, P.; Cahané, D.; Dachary, J.; Clozel, T.; Wainrib, G.; Keime-Guibert, F.; Lalande, A.; Pueyo, M.; et al. A deep learning method for predicting knee osteoarthritis radiographic progression from MRI. Arthritis Res. Ther. 2021, 23, 262. [Google Scholar] [CrossRef]
- Bonakdari, H.; Pelletier, J.P.; Abram, F.; Martel-Pelletier, J. A machine learning model to predict knee osteoarthritis cartilage volume changes over time using baseline bone curvature. Biomedicines 2022, 10, 1247. [Google Scholar] [CrossRef]
- Dore, D.; Martens, A.; Quinn, S.; Ding, C.; Winzenberg, T.; Zhai, G.; Pelletier, J.P.; Martel-Pelletier, J.; Abram, F.; Cicuttini, F.; et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: A prospective study in older adults. Arthritis Res. Ther. 2010, 12, R222. [Google Scholar] [CrossRef]
- Hu, K.; Wu, W.; Li, W.; Simic, M.; Zomaya, A.; Wang, Z. Adversarial evolving neural network for longitudinal knee osteoarthritis prediction. IEEE Trans. Med. Imaging 2022, 41, 3207–3217. [Google Scholar] [CrossRef]
- Panfilov, E.; Saarakkala, S.; Nieminen, M.T.; Tiulpin, A. Predicting knee osteoarthritis progression from structural MRI using deep learning. In Proceedings of the 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI), Kolkata, India, 28–31 March 2022; pp. 1–5. [Google Scholar]
- Costello, K.E.; Felson, D.T.; Jafarzadeh, S.R.; Guermazi, A.; Roemer, F.W.; Segal, N.A.; Lewis, C.E.; Nevitt, M.C.; Lewis, C.L.; Kolachalama, V.B.; et al. Gait, physical activity and tibiofemoral cartilage damage: A longitudinal machine learning analysis in the Multicenter Osteoarthritis Study. Br. J. Sports Med. 2023, 57, 1018–1024. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, C.; Yu, Q.; Zhong, L.; Yu, K.; Chen, Y.; Wang, Z.; Zhang, B.; Dou, Q.; Zhang, X. DeepKOA: A deep-learning model for predicting progression in knee osteoarthritis using multimodal magnetic resonance images from the osteoarthritis initiative. Quant. Imaging Med. Surg. 2023, 13, 4852–4866. [Google Scholar] [CrossRef]
- Jansen, M.P.; Wirth, W.; Bacardit, J.; van Helvoort, E.M.; Marijnissen, A.C.A.; Kloppenburg, M.; Blanco, F.J.; Haugen, I.K.; Berenbaum, F.; Ladel, C.H.; et al. Machine-learning predicted and actual 2-year structural progression in the IMI-APPROACH cohort. Quant. Imaging Med. Surg. 2023, 13, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Peng, J.; Hu, J.; Lu, Y.; Zhou, Z.; Xu, H.; Xing, K.; Zhang, X.; Lu, L. LMSST-GCN: Longitudinal MRI sub-structural texture guided graph convolution network for improved progression prediction of knee osteoarthritis. Comput. Methods Programs Biomed. 2025, 261, 108600. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Paiement, P.; Pelletier, J.P. Magnetic resonance imaging assessments for knee segmentation and their use in combination with machine/deep learning as predictors of early osteoarthritis diagnosis and prognosis. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720x231165560. [Google Scholar] [CrossRef] [PubMed]
- 66 Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Köse, Ö.; Acar, B.; Çay, F.; Yilmaz, B.; Güler, F.; Yüksel, H.Y. Inter- and intraobserver reliabilities of four different radiographic grading scales of osteoarthritis of the knee joint. J. Knee Surg. 2018, 31, 247–253. [Google Scholar] [CrossRef]
- Cheung, J.C.; Tam, A.Y.; Chan, L.C.; Chan, P.K.; Wen, C. Superiority of multiple-joint space width over minimum-joint space width approach in the machine learning for radiographic severity and knee osteoarthritis progression. Biology 2021, 10, 1107. [Google Scholar] [CrossRef]
