Biosynthesized Calcium Peroxide Nanoparticles as a Multifunctional Platform for Liver Cancer Therapy
Abstract
1. Introduction
2. Results
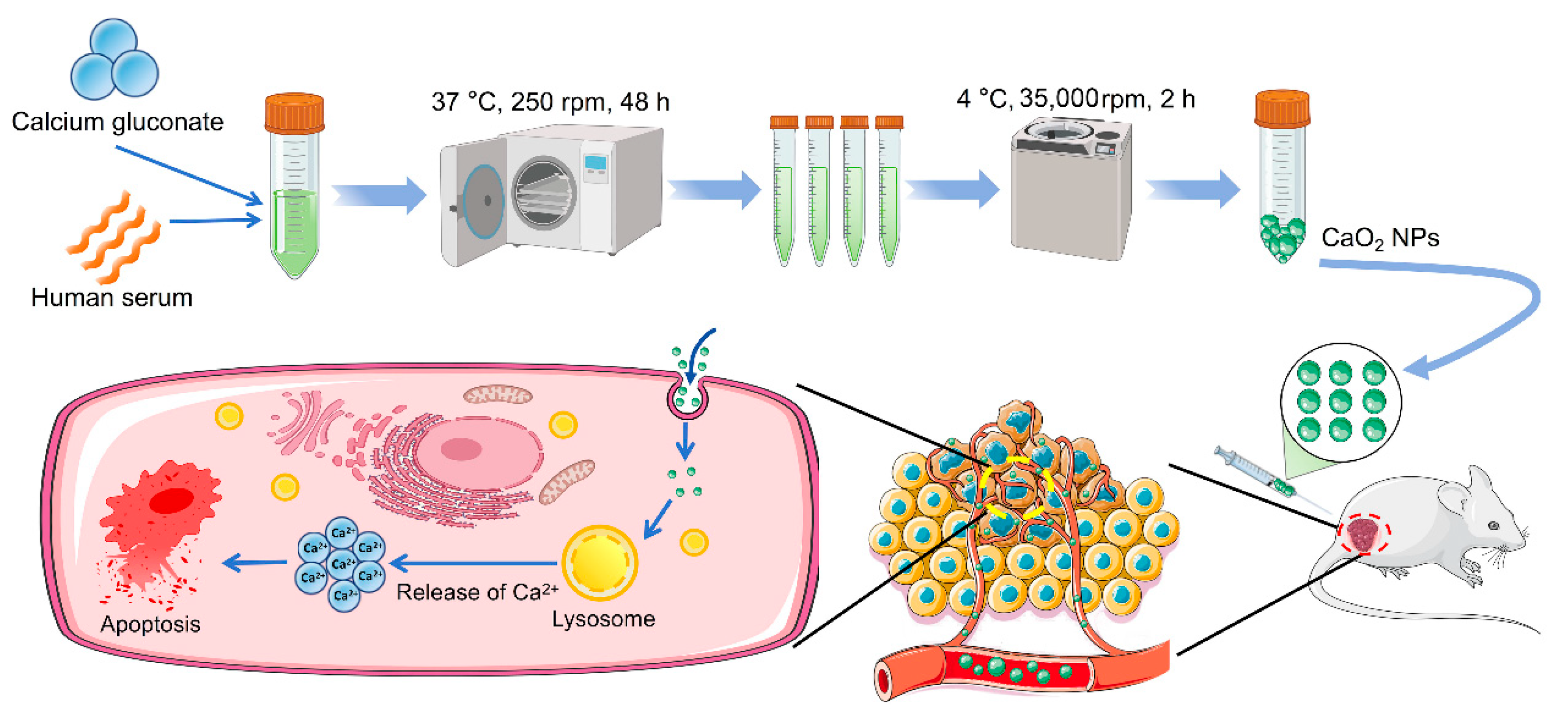
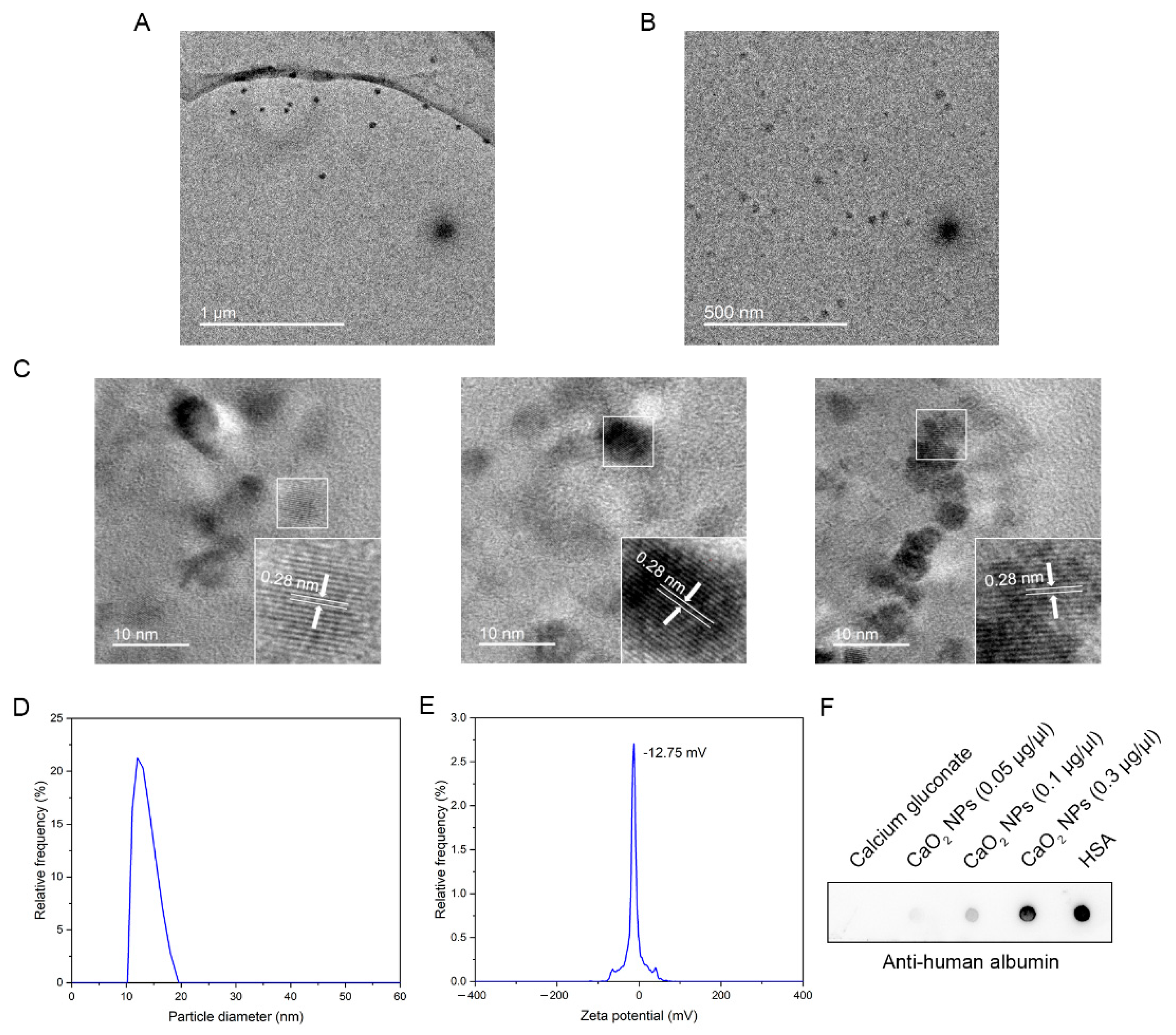
2.1. Biosynthesis and Characterization of CaO2 NPs
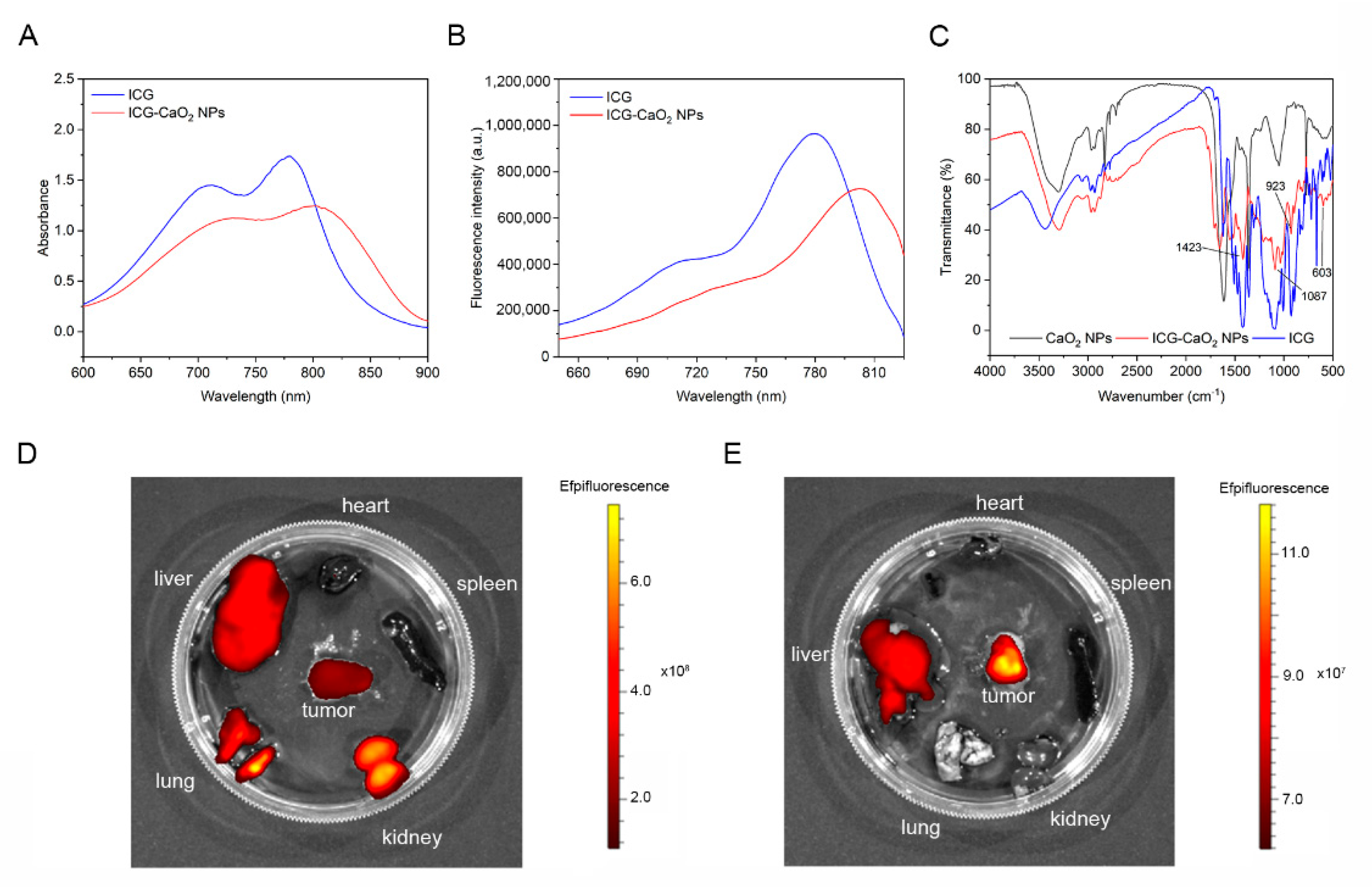
2.2. CaO2 NPs Exhibit Prolonged Retention at the Tumor Site
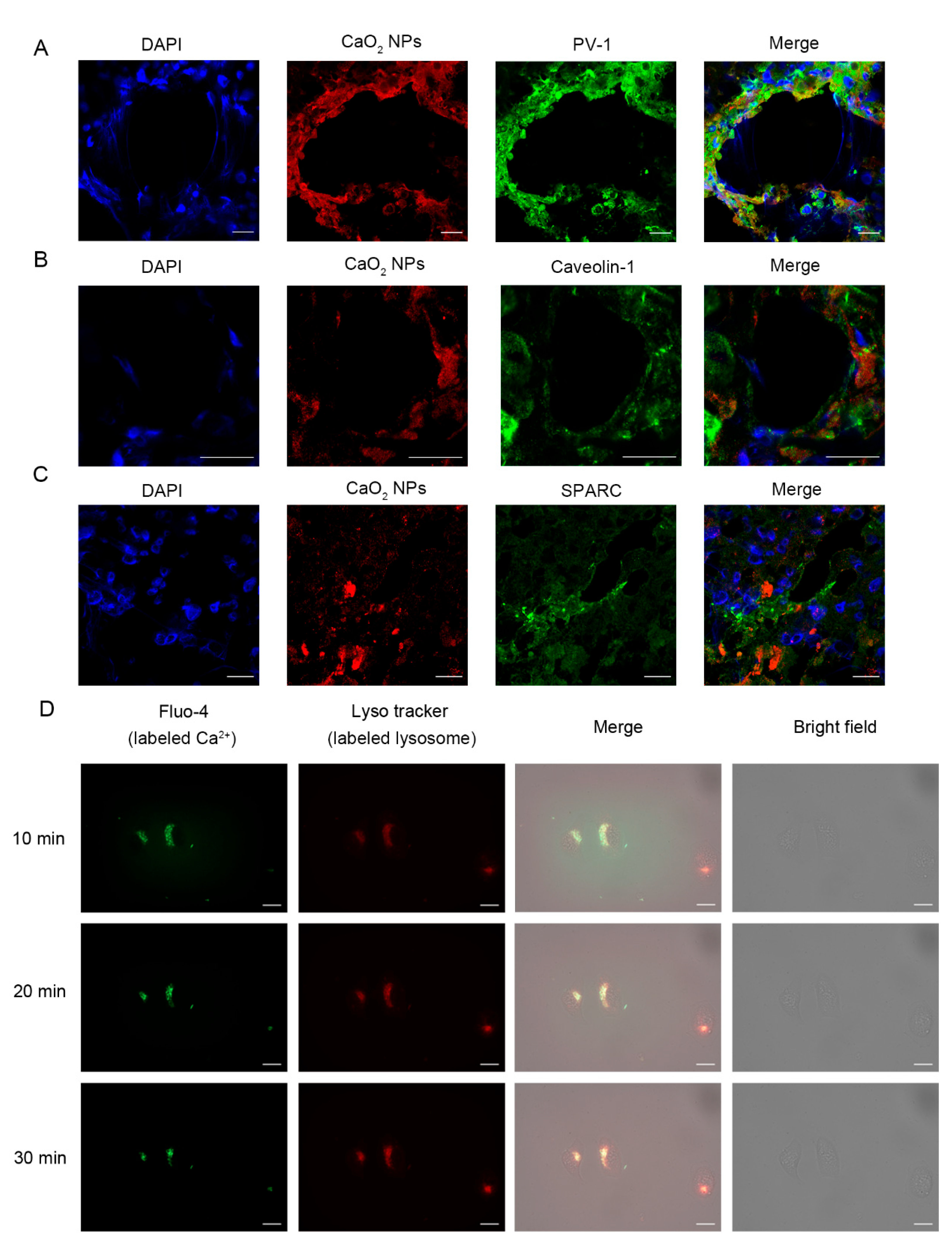
2.3. CaO2 NPs Enter Tumor Tissues Through Active Pathways
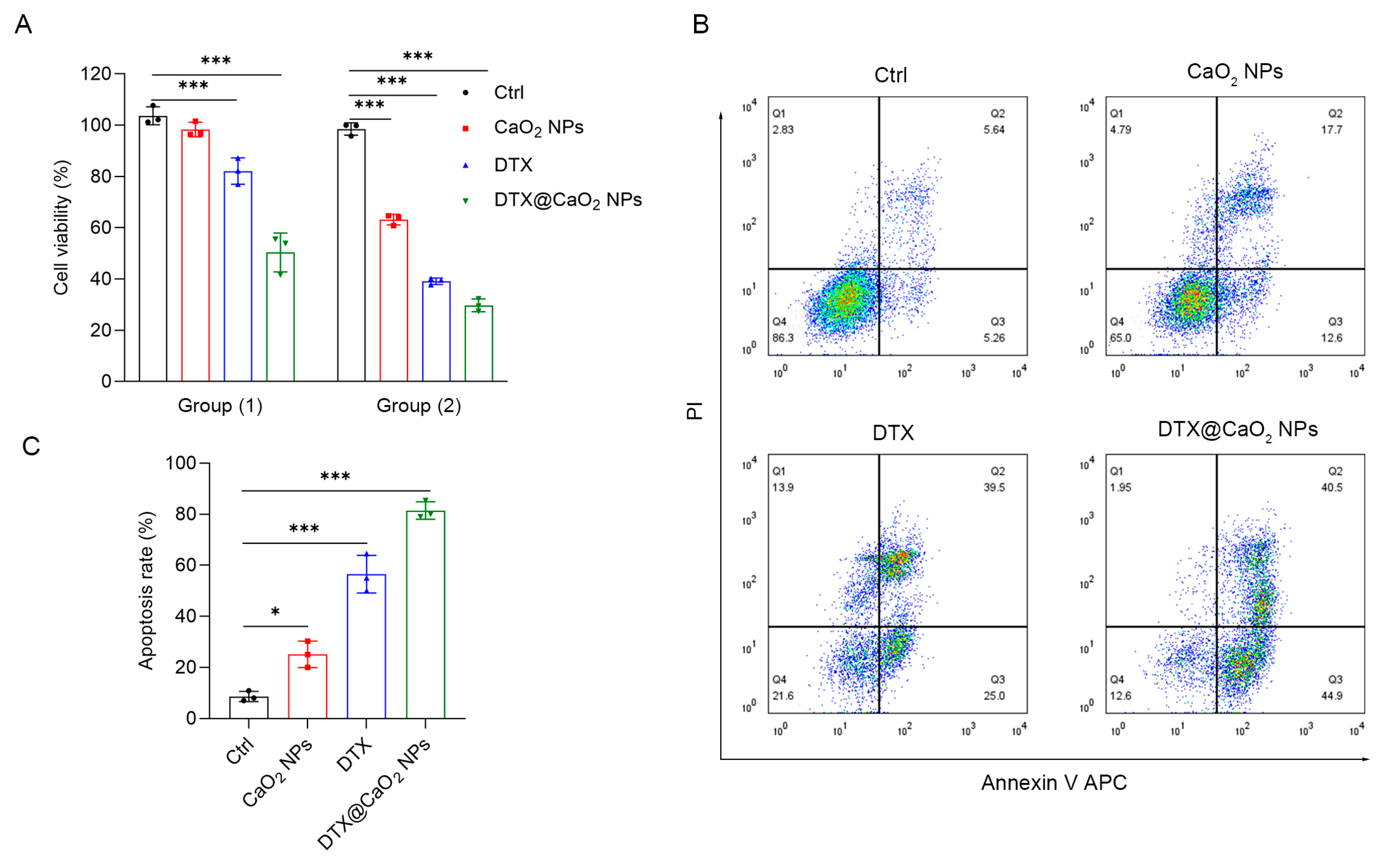
2.4. The Synergistic Cytotoxic Effect of CaO2 NPs as Nanocarriers in Liver Cancer Cells
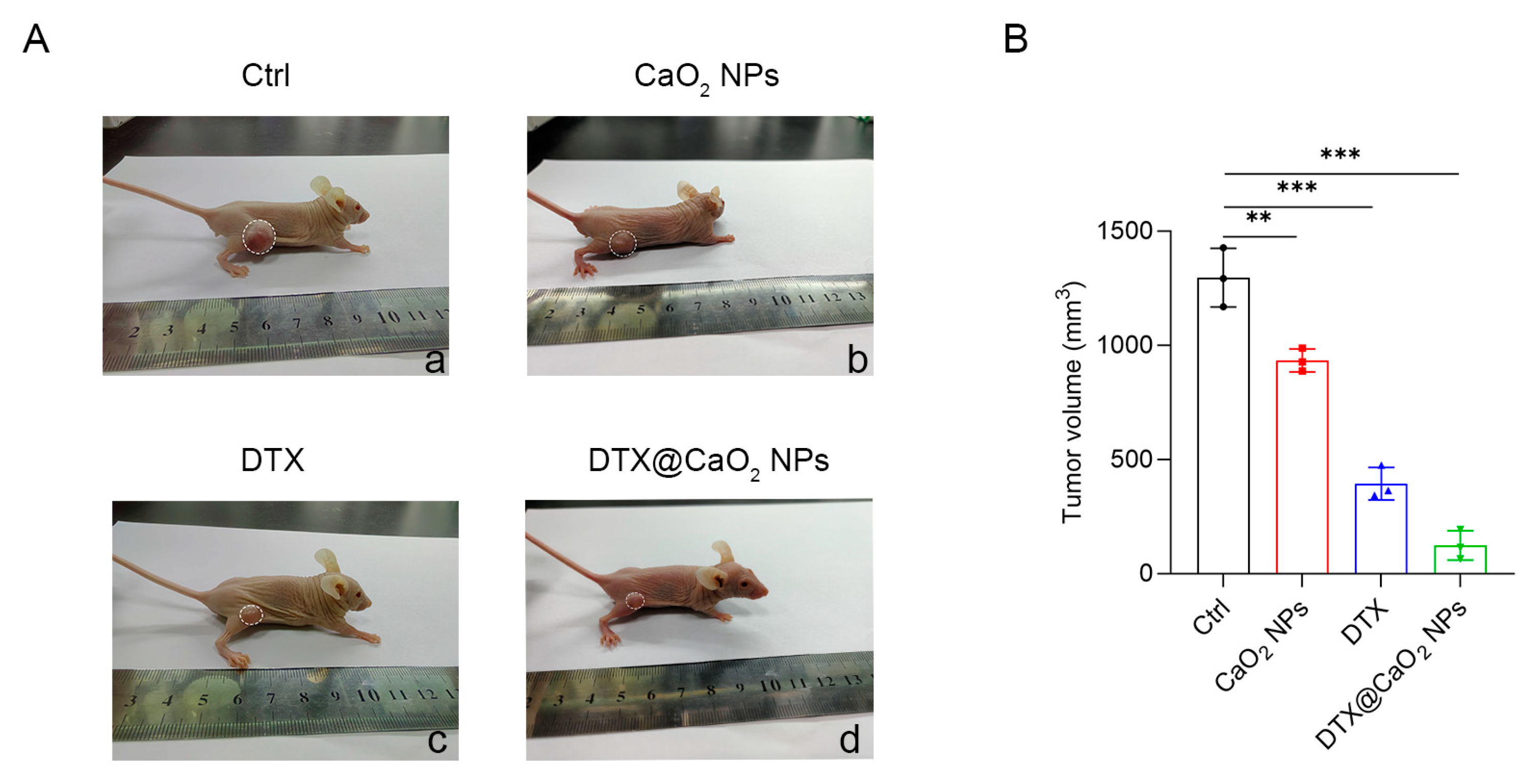
2.5. The Synergistic Therapeutic Effect of CaO2 NPs as Nanocarriers In Vivo
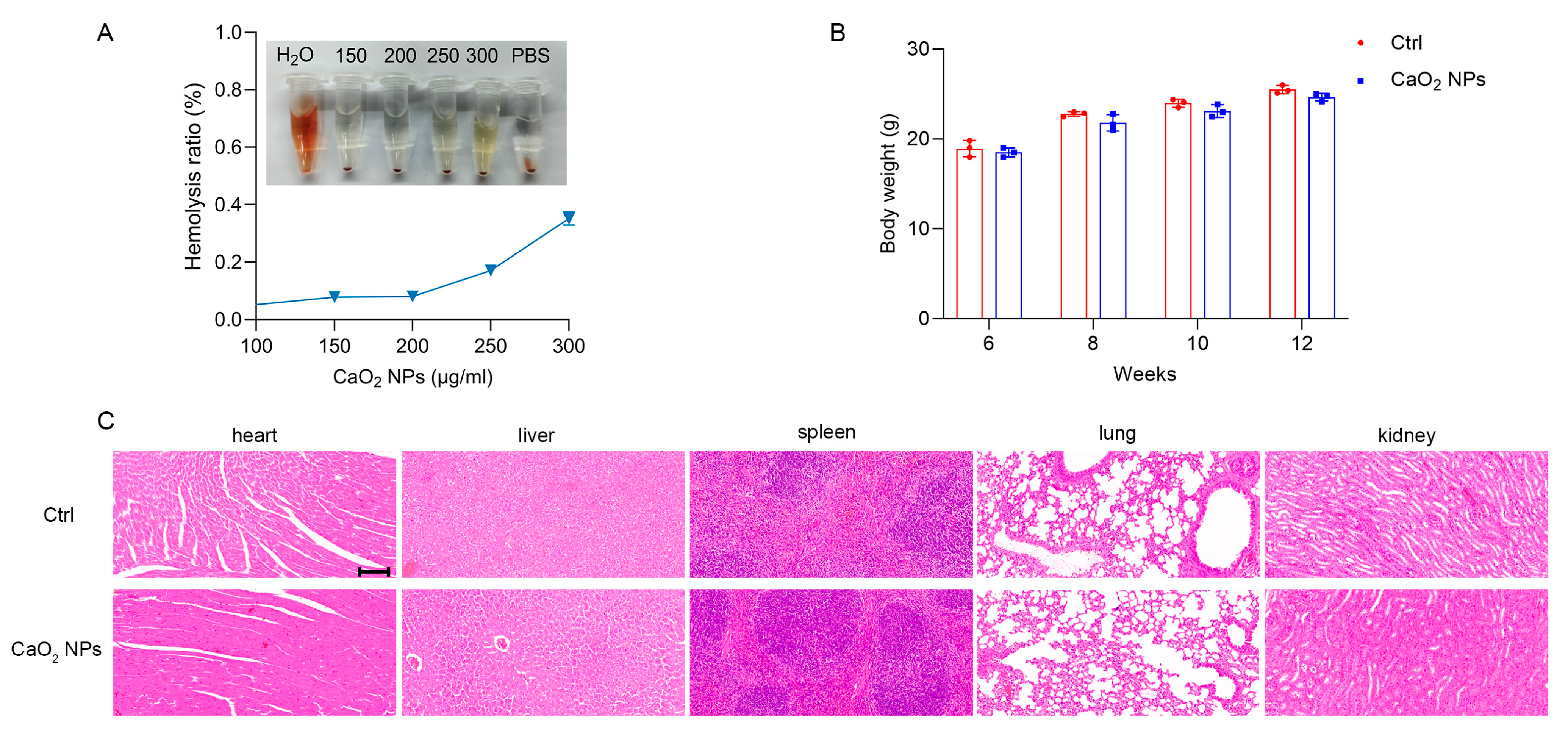
2.6. Biocompatibility and Safety of CaO2 NPs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Biosynthesis of CaO2 NPs
4.4. Preparation of DTX-Loaded CaO2 NPs
4.5. Characterization of CaO2 NPs
4.6. Dot Blot Assay
4.7. Live-Cell Imaging
4.8. Cytotoxicity Assay
4.9. Flow Cytometry
4.10. CaO2 NPs Crosslink ICG
4.11. Investigation of Targeting Ability of CaO2 NPs
4.12. Immunofluorescence Staining
4.13. In Vivo Antitumor Activity Study
4.14. Hemolysis Assay
4.15. Biosafety Assessment of CaO2 NPs
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021, 19, 311. [Google Scholar] [CrossRef]
