Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets
Abstract
1. Introduction
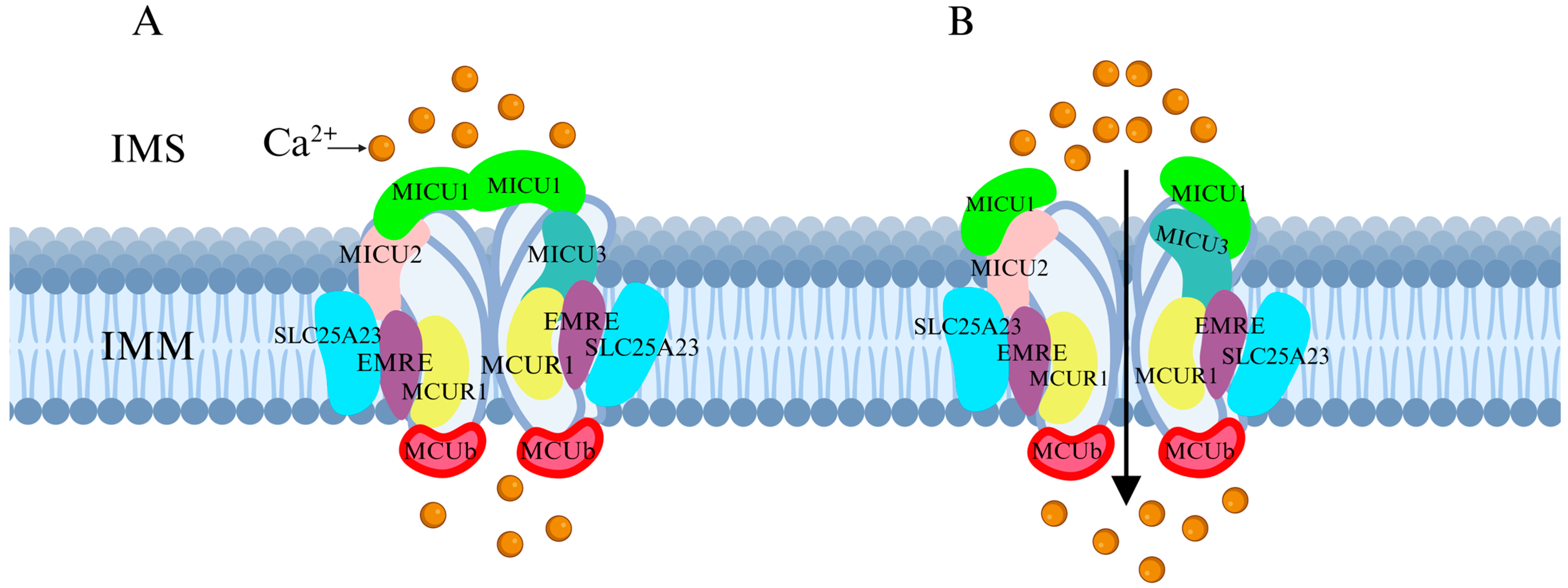
2. Structure and Function of MCU
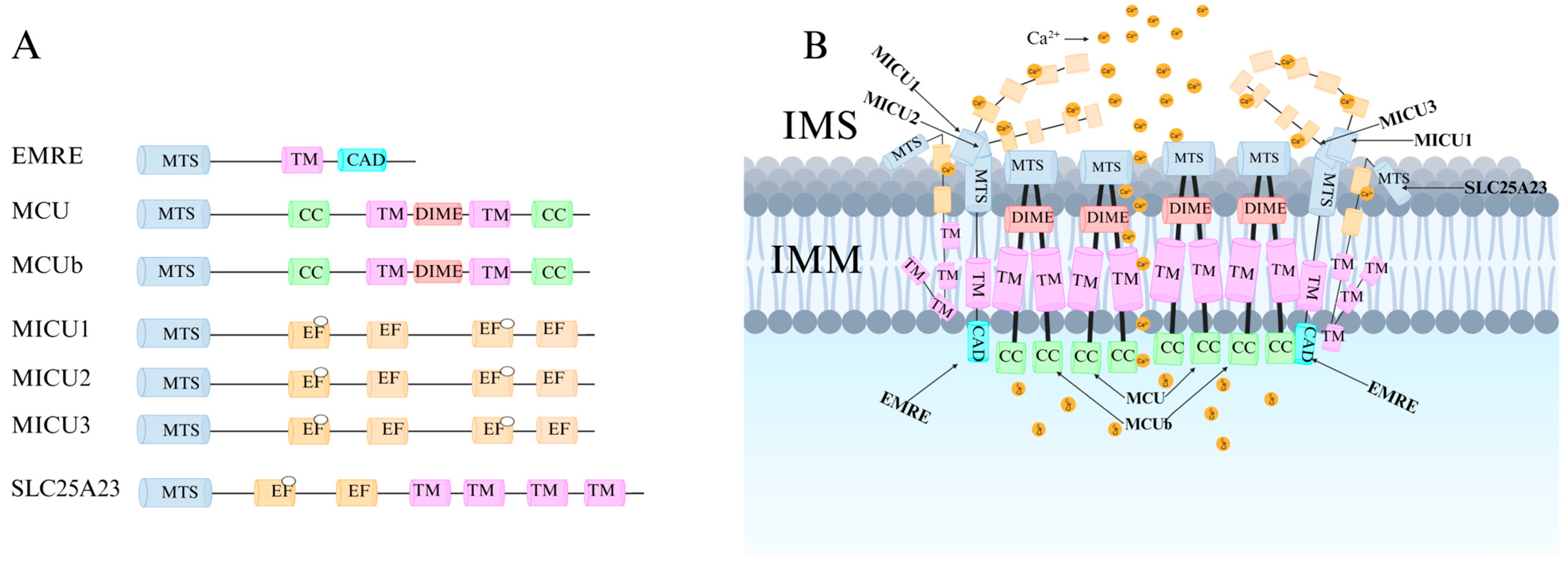
2.1. Structure of MCU
2.2. Function of MCU
3. Regulatory Mechanisms of MCU
3.1. Regulation of MCU by Associated Proteins
3.2. Other Regulatory Mechanisms of MCU
3.2.1. Regulation via the CaMKII-CREB Signaling Pathway
3.2.2. Kinase-Mediated Post-Translational Modifications of MCU
3.2.3. MicroRNA-Mediated Post-Transcriptional Regulatory Network
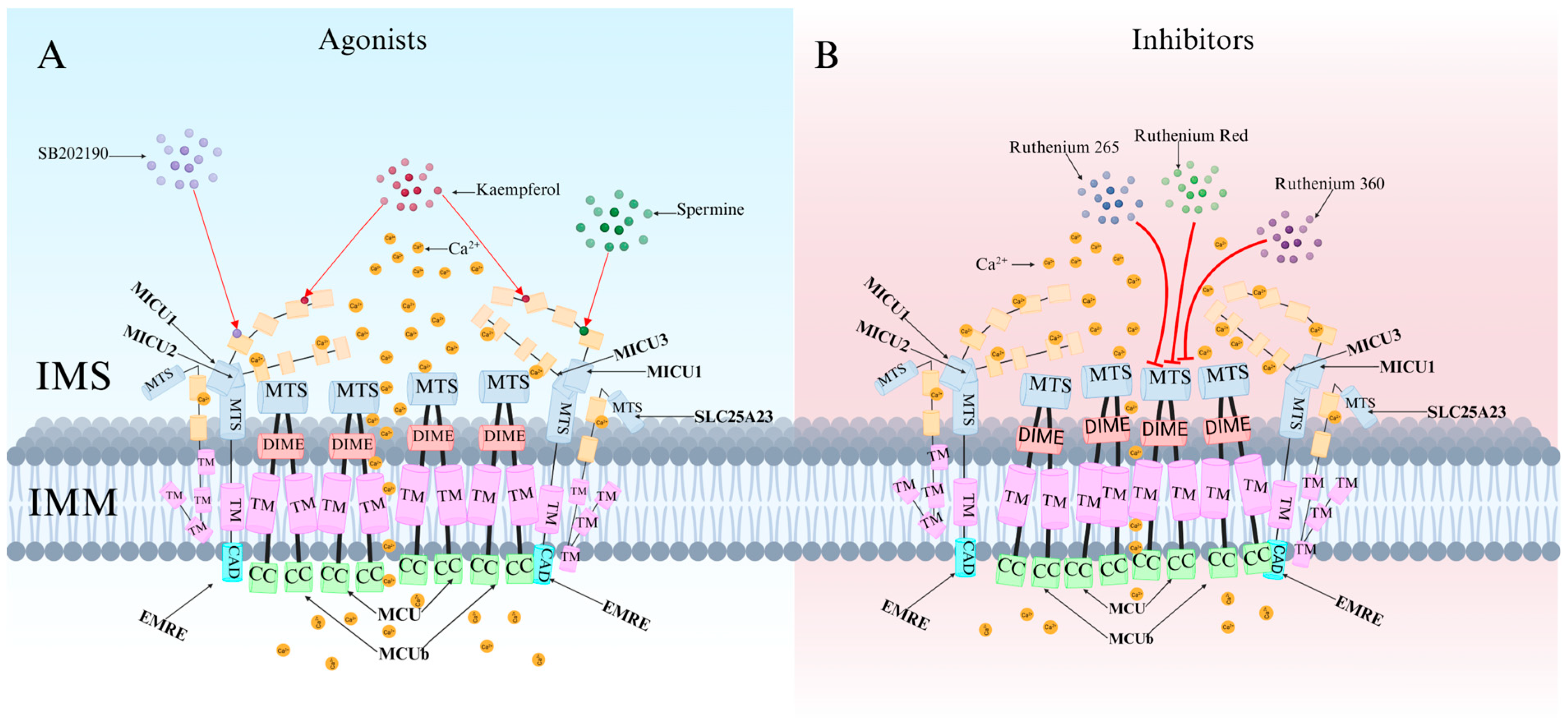
4. Specific Agonists and Inhibitors of MCU
4.1. MCU-Specific Agonists and Their Mechanisms of Action
4.2. Classification and Characteristics of MCU-Specific Inhibitors
5. Research on MCU in Neurotoxic Damage Induced by Psychoactive Substances
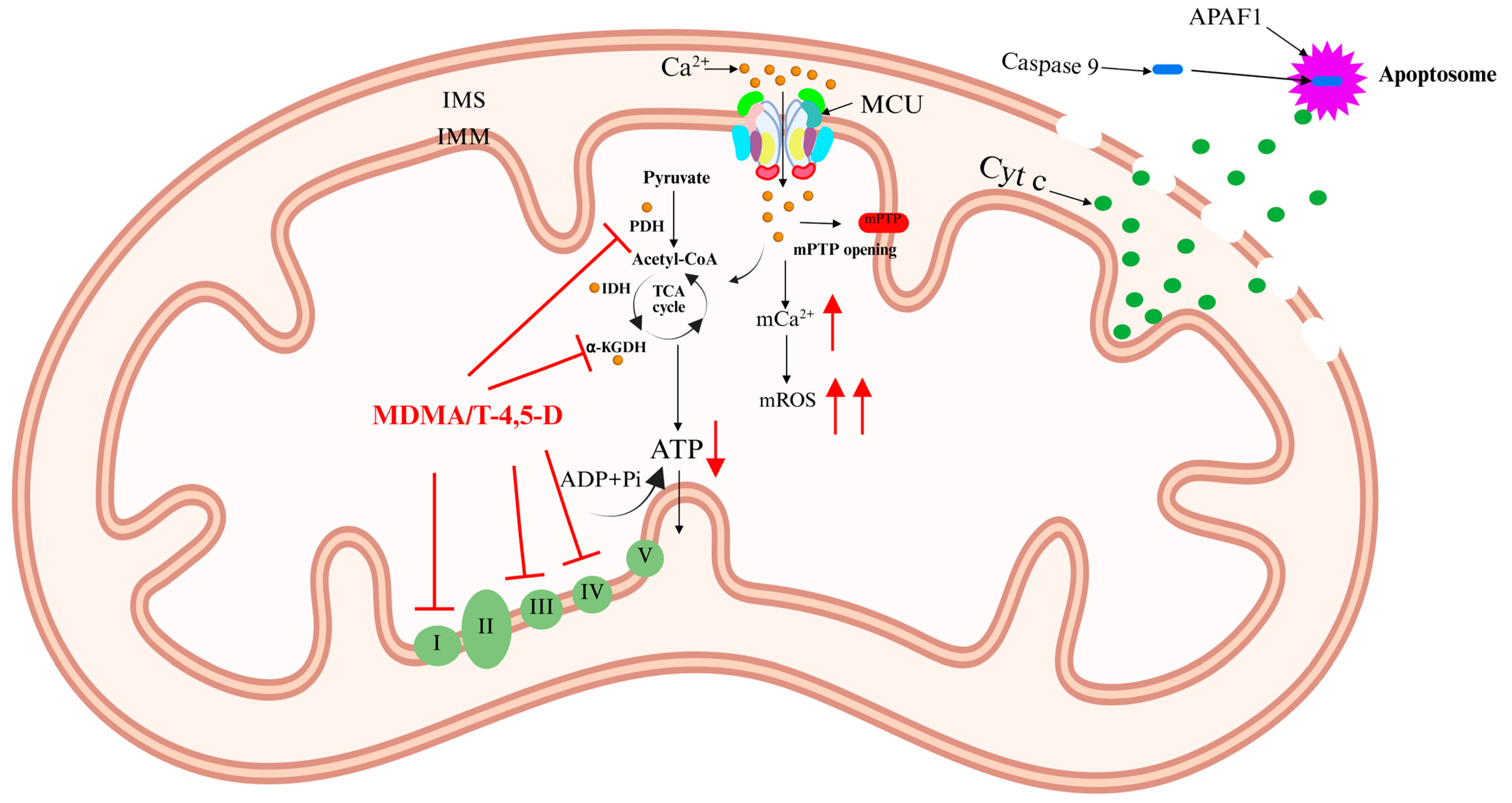
5.1. 3,4-Methylenedioxymethamphetamine (MDMA)
5.2. Cocaine
5.3. Morphine
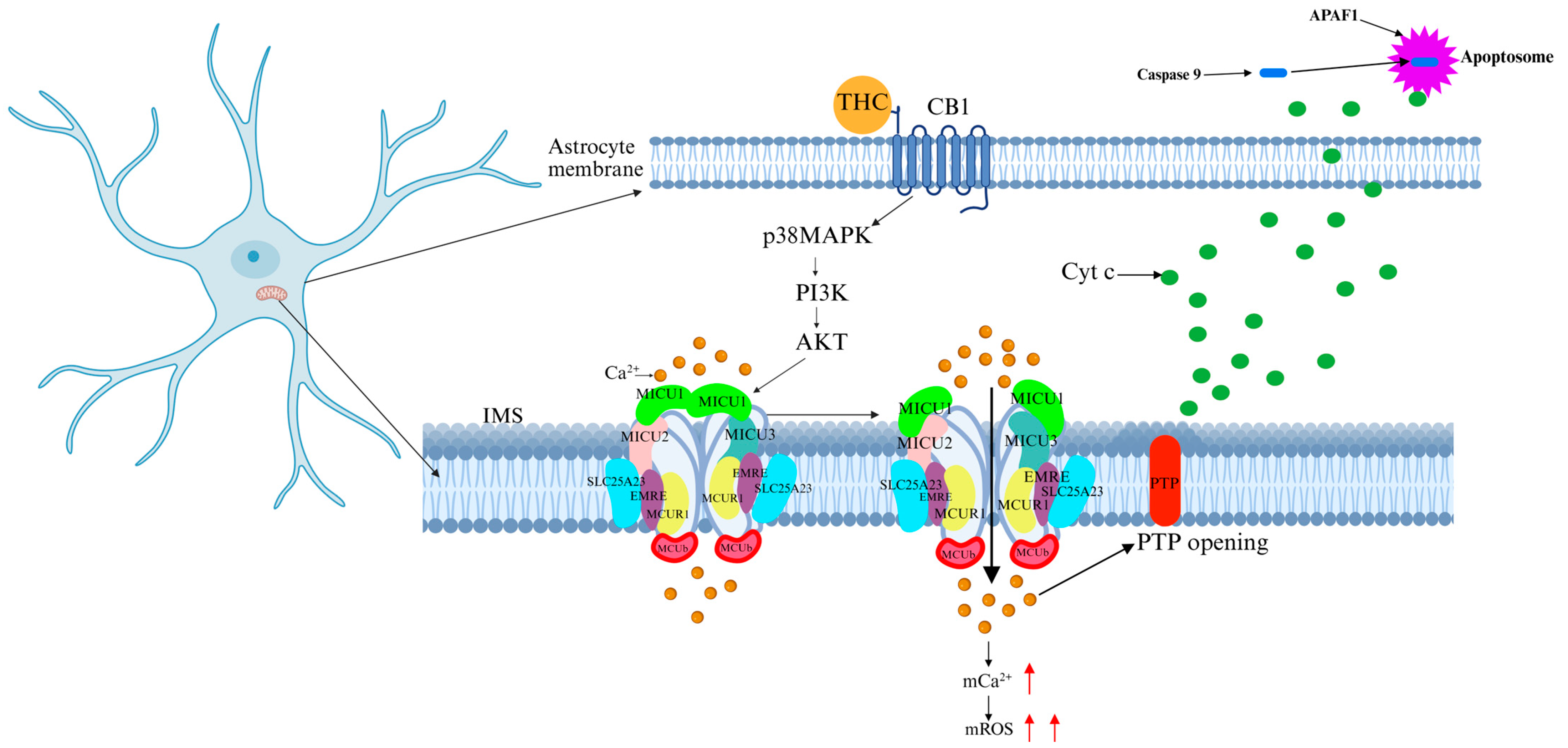
5.4. Cannabis
5.5. Methamphetamine (METH)
5.6. Ketamine
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, W.; Yuan, P.; Zhu, P.; Fan, K.; Xia, Z.; Xu, S. The mechanism of CaMK2α-MCU-mitochondrial oxidative stress in bupivacaine-induced neurotoxicity. Free Radic. Biol. Med. 2020, 152, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Hu, H.; Chen, L. MCU complex: Exploring emerging targets and mechanisms of mitochondrial physiology and pathology. J. Adv. Res. 2025, 68, 271–298. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Baradaran, R.; Wang, C.; Siliciano, A.F.; Long, S.B. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 2018, 559, 580–584. [Google Scholar] [CrossRef]
- Woods, J.J.; Wilson, J.J. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr. Opin. Chem. Biol. 2020, 55, 9–18. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, M.; Wang, J.; Nie, Z.; Wu, G.; Yang, X.; Shen, Y. Dimerization of MICU Proteins Controls Ca2+ Influx through the Mitochondrial Ca2+ Uniporter. Cell Rep. 2019, 26, 1203–1212. [Google Scholar] [CrossRef]
- Twyning, M.J.; Tufi, R.; Gleeson, T.P.; Kolodziej, K.M.; Campesan, S.; Terriente-Felix, A.; Collins, L.; De Lazzari, F.; Giorgini, F.; Whitworth, A.J. Partial loss of MCU mitigates pathology in vivo across a diverse range of neurodegenerative disease models. Cell Rep. 2024, 43, 113681. [Google Scholar] [CrossRef]