- Chen, P.; Gao, L.; Shi, X.; Allen, K.; Yang, L. Fully automatic knee osteoarthritis severity grading using deep neural networks with a novel ordinal loss. Comput. Med. Imaging Graph. 2019, 75, 84–92. [Google Scholar] [CrossRef]
- Wang, C.T.; Huang, B.; Thogiti, N.; Zhu, W.X.; Chang, C.H.; Pao, J.L.; Lai, F. Successful real-world application of an osteoarthritis classification deep-learning model using 9210 knees-An orthopedic surgeon’s view. J. Orthop. Res. 2023, 41, 737–746. [Google Scholar] [CrossRef]
- Pan, J.; Wu, Y.; Tang, Z.; Sun, K.; Li, M.; Sun, J.; Liu, J.; Tian, J.; Shen, B. Automatic knee osteoarthritis severity grading based on X-ray images using a hierarchical classification method. Arthritis Res. Ther. 2024, 26, 203. [Google Scholar] [CrossRef]
- Anifah, L.; Purnama, I.K.; Hariadi, M.; Purnomo, M.H. Osteoarthritis classification using self organizing map based on gabor kernel and contrast-limited adaptive histogram equalization. Open Biomed. Eng. J. 2013, 7, 18–28. [Google Scholar] [CrossRef]
- Štrumbelj, E.; Kononenko, I. Explaining prediction models and individual predictions with feature contributions. Knowl. Inf. Syst. 2013, 41, 647–665. [Google Scholar] [CrossRef]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why should I trust you?”: Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar] [CrossRef]
- FDA. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. U.S. Food & Drug Administration. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 30 April 2025).
- FDA. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SAMD)—Discussion Paper and Request for Feedback. U.S. Food and Drug Administration. Available online: https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf (accessed on 30 April 2025).
- Health Canada. Guidance Document: Software as a Medical Device (SaMD): Definition and Classification. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/software-medical-device-guidance-document.html (accessed on 30 April 2025).
- European Parliament 2019–2024. Provisional Agreement Resulting from Interinstitutional. Proposal for a Regulation Laying down Harmonised Rules on Artificial Intelligence (Artificial Intelligence Act) and Amending Certain Union Legislative Acts 2021/0106(COD). Available online: https://www.europarl.europa.eu/meetdocs/2014_2019/plmrep/COMMITTEES/CJ40/AG/2024/02-13/1296003EN.pdf (accessed on 30 April 2025).
- Sendak, M.P.; Ratliff, W.; Sarro, D.; Alderton, E.; Futoma, J.; Gao, M.; Nichols, M.; Revoir, M.; Yashar, F.; Miller, C.; et al. Real-world integration of a sepsis deep learning technology into routine clinical care: Implementation study. JMIR Med. Inf. 2020, 8, e15182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martel-Pelletier, J.; Pelletier, J.-P. Next-Level Prediction of Structural Progression in Knee Osteoarthritis: A Perspective. Int. J. Mol. Sci. 2025, 26, 4748. https://doi.org/10.3390/ijms26104748
Martel-Pelletier J, Pelletier J-P. Next-Level Prediction of Structural Progression in Knee Osteoarthritis: A Perspective. International Journal of Molecular Sciences. 2025; 26(10):4748. https://doi.org/10.3390/ijms26104748
Chicago/Turabian StyleMartel-Pelletier, Johanne, and Jean-Pierre Pelletier. 2025. "Next-Level Prediction of Structural Progression in Knee Osteoarthritis: A Perspective" International Journal of Molecular Sciences 26, no. 10: 4748. https://doi.org/10.3390/ijms26104748
APA StyleMartel-Pelletier, J., & Pelletier, J.-P. (2025). Next-Level Prediction of Structural Progression in Knee Osteoarthritis: A Perspective. International Journal of Molecular Sciences, 26(10), 4748. https://doi.org/10.3390/ijms26104748