- Suliman, I.H.; Kim, K.; Chen, W.; Kim, Y.; Moon, J.H.; Son, S.; Nam, J. Metal-Based Nanoparticles for Cancer Metalloimmunotherapy. Pharmaceutics 2023, 15, 2003. [Google Scholar] [CrossRef] [PubMed]
- Chandoliya, R.; Sharma, S.; Sharma, V.; Joshi, R.; Sivanesan, I. Titanium Dioxide Nanoparticle: A Comprehensive Review on Synthesis, Applications and Toxicity. Plants 2024, 13, 2964. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Wartena, R.; Yoo, P.J.; Liau, F.W.; Lee, Y.J.; Chiang, Y.M.; Hammond, P.T.; Belcher, A.M. Stamped microbattery electrodes based on self-assembled M13 viruses. Proc. Natl. Acad. Sci. USA 2008, 105, 17227–17231. [Google Scholar] [CrossRef]
- Stürzenbaum, S.R.; Höckner, M.; Panneerselvam, A.; Levitt, J.; Bouillard, J.S.; Taniguchi, S.; Dailey, L.A.; Ahmad Khanbeigi, R.; Rosca, E.V.; Thanou, M.; et al. Biosynthesis of luminescent quantum dots in an earthworm. Nat. Nanotechnol. 2013, 8, 57–60. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Sun, J.; Yang, H.; Zhao, X.; He, D.; Pu, M.; Zhang, G.; He, N.; Zeng, X. Biosynthetic Mechanism of Luminescent ZnO Nanocrystals in the Mammalian Blood Circulation and Their Functionalization for Tumor Therapy. ACS Appl. Mater. Interfaces 2018, 10, 105–113. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Li, S.; Shi, J.; Gao, H.; Sun Leong, W.; Wu, Y.; Li, M.; Liu, C.; Li, P.; et al. Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent. Nat. Commun. 2020, 11, 567. [Google Scholar] [CrossRef]