- D’Angelo, D.; Rizzuto, R. The Mitochondrial Calcium Uniporter (MCU): Molecular Identity and Role in Human Diseases. Biomolecules 2023, 13, 1304. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.G.; Denton, R.M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979, 180, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.E.; Gibson, G.E. The α-Ketoglutarate Dehydrogenase Complex as a Hub of Plasticity in Neurodegeneration and Regeneration. Int. J. Mol. Sci. 2022, 23, 12403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse functions of cytochrome c in cell death and disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, Y.; Lin, W.; Huang, R.; Li, K.; Han, X.; Ren, Y. Neurotoxic effects induced by flunitrazepam and its metabolites in zebrafish: Oxidative stress, apoptosis, and histone hypoacetylation. Sci. Total Environ. 2024, 917, 170521. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, K.H.; DasGupta, A.; Potus, F.; Dunham-Snary, K.; Bonnet, S.; Tian, L.; Fu, J.; Breuils-Bonnet, S.; Provencher, S.; et al. MicroRNA-138 and MicroRNA-25 Down-regulate Mitochondrial Calcium Uniporter.; Causing the Pulmonary Arterial Hypertension Cancer Phenotype. Am. J. Respir. Crit. Care Med. 2017, 195, 515–529. [Google Scholar] [CrossRef]
- Liu, T.; Yang, N.; Sidor, A.; O’Rourke, B. MCU Overexpression Rescues Inotropy and Reverses Heart Failure by Reducing SR Ca2+ Leak. Circ. Res. 2021, 128, 1191–1204. [Google Scholar] [CrossRef]
- Tosatto, A.; Sommaggio, R.; Kummerow, C.; Bentham, R.B.; Blacker, T.S.; Berecz, T.; Duchen, M.R.; Rosato, A.; Bogeski, I.; Szabadkai, G.; et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol. Med. 2016, 8, 569–585. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, Z.; Lin, S.; Wang, H.; Sun, J.; Lan, C.; Wu, L.; Sun, D.; Huang, C.; et al. Mitochondrial Calcium Uniporter Drives Metastasis and Confers a Targetable Cystine Dependency in Pancreatic Cancer. Cancer Res. 2022, 82, 2254–2268. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nascimento Da Conceicao, V.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium Signaling Regulates Autophagy and Apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Egunlusi, A.O.; Malan, S.F.; Palchykov, V.A.; Joubert, J. Calcium Modulating Effect of Polycyclic Cages: A Suitable Therapeutic Approach Against Excitotoxic-induced Neurodegeneration. Mini Rev. Med. Chem. 2024, 24, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, M.; O’Neill, N.; Lariccia, V.; Amoroso, S.; Sylantyev, S.; Angelova, P.R.; Abramov, A.Y. Inorganic Polyphosphate Regulates AMPA and NMDA Receptors and Protects Against Glutamate Excitotoxicity via Activation of P2Y Receptors. J. Neurosci. 2019, 39, 6038–6048. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.L.; Cohen, H.M.; Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium uniporter channel gatekeeping in cardiovascular disease. Nat. Cardiovasc. Res. 2024, 3, 500–514. [Google Scholar] [CrossRef]
