Anti-IL-4, Anti-IL-17, and Anti-IFN-Gamma Activity in the Saliva of Amblyomma sculptum Ticks
Abstract
1. Introduction
2. Results
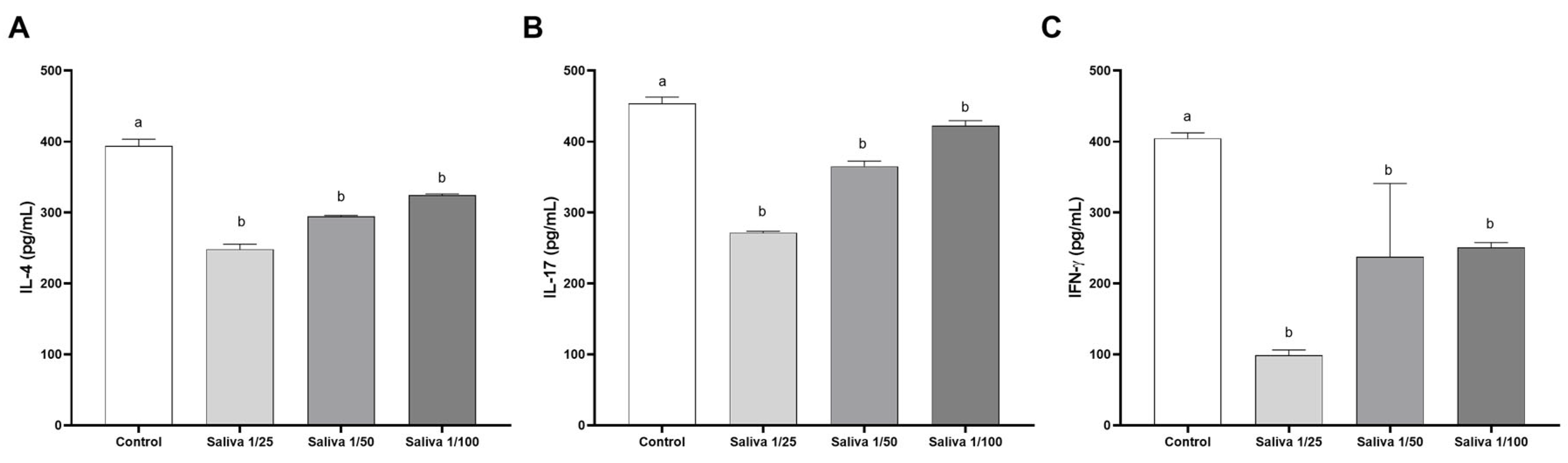
2.1. Amblyomma sculptum Saliva Binds to Human IL-4, IL-17, and IFN-γ Cytokines
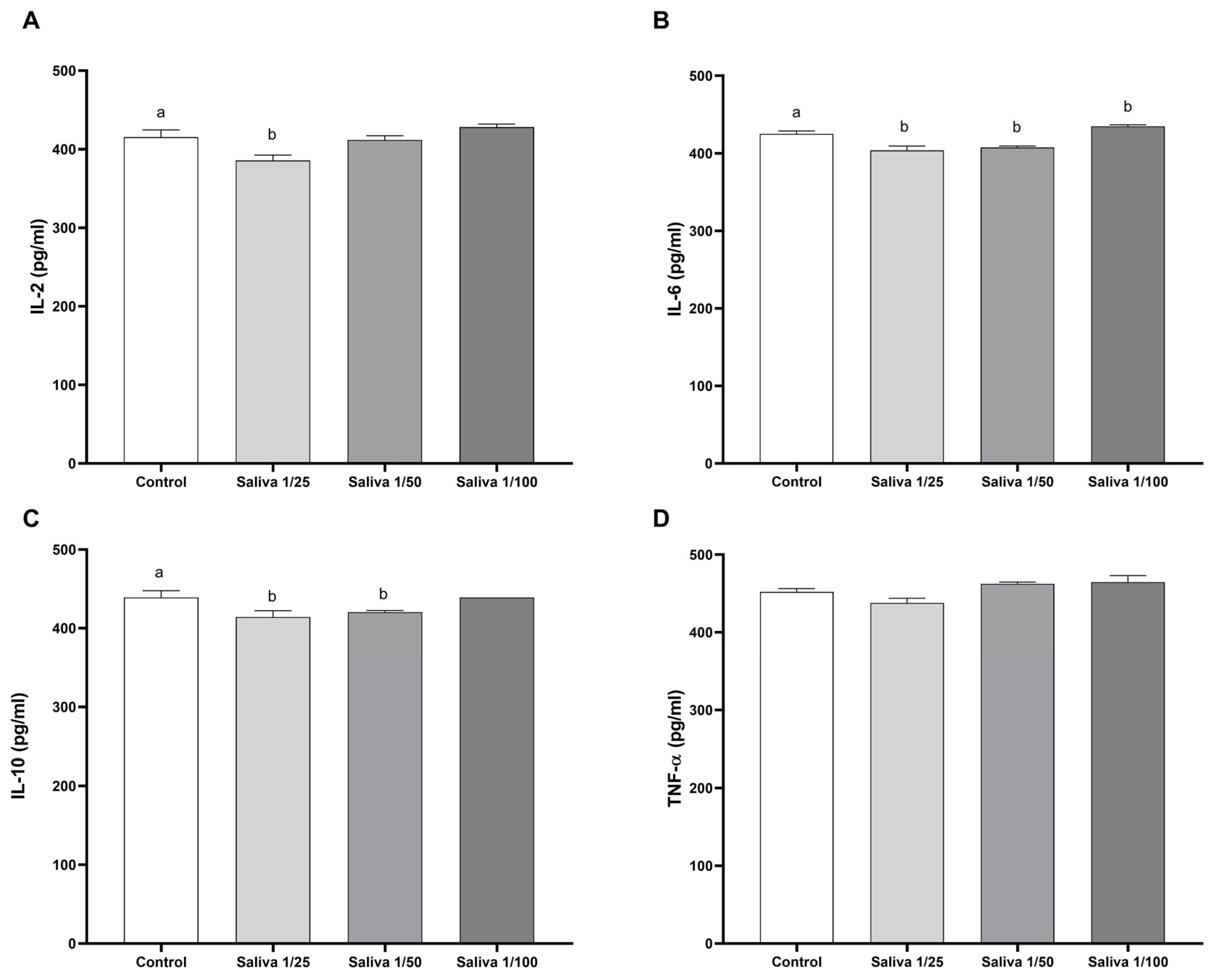
2.2. Amblyomma sculptum Saliva Does Not Show Effective Binding Activity Against Cytokines IL-2, IL-6, IL-10, and TNF-α
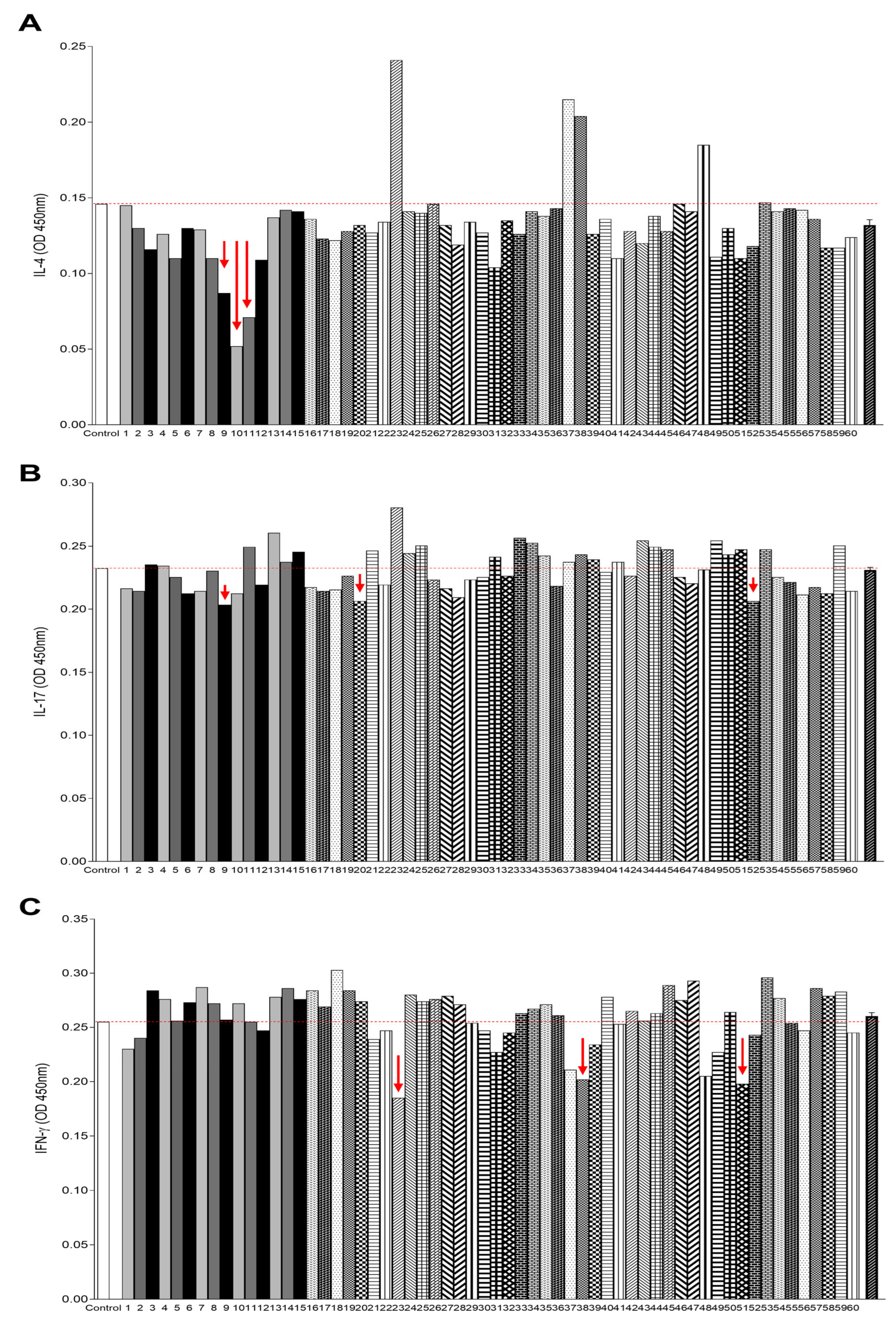
2.3. Anti-IL-4, Anti-IL-17, and Anti-IFN-γ Binding Activity in A. sculptum Tick Saliva Fractions
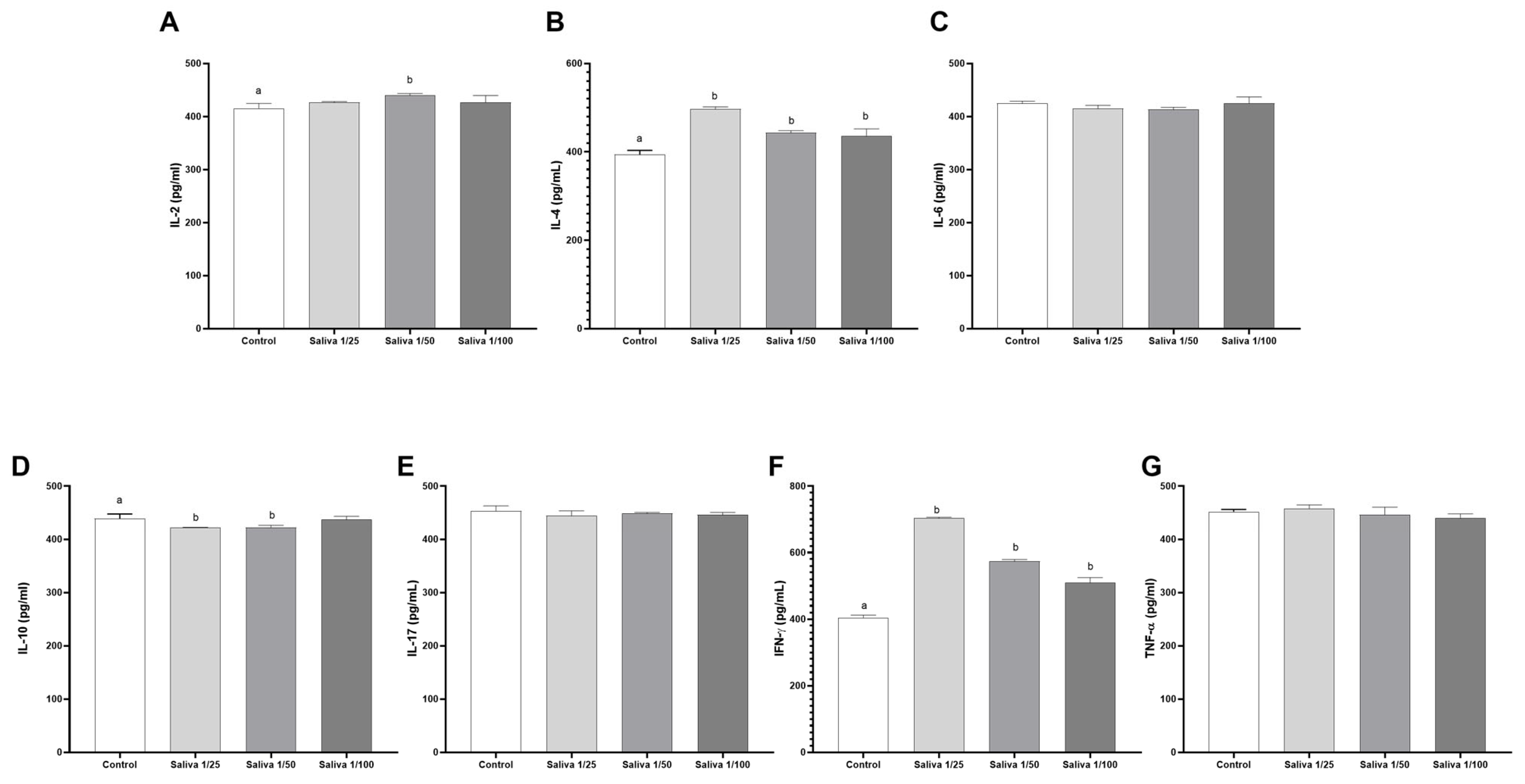
2.4. The Anti-Cytokine Binding Activity Was Not Evidenced in Triatomine Saliva
3. Discussion
4. Materials and Methods
4.1. Tick and Triatomine Saliva
4.2. Binding Effect of Tick and Triatomines Saliva Molecules on Cytokines
4.3. HPLC Procedures
4.4. Activity of Saliva Fractions on Cytokines
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Famurewa, A.C.; Oprea, E. Natural Bioactive Compounds and Human Health. Molecules 2024, 29, 3372. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91 (Suppl. S3), e20190105. [Google Scholar] [CrossRef]
- Desiderio, C.S.; Flavio-Reis, V.H.P.; Pessoa-Goncalves, Y.M.; Tiveron, R.D.R.; Sales-Campos, H.; Felice, A.G.; Soares, S.C.; Guillermo-Ferreira, R.; Rodrigues, W.F.; Oliveira, C.J.F. Binding Molecules in Tick Saliva for Targeting Host Cytokines, Chemokines, and Beyond. Biomolecules 2024, 14, 1647. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Sa-Nunes, A.; Mans, B.J.; Santos, I.M.; Ribeiro, J.M. The role of saliva in tick feeding. Front. Biosci. (Landmark Ed) 2009, 14, 2051–2088. [Google Scholar] [CrossRef]