- O’Grady, S.; Morgan, M.P. Calcium transport and signalling in breast cancer: Functional and prognostic significance. Semin. Cancer Biol. 2021, 72, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, L.; Ma, C.; Zhang, S.; Lin, S.; Zhang, L.W.; Wang, Y.; Gao, M. Chemotherapy-Sensitized In Situ Vaccination for Malignant Osteosarcoma Enabled by Bioinspired Calcium Phosphonate Nanoagents. ACS Nano 2023, 17, 6247–6260. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Ren, Y.; Yuan, Q.; Zhang, Z.; Li, L.; Bao, B.; Jia, W.; Zhang, X.; Li, M.; et al. Calcium-Mediated Cell Adhesion Enhancement-Based Antimetastasis and Synergistic Antitumor Therapy by Conjugated Polymer-Calcium Composite Nanoparticles. ACS Nano 2024, 18, 24953–24967. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Leo, S.; Rimessi, A.; Wieckowski, M.R.; Fiorica, F.; Giorgi, C.; Pinton, P. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119061. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yuan, L.; Zhang, Y.; Kuang, J.; Song, W.; Lou, X.; Xia, F.; Yoon, J. Photo-Controlled Calcium Overload from Endogenous Sources for Tumor Therapy. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317578. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, J.; Xiao, Y.; Zhang, Z.; Yang, X.; Liang, X.; Yang, J.; Zhou, X.; Li, C. Advances in nanotherapeutics for tumor treatment by targeting calcium overload. Colloids Surf. B Biointerfaces 2025, 245, 114190. [Google Scholar] [CrossRef]
- Che, Y.; Chen, G.; Guo, Q.; Duan, Y.; Feng, H.; Xia, Q. Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology 2023, 78, 88–102. [Google Scholar] [CrossRef]
- Guo, D.; Dai, X.; Liu, K.; Liu, Y.; Wu, J.; Wang, K.; Jiang, S.; Sun, F.; Wang, L.; Guo, B.; et al. A Self-Reinforcing Nanoplatform for Highly Effective Synergistic Targeted Combinatary Calcium-Overload and Photodynamic Therapy of Cancer. Adv. Healthc. Mater. 2023, 12, e2202424. [Google Scholar] [CrossRef]
- Liu, H.; Ai, R.; Liu, B.; Cao, X.; He, L. Tumor-Targeting CaO2-Based Nanoparticles for Cancer Therapy. ACS Appl. Nano Mater. 2024, 7, 26276–26286. [Google Scholar] [CrossRef]
- Cai, T.; Zheng, W.; Chang, Q.; Li, N.; Yang, J.; Hu, S. Carbon dot-boosted catalytic activity of CaO2 by tuning visible light conversion. J. Mater. Chem. A 2022, 10, 7792–7799. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Z.; Zhao, A.; Wu, Y.; Wang, Z.; Wu, A.; Wang, Q.; Xia, X.; Chen, X.; Zhao, W.; et al. Zinc nanoparticles from oral supplements accumulate in renal tumours and stimulate antitumour immune responses. Nat. Mater. 2025, 24, 287–296. [Google Scholar] [CrossRef]