- Tomar, D.; Thomas, M.; Garbincius, J.F.; Kolmetzky, D.W.; Salik, O.; Jadiya, P.; Joseph, S.K.; Carpenter, A.C.; Hajnóczky, G.; Elrod, J.W. MICU1 regulates mitochondrial cristae structure and function independently of the mitochondrial Ca2+ uniporter channel. Sci. Signal. 2023, 16, eabi8948. [Google Scholar] [CrossRef]
- Wang, C.; Jacewicz, A.; Delgado, B.D.; Baradaran, R.; Long, S.B. Structures reveal gatekeeping of the mitochondrial Ca2+ uniporter by MICU1-MICU2. eLife 2020, 9, e59991. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, J.; Jia, Z. Structural characterization of the mitochondrial Ca2+ uniporter provides insights into Ca2+ uptake and regulation. iScience 2021, 24, 102895. [Google Scholar] [CrossRef]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular Tuning of the Axonal Mitochondrial Ca2+ Uniporter Ensures Metabolic Flexibility of Neurotransmission. Neuron 2020, 105, 678–687. [Google Scholar] [CrossRef]
- Lambert, J.P.; Luongo, T.S.; Tomar, D.; Jadiya, P.; Gao, E.; Zhang, X.; Lucchese, A.M.; Kolmetzky, D.W.; Shah, N.S.; Elrod, J.W. MCUB Regulates the Molecular Composition of the Mitochondrial Calcium Uniporter Channel to Limit Mitochondrial Calcium Overload During Stress. Circulation 2019, 140, 1720–1733. [Google Scholar] [CrossRef]
- Rodríguez-Prados, M.; Huang, K.T.; Márta, K.; Paillard, M.; Csordás, G.; Joseph, S.K.; Hajnóczky, G. MICU1 controls the sensitivity of the mitochondrial Ca2+ uniporter to activators and inhibitors. Cell Chem. Biol. 2023, 30, 606–617. [Google Scholar] [CrossRef]
- Paupe, V.; Prudent, J.; Dassa, E.P.; Rendon, O.Z.; Shoubridge, E.A. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015, 21, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Artiga, D.J.; Abiria, S.A.; Clapham, D.E. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc. Natl. Acad. Sci. USA 2016, 113, E1872–E1880. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamagoshi, R.; Harada, K.; Kawano, M.; Minami, N.; Ido, Y.; Kuwahara, K.; Fujita, A.; Ozono, M.; Watanabe, A.; et al. Analysis of the structure and function of EMRE in a yeast expression system. Biochim. Biophys. Acta 2016, 1857, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nguyen, N.X.; She, J.; Zeng, W.; Yang, Y.; Bai, X.C.; Jiang, Y. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell 2019, 177, 1252–1261. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; She, J.; Nguyen, N.X.; Mootha, V.K.; Bai, X.C.; Jiang, Y. Structural insights into the Ca2+-dependent gating of the human mitochondrial calcium uniporter. eLife 2020, 9, e60513. [Google Scholar] [CrossRef]