- Bonvin, P.; Power, C.A.; Proudfoot, A.E. Evasins: Therapeutic Potential of a New Family of Chemokine-Binding Proteins from Ticks. Front. Immunol. 2016, 7, 208. [Google Scholar] [CrossRef]
- Torina, A.; Villari, S.; Blanda, V.; Vullo, S.; La Manna, M.P.; Shekarkar Azgomi, M.; Di Liberto, D.; de la Fuente, J.; Sireci, G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 2020, 21, 5437. [Google Scholar] [CrossRef]
- Wikel, S.K. Modulation of the Host Immune System by Ectoparasitic Arthropods. BioScience 1999, 49, 311–320. [Google Scholar] [CrossRef]
- Hajnicka, V.; Vancova, I.; Kocakova, P.; Slovak, M.; Gasperik, J.; Slavikova, M.; Hails, R.S.; Labuda, M.; Nuttall, P.A. Manipulation of host cytokine network by ticks: A potential gateway for pathogen transmission. Parasitology 2005, 130 Pt 3, 333–342. [Google Scholar] [CrossRef]
- Rezkova, M.; Kopecky, J. Anti-tumour necrosis factor activity in saliva of various tick species and its appearance during the feeding period. Folia Parasitol. 2017, 64, 032. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Fernandes Martins, T.; Munoz-Leal, S.; Onofrio, V.C.; Barros-Battesti, D.M. Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick Borne Dis. 2019, 10, 101252. [Google Scholar] [CrossRef] [PubMed]
- Fogaca, A.C.; Sousa, G.; Pavanelo, D.B.; Esteves, E.; Martins, L.A.; Urbanova, V.; Kopacek, P.; Daffre, S. Tick Immune System: What Is Known, the Interconnections, the Gaps, and the Challenges. Front. Immunol. 2021, 12, 628054. [Google Scholar] [CrossRef]