- Moe, S.M. Rationale to reduce calcium intake in adult patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2018, 27, 251–257. [Google Scholar] [CrossRef]
- Borkowska, M.; Siek, M.; Kolygina, D.V.; Sobolev, Y.I.; Lach, S.; Kumar, S.; Cho, Y.K.; Kandere-Grzybowska, K.; Grzybowski, B.A. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 2020, 15, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yang, J.; Peng, A.; Qian, Y.; Liu, Y.; Pan, P.; Liu, Q. Lysosome Targeted Nanoparticle Aggregation Reverses Immunosuppressive Tumor Microenvironment for Cancer Immunotherapy. Adv. Mater. 2024, 36, e2412730. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Wu, W.; Feng, G.; Wang, Z.; Liu, B.; Tang, B.Z. Size Optimization of Organic Nanoparticles with Aggregation-Induced Emission Characteristics for Improved ROS Generation and Photodynamic Cancer Cell Ablation. Small 2022, 18, e2202242. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Ascensão, L.; Viana, A.S.; Carvalho, L.; Catarino, J.; Faísca, P.; Oliva, A.; de Barros, D.P.C.; Rodrigues, C.M.P.; et al. Safety of Gold Nanoparticles: From In Vitro to In Vivo Testing Array Checklist. Pharmaceutics 2023, 15, 1120. [Google