- Watanabe, A.; Maeda, K.; Nara, A.; Hashida, M.; Ozono, M.; Nakao, A.; Yamada, A.; Shinohara, Y.; Yamamoto, T. Quantitative analysis of mitochondrial calcium uniporter (MCU) and essential MCU regulator (EMRE) in mitochondria from mouse tissues and HeLa cells. FEBS Open Bio 2022, 12, 811–826. [Google Scholar] [CrossRef]
- Alevriadou, B.R.; Patel, A.; Noble, M.; Ghosh, S.; Gohil, V.M.; Stathopulos, P.B.; Madesh, M. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am. J. Physiol. Cell Physiol. 2021, 320, C465–C482. [Google Scholar] [CrossRef]
- Hoffman, N.E.; Chandramoorthy, H.C.; Shanmughapriya, S.; Zhang, X.Q.; Vallem, S.; Doonan, P.J.; Malliankaraman, K.; Guo, S.; Rajan, S.; Elrod, J.W.; et al. SLC25A23 augments mitochondrial Ca2+ uptake.; interacts with MCU.; and induces oxidative stress-mediated cell death. Mol. Biol. Cell. 2014, 25, 936–947. [Google Scholar] [CrossRef]
- Bassi, M.T.; Manzoni, M.; Bresciani, R.; Pizzo, M.T.; Della, M.A.; Barlati, S.; Monti, E.; Borsani, G. Cellular expression and alternative splicing of SLC25A23, a member of the mitochondrial Ca2+-dependent solute carrier gene family. Gene 2005, 345, 173–182. [Google Scholar] [CrossRef]
- Feno, S.; Rizzuto, R.; Raffaello, A.; Vecellio, R.D. The molecular complexity of the Mitochondrial Calcium Uniporter. Cell Calcium 2021, 93, 102322. [Google Scholar] [CrossRef]
- Kreusser, M.M.; Backs, J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 2014, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Faccenda, D.; Gorini, G.; Jones, A.; Thornton, C.; Baracca, A.; Solaini, G.; Campanella, M. The ATPase Inhibitory Factor 1 (IF1) regulates the expression of the mitochondrial Ca2+ uniporter (MCU) via the AMPK/CREB pathway. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118860. [Google Scholar] [CrossRef] [PubMed]
- O-Uchi, J.; Jhun, B.S.; Xu, S.; Hurst, S.; Raffaello, A.; Liu, X.; Yi, B.; Zhang, H.; Gross, P.; Mishra, J.; et al. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid. Redox Signal. 2014, 21, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, T.; Wang, K.; Zhao, F.; Chen, J.; Xu, G.; Zhao, J.; Li, T.; Chen, L.; Li, L.; et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat. Cell Biol. 2019, 21, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; You, C. miR-129-3p Targeting of MCU Protects Against Glucose Fluctuation-Mediated Neuronal Damage via a Mitochondrial-Dependent Intrinsic Apoptotic Pathway. Diabetes Metab. Syndr. Obes. 2021, 14, 153–163. [Google Scholar] [CrossRef]