- de Paula, L.G.F.; do Nascimento, R.M.; Franco, A.O.; Szabo, M.P.J.; Labruna, M.B.; Monteiro, C.; Krawczak, F.D.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasit. Vectors 2022, 15, 193. [Google Scholar] [CrossRef]
- Esteves, E.; Bizzarro, B.; Costa, F.B.; Ramirez-Hernandez, A.; Peti, A.P.F.; Cataneo, A.H.D.; Wowk, P.F.; Timoteo, R.P.; Labruna, M.B.; Silva Junior, P.I.; et al. Amblyomma sculptum Salivary PGE(2) Modulates the Dendritic Cell-Rickettsia rickettsii Interactions in vitro and in vivo. Front. Immunol. 2019, 10, 118. [Google Scholar] [CrossRef]
- Schoeler, G.B.; Wikel, S.K. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 2001, 95, 755–771. [Google Scholar] [CrossRef]
- Scholl, D.C.; Embers, M.E.; Caskey, J.R.; Kaushal, D.; Mather, T.N.; Buck, W.R.; Morici, L.A.; Philipp, M.T. Immunomodulatory effects of tick saliva on dermal cells exposed to Borrelia burgdorferi, the agent of Lyme disease. Parasit. Vectors 2016, 9, 394. [Google Scholar] [CrossRef]
- Santiago, P.B.; de Araujo, C.N.; Motta, F.N.; Praca, Y.R.; Charneau, S.; Bastos, I.M.; Santana, J.M. Proteases of haematophagous arthropod vectors are involved in blood-feeding, yolk formation and immunity—A review. Parasit Vectors 2017, 10, 79. [Google Scholar] [CrossRef]
- Tirloni, L.; Kim, T.K.; Pinto, A.F.M.; Yates, J.R., 3rd; da Silva Vaz, I., Jr.; Mulenga, A. Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts. Front. Cell. Infect. Microbiol. 2017, 7, 517. [Google Scholar] [CrossRef]
- Tirloni, L.; Reck, J.; Terra, R.M.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R., 3rd; Termignoni, C.; Pinto, A.F.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef]