Scholar] [CrossRef]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, X.; Xu, Y.; Zhu, L.; Cheng, J.; Qiao, Y.; Dai, S.; Zhu, J.; Jiang, N.; Wu, H.; et al. The influence of surface charge on the tumor-targeting behavior of Fe3O4 nanoparticles for MRI. J. Mater. Chem. B 2022, 10, 646–655. [Google Scholar] [CrossRef]
- Mulenos, M.R.; Lujan, H.; Pitts, L.R.; Sayes, C.M. Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials 2020, 10, 1953. [Google Scholar] [CrossRef]
- Dong, X.; Zang, C.; Sun, Y.; Zhang, S.; Liu, C.; Qian, J. Hydroxyapatite nanoparticles induced calcium overload-initiated cancer cell-specific apoptosis through inhibition of PMCA and activation of calpain. J. Mater. Chem. B 2023, 11, 7609–7622. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Dai, X.; Xu, T.; Qian, X.; Chang, M.; Chen, Y. 2D Catalytic Nanozyme Enables Cascade Enzyodynamic Effect-Boosted and Ca(2+) Overload-Induced Synergistic Ferroptosis/Apoptosis in Tumor. Adv. Mater. 2024, 36, e2312316. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chen, M.; Zeng, M. Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol. Environ. Saf. 2021, 208, 111391. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Pena-Ramos, O.; Li, Z.; Chiao, L.; Zhou, Z. Calcium ions trigger the exposure of phosphatidylserine on the surface of necrotic cells. PLoS Genet. 2021, 17, e1009066. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bi, Z.; Hu, Y.; Sun, L.; Song, Y.; Chen, S.; Mo, F.; Yang, J.; Wei, Y.; Wei, X. Silver nanoparticles and silver ions cause inflammatory response through induction of cell necrosis and the release of mitochondria in vivo and in vitro. Cell Biol. Toxicol. 2021, 37, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Artico, L.L.; Arruda, A.P. CaMKIIβ Ca(2+)ptures ER signals to initiate autophagosome biogenesis. Mol. Cell 2025, 85, 464–465. [Google Scholar] [CrossRef]
- Brunello, L. Autophagosomes need calcium. Nat. Struct. Mol. Biol. 2022, 29, 1037. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Wang, Y.; Lei, Y.; Huang, T.; Zhang, Y.; Lam, S.M.; Li, H.; Qi, S.; Geng, J.; et al. The ER calcium channel Csg2 integrates sphingolipid metabolism with autophagy. Nat. Commun. 2023, 14, 3725. [Google Scholar] [CrossRef]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Li, C.; Hou, Y.; He, M.; Lv, L.; Zhang, Y.; Sun, S.; Zhao, Y.; Liu, X.; Ma, P.; Wang, X.; et al. Laponite Lights Calcium Flickers by Reprogramming Lysosomes to Steer DC Migration for An Effective Antiviral CD8(+) T-Cell Response. Adv. Sci. 2023, 10, e2303006. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Abrahamian, C.; Grimm, C. Endolysosomal Cation Channels and MITF in Melanocytes and Melanoma. Biomolecules 2021, 11, 1021. [Google Scholar] [CrossRef]