- Zaglia, T.; Ceriotti, P.; Campo, A.; Borile, G.; Armani, A.; Carullo, P.; Prando, V.; Coppini, R.; Vida, V.; Stølen, T.O.; et al. Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA-1 in physiologic and pathologic hypertrophy. Proc. Natl. Acad. Sci. USA 2017, 114, E9006–E9015. [Google Scholar] [CrossRef]
- Sharma, Y.; Garabadu, D. Ruthenium red, mitochondrial calcium uniporter inhibitor, attenuates cognitive deficits in STZ-ICV challenged experimental animals. Brain Res. Bull. 2020, 164, 121–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Liu, T.; Yang, Y.; Guo, J.; He, Y.; Xi, J. Zn2+ protect cardiac H9c2 cells from endoplasmic reticulum stress by preventing mPTP opening through MCU. Cell. Signal. 2022, 100, 110467. [Google Scholar] [CrossRef]
- Broekemeier, K.M.; Krebsbach, R.J.; Pfeiffer, D.R. Inhibition of the mitochondrial Ca2+ uniporter by pure and impure ruthenium red. Mol. Cell Biochem. 1994, 139, 33–40. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, L.; Qin, H.; Su, H.; Zhang, J.; Wang, S.; Xu, X.; Zhao, Z.; Mao, G.; Chen, J. Inhibition of mitochondrial calcium uptake by Ru360 enhances the effect of 1800 MHz radio-frequency electromagnetic fields on DNA damage. Ecotoxicol. Environ. Saf. 2023, 264, 115472. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim. Biophys. Acta. 2008, 1777, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Wu, C.; Wang, C.; Cheng, X.; Zhang, L.; Zhang, H.; Lian, Y. Inhibition of the mitochondrial calcium uniporter inhibits Aβ-induced apoptosis by reducing reactive oxygen species-mediated endoplasmic reticulum stress in cultured microglia. Brain Res. 2017, 1676, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Shehwar, D.; Barki, S.; Aliotta, A.; Veuthey, L.; Bertaggia, C.D.; Alberio, L.; Alam, M.R. Inhibition of mitochondrial calcium transporters alters adp-induced platelet responses. Mol. Biol. Rep. 2024, 51, 177. [Google Scholar] [CrossRef]
- Kon, N.; Murakoshi, M.; Isobe, A.; Kagechika, K.; Miyoshi, N.; Nagayama, T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017, 3, 17045. [Google Scholar] [CrossRef]
- Di, M.G.; Vallese, F.; Jourde, B.; Bergsdorf, C.; Sturlese, M.; De, M.A.; Techer-Etienne, V.; Haasen, D.; Oberhauser, B.; Schleeger, S.; et al. A High-Throughput Screening Identifies MICU1 Targeting Compounds. Cell Rep. 2020, 30, 2321–2331. [Google Scholar]
- Holze, F.; Vizeli, P.; Müller, F.; Ley, L.; Duerig, R.; Varghese, N.; Eckert, A.; Borgwardt, S.; Liechti, M.E. Distinct acute effects of LSD.; MDMA.; and D-amphetamine in healthy subjects. Neuropsychopharmacology 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Costa, G.; Gołembiowska, K. Neurotoxicity of MDMA: Main effects and mechanisms. Exp. Neurol. 2022, 347, 113894. [Google Scholar] [CrossRef]