- Denisov, S.S.; Dijkgraaf, I. Immunomodulatory Proteins in Tick Saliva From a Structural Perspective. Front. Cell. Infect. Microbiol. 2021, 11, 769574. [Google Scholar] [CrossRef] [PubMed]
- Mejri, N.; Rutti, B.; Brossard, M. Immunosuppressive effects of ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol. Res. 2002, 88, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sa-Nunes, A.; Oliveira, C.J.F.; Ribeiro, J.M. Mast Cells and Basophils: From Malevolent Design to Coevolutionary Arms Race. Trends Parasitol. 2020, 36, 655–659. [Google Scholar] [CrossRef]
- Alarcon-Chaidez, F.J.; Boppana, V.D.; Hagymasi, A.T.; Adler, A.J.; Wikel, S.K. A novel sphingomyelinase-like enzyme in Ixodes scapularis tick saliva drives host CD4 T cells to express IL-4. Parasite Immunol. 2009, 31, 210–219. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Theodos, C.M.; Titus, R.G. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 1993, 15, 481–487. [Google Scholar] [CrossRef]
- Ferreira, B.R.; Silva, J.S. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-gamma-induced macrophage microbicidal activity. Vet. Immunol. Immunopathol. 1998, 64, 279–293. [Google Scholar] [CrossRef]
- Ganapamo, F.; Rutti, B.; Brossard, M. In Vitro production of interleukin-4 and interferon-gamma by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology 1995, 85, 120–124. [Google Scholar]
- Lieskovska, J.; Palenikova, J.; Sirmarova, J.; Elsterova, J.; Kotsyfakis, M.; Campos Chagas, A.; Calvo, E.; Ruzek, D.; Kopecky, J. Tick salivary cystatin sialostatin L2 suppresses IFN responses in mouse dendritic cells. Parasite Immunol. 2015, 37, 70–78. [Google Scholar] [CrossRef]