- Siow, W.X.; Kabiri, Y.; Tang, R.; Chao, Y.K.; Plesch, E.; Eberhagen, C.; Flenkenthaler, F.; Fröhlich, T.; Bracher, F.; Grimm, C.; et al. Lysosomal TRPML1 regulates mitochondrial function in hepatocellular carcinoma cells. J. Cell Sci. 2022, 135, jcs259455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Li, S.; Xia, X.; Zhang, G.; Wang, T. Biosynthesized Calcium Peroxide Nanoparticles as a Multifunctional Platform for Liver Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 4696. https://doi.org/10.3390/ijms26104696
Wu S, Li S, Xia X, Zhang G, Wang T. Biosynthesized Calcium Peroxide Nanoparticles as a Multifunctional Platform for Liver Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(10):4696. https://doi.org/10.3390/ijms26104696
Chicago/Turabian StyleWu, Sen, Siqi Li, Xin Xia, Gen Zhang, and Ting Wang. 2025. "Biosynthesized Calcium Peroxide Nanoparticles as a Multifunctional Platform for Liver Cancer Therapy" International Journal of Molecular Sciences 26, no. 10: 4696. https://doi.org/10.3390/ijms26104696
APA StyleWu, S., Li, S., Xia, X., Zhang, G., & Wang, T. (2025). Biosynthesized Calcium Peroxide Nanoparticles as a Multifunctional Platform for Liver Cancer Therapy. International Journal of Molecular Sciences, 26(10), 4696. https://doi.org/10.3390/ijms26104696