- Capela, J.P.; Carvalho, F.D. A review on the mitochondrial toxicity of “ecstasy” (3, 4-methylenedioxymethamphetamine, MDMA). Curr. Res. Toxicol. 2022, 3, 100075. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Capela, J.P.; Feio-Azevedo, R.; Teixeira-Gomes, A.; Bastos, M.; Carvalho, F. Mitochondria: Key players in the neurotoxic effects of amphetamines. Arch. Toxicol. 2015, 89, 1695–1725. [Google Scholar] [CrossRef]
- Jiang, X.R.; Dryhurst, G. Inhibition of the alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes by a putative aberrant metabolite of serotonin, tryptamine-4,5-dione. Chem. Res. Toxicol. 2002, 15, 1242–1247. [Google Scholar] [CrossRef]
- Natarajaseenivasan, K.; Cotto, B.; Shanmughapriya, S.; Lombardi, A.A.; Datta, P.K.; Madesh, M.; Elrod, J.W.; Khalili, K.; Langford, D. Astrocytic metabolic switch is a novel etiology for Cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 2018, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.; Ouyang, J.; Parakhia, R.A.; Pitake, S.; Ochs, R.S. Ca2+ effects on glucose transport and fatty acid oxidation in L6 skeletal muscle cell cultures. Biochem. Biophys. Rep. 2016, 5, 365–373. [Google Scholar] [PubMed]
- Mao, J.; Sung, B.; Ji, R.R.; Lim, G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J. Neurosci. 2002, 22, 7650–7661. [Google Scholar] [CrossRef]
- Ko, S.W.; Jia, Y.; Xu, H.; Yim, S.J.; Jang, D.H.; Lee, Y.S.; Zhao, M.G.; Toyoda, H.; Wu, L.J.; Chatila, T.; et al. Evidence for a role of CaMKIV in the development of opioid analgesic tolerance. Eur. J. Neurosci. 2006, 23, 2158–2168. [Google Scholar] [CrossRef]
- Ivshina, M.; Lasko, P.; Richter, J.D. Cytoplasmic polyadenylation element binding proteins in development.; health.; and disease. Annu. Rev. Cell Dev. Biol. 2014, 30, 393–415. [Google Scholar] [CrossRef]
- Takahashi, K.; Yi, H.; Gu, J.; Ikegami, D.; Liu, S.; Iida, T.; Kashiwagi, Y.; Dong, C.; Kunisawa, T.; Hao, S. The mitochondrial calcium uniporter contributes to morphine tolerance through pCREB and CPEB1 in rat spinal cord dorsal horn. Br. J. Anaesth. 2019, 123, e226–e238. [Google Scholar] [CrossRef]
- Chiu, P.; Karler, R.; Craven, C.; Olsen, D.M.; Turkanis, S.A. The influence of delta9-tetrahydrocannabinol.; cannabinol and cannabidiol on tissue oxygen consumption. Res. Commun. Chem. Pathol. Pharmacol. 1975, 12, 267–286. [Google Scholar]