- Garcia, G.R.; Chaves Ribeiro, J.M.; Maruyama, S.R.; Gardinassi, L.G.; Nelson, K.; Ferreira, B.R.; Andrade, T.G.; de Miranda Santos, I.K.F. A transcriptome and proteome of the tick Rhipicephalus microplus shaped by the genetic composition of its hosts and developmental stage. Sci. Rep. 2020, 10, 12857. [Google Scholar] [CrossRef] [PubMed]
- Vancova, I.; Hajnicka, V.; Slovak, M.; Nuttall, P.A. Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Vet. Parasitol. 2010, 167, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Hajnicka, V.; Vancova-Stibraniova, I.; Slovak, M.; Kocakova, P.; Nuttall, P.A. Ixodid tick salivary gland products target host wound healing growth factors. Int. J. Parasitol. 2011, 41, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Castagnolli, K.C.; Ferreira, B.R.; Franzin, A.M.; de Castro, M.B.; Szabo, M.P. Effect of Amblyomma cajennense ticks on the immune response of BALB/c mice and horses. Ann. N. Y. Acad. Sci. 2008, 1149, 230–234. [Google Scholar] [CrossRef]
- Mesquita, R.D.; Carneiro, A.B.; Bafica, A.; Gazos-Lopes, F.; Takiya, C.M.; Souto-Padron, T.; Vieira, D.P.; Ferreira-Pereira, A.; Almeida, I.C.; Figueiredo, R.T.; et al. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect. Immun. 2008, 76, 5543–5552. [Google Scholar] [CrossRef]
- Adib, N.; Zahmatkesh, A.; Bagheri, M. A simple cost-effective method for purification of Clostridium chauvoei cell-surface proteins for detection of antibodies against blackleg disease vaccine. Vet. Res. Forum 2025, 16, 57–61. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales-Campos, H.; Desidério, C.S.; Trevisan, R.O.; Timóteo, R.P.; Flávio-Reis, V.H.P.; Pessoa-Gonçalves, Y.M.; da Silva, M.V.; Esteves, E.; de Jesus Oliveira, T.; da Silva Junior, P.I.; et al. Anti-IL-4, Anti-IL-17, and Anti-IFN-Gamma Activity in the Saliva of Amblyomma sculptum Ticks. Int. J. Mol. Sci. 2025, 26, 4734. https://doi.org/10.3390/ijms26104734
Sales-Campos H, Desidério CS, Trevisan RO, Timóteo RP, Flávio-Reis VHP, Pessoa-Gonçalves YM, da Silva MV, Esteves E, de Jesus Oliveira T, da Silva Junior PI, et al. Anti-IL-4, Anti-IL-17, and Anti-IFN-Gamma Activity in the Saliva of Amblyomma sculptum Ticks. International Journal of Molecular Sciences. 2025; 26(10):4734. https://doi.org/10.3390/ijms26104734
Chicago/Turabian StyleSales-Campos, Helioswilton, Chamberttan Souza Desidério, Rafael Obata Trevisan, Rodolfo Pessato Timóteo, Victor Hugo Palhares Flávio-Reis, Yago Marcos Pessoa-Gonçalves, Marcos Vinicius da Silva, Eliane Esteves, Thiago de Jesus Oliveira, Pedro Ismael da Silva Junior, and et al. 2025. "Anti-IL-4, Anti-IL-17, and Anti-IFN-Gamma Activity in the Saliva of Amblyomma sculptum Ticks" International Journal of Molecular Sciences 26, no. 10: 4734. https://doi.org/10.3390/ijms26104734
APA StyleSales-Campos, H., Desidério, C. S., Trevisan, R. O., Timóteo, R. P., Flávio-Reis, V. H. P., Pessoa-Gonçalves, Y. M., da Silva, M. V., Esteves, E., de Jesus Oliveira, T., da Silva Junior, P. I., & Oliveira, C. J. F. (2025). Anti-IL-4, Anti-IL-17, and Anti-IFN-Gamma Activity in the Saliva of Amblyomma sculptum Ticks. International Journal of Molecular Sciences, 26(10), 4734. https://doi.org/10.3390/ijms26104734