- Hempel, B.; Xi, Z.X. Receptor mechanisms underlying the CNS effects of cannabinoids: CB1 receptor and beyond. Adv. Pharmacol. 2022, 93, 275–333. [Google Scholar]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef]
- Serrat, R.; Covelo, A.; Kouskoff, V.; Delcasso, S.; Ruiz-Calvo, A.; Chenouard, N.; Stella, C.; Blancard, C.; Salin, B.; Julio-Kalajzić, F.; et al. Astroglial ER-mitochondria calcium transfer mediates endocannabinoid-dependent synaptic integration. Cell Rep. 2021, 37, 110133. [Google Scholar] [CrossRef]
- Soria-Gomez, E.; Zottola, A.C.P.; Mariani, Y.; Desprez, T.; Barresi, M.; Bonilla-Del Río, I.; Muguruza, C.; Le Bon-Jego, M.; Julio-Kalajzić, F.; Flynn, R.; et al. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron 2021, 109, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Corricelli, M.; Branchini, A.; Missiroli, S.; Morciano, G.; Perrone, M.; Ferrarese, M.; Giorgi, C.; Pinotti, M.; Galluzzi, L.; et al. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca2+ levels and tumor growth. EMBO J. 2019, 38, e99435. [Google Scholar] [CrossRef] [PubMed]
- Flavin, A.; Azizi, P.; Murataeva, N.; Yust, K.; Du, W.; Ross, R.; Greig, I.; Nguyen, T.; Zhang, Y.; Mackie, K.; et al. CB1 Receptor Negative Allosteric Modulators as a Potential Tool to Reverse Cannabinoid Toxicity. Molecules 2024, 29, 1881. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Cadet, J.L. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009, 60, 379–407. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, R.; Yang, G.; Peng, Y.; Nie, Q.; Yu, H.; Dong, W.; Chen, B.; Song, C.; Tian, Y.; et al. Cannabidiol prevents methamphetamine-induced neurotoxicity by modulating dopamine receptor D1-mediated calcium-dependent phosphorylation of methyl-CpG-binding protein 2. Front. Pharmacol. 2022, 13, 972828. [Google Scholar] [CrossRef]
- Bustamante, J.; Acosta, L.; Karadayian, A.G.; Lores-Arnaiz, S. Ketamine induced cell death can be mediated by voltage dependent calcium channels in PC12 cells. Exp. Mol. Pathol. 2019, 111, 104318. [Google Scholar] [CrossRef]
- Czerniczyniec, A.; Karadayian, A.G.; Bustamante, J.; Lores-Arnaiz, S. Ketamine treatment affects hippocampal but not cortical mitochondrial function in prepubertal rats. Int. J. Dev. Neurosci. 2020, 80, 175–187. [Google Scholar] [CrossRef]
- Han, W.M.; Hao, X.B.; Hong, Y.X.; Zhao, S.S.; Chen, X.C.; Wang, R.; Wang, Y.; Li, G. NMDARs antagonist MK801 suppresses LPS-induced apoptosis and mitochondrial dysfunction by regulating subunits of NMDARs via the CaM/CaMKII/ERK pathway. Cell Death Discov. 2023, 9, 59. [Google Scholar] [CrossRef]
- Haynes, V.; Giulivi, C. Calcium-Dependent Interaction of Nitric Oxide Synthase with Cytochrome c Oxidase: Implications for Brain Bioenergetics. Brain Sci. 2023, 13, 1534. [Google Scholar] [CrossRef]
- Li, Y.; Ding, R.; Ren, X.; Wen, G.; Dong, Z.; Yao, H.; Tan, Y.; Yu, H.; Wang, X.; Zhan, X.; et al. Long-term ketamine administration causes Tau protein phosphorylation and Tau protein-dependent AMPA receptor reduction in the hippocampus of mice. Toxicol. Lett. 2019, 315, 107–111. [Google Scholar] [CrossRef]


| Drugs | MCU-Related Mechanisms | Final Effects |
|---|---|---|
| MDMA | Inhibits mitochondrial complexes I/III → ROS↑, Ca2+ overload → ATP depletion → apoptosis. Metabolite T-4,5-D inhibits PDH/α-KGDH and inactivates complexes I/IV. | Mitochondrial Ca2+ overload → apoptosis |
| Cocaine | Astrocyte acetyl-CoA↑ → GPCR activation → ↑MCU-mediated mitochondrial Ca2+ uptake → mROS↑→ neurodegeneration. MCU knockdown activates AMPK-dependent glycolysis. | Metabolic imbalance → neurodegeneration |
| Morphine | Epigenetic MCU upregulation Via pCREB/CPEB1 → mitochondrial Ca2+ overload → tolerance-related neurotoxicity. CPEB1 stabilizes polyadenylation complexes. | Morphine tolerance → neuronal damage |
| Cannabis | CB1 activation → AKT-mediated MICU1 phosphorylation → ↑MCU-driven Ca2+ influx → mitochondrial depolarization → mPTP opening → apoptosis. | Mitochondrial Ca2+ overload → apoptosis |
| METH | MCU overactivation → mitochondrial Ca2+ overload → Bax activation → mPTP opening → Cyt c/AIF release → caspase-dependent/independent apoptosis. | Mitochondrial stress → caspase-dependent/independent apoptosis |
| Ketamine | ROS↑ Via CaMKII/CaM/ERK → MCU activation → mitochondrial Ca2+ overload → Cyt c release → apoptosis. NMDAR NR1 subunit upregulation → glutamate excitotoxicity → Tau hyperphosphorylation. | Oxidative phosphorylation uncoupling → apoptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, Y.; Zheng, G.; Nie, Q.; Zhang, P. Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 4732. https://doi.org/10.3390/ijms26104732
Yang X, Chen Y, Zheng G, Nie Q, Zhang P. Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(10):4732. https://doi.org/10.3390/ijms26104732
Chicago/Turabian StyleYang, Xinyan, Yinyu Chen, Gaolin Zheng, Qianyun Nie, and Peng Zhang. 2025. "Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets" International Journal of Molecular Sciences 26, no. 10: 4732. https://doi.org/10.3390/ijms26104732
APA StyleYang, X., Chen, Y., Zheng, G., Nie, Q., & Zhang, P. (2025). Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets. International Journal of Molecular Sciences, 26(10), 4732. https://doi.org/10.3390/ijms26104732







