GPR55 Antagonist CID16020046 Suppresses Collagen-Induced Rheumatoid Arthritis by Suppressing Th1/Th17 Cells in Mice
Abstract
:1. Introduction
2. Results
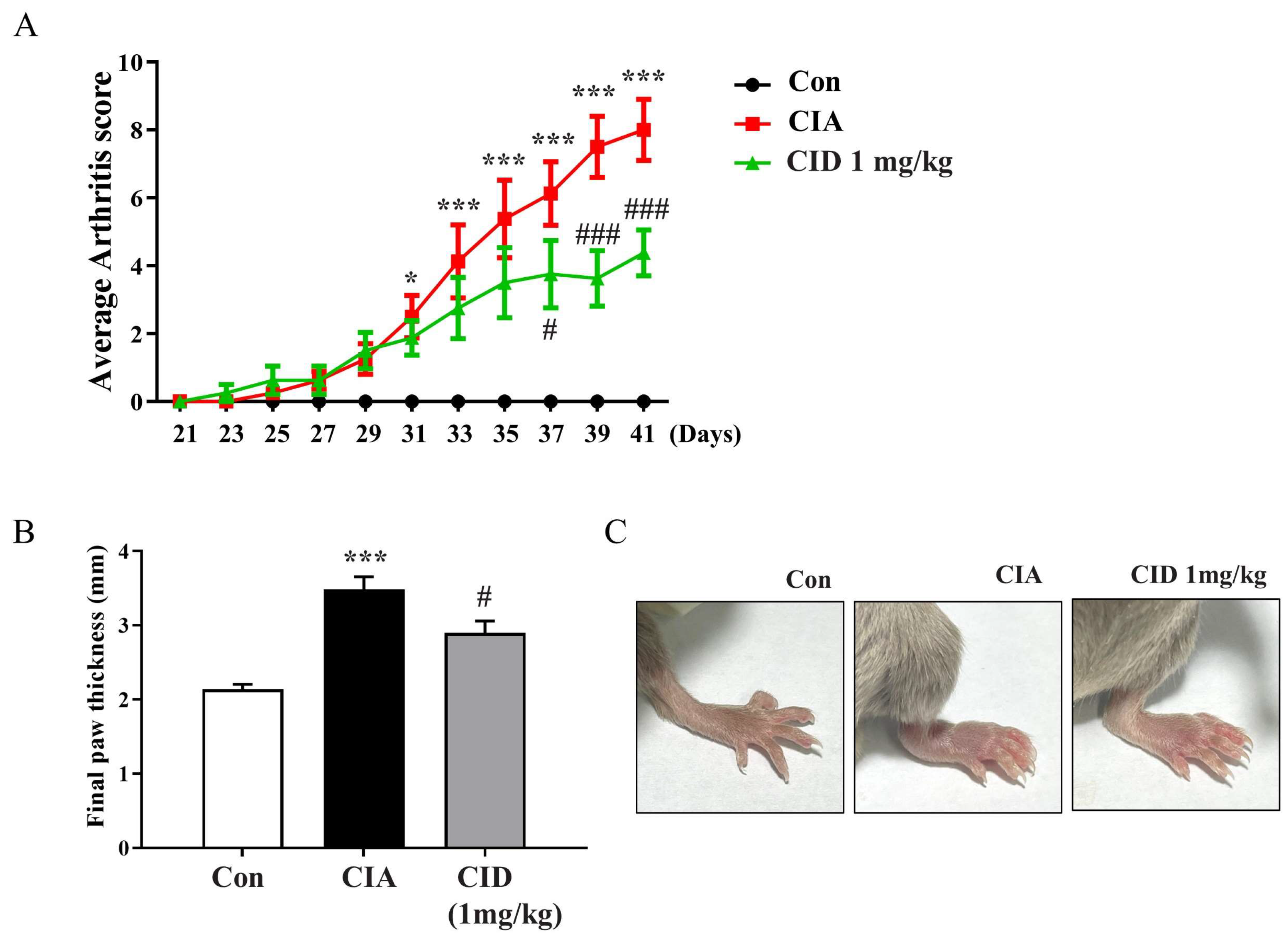
2.1. CID16020046 Treatment Inhibited the Progression of Arthritis and the Thickening of the Feet in DBA-1J Mice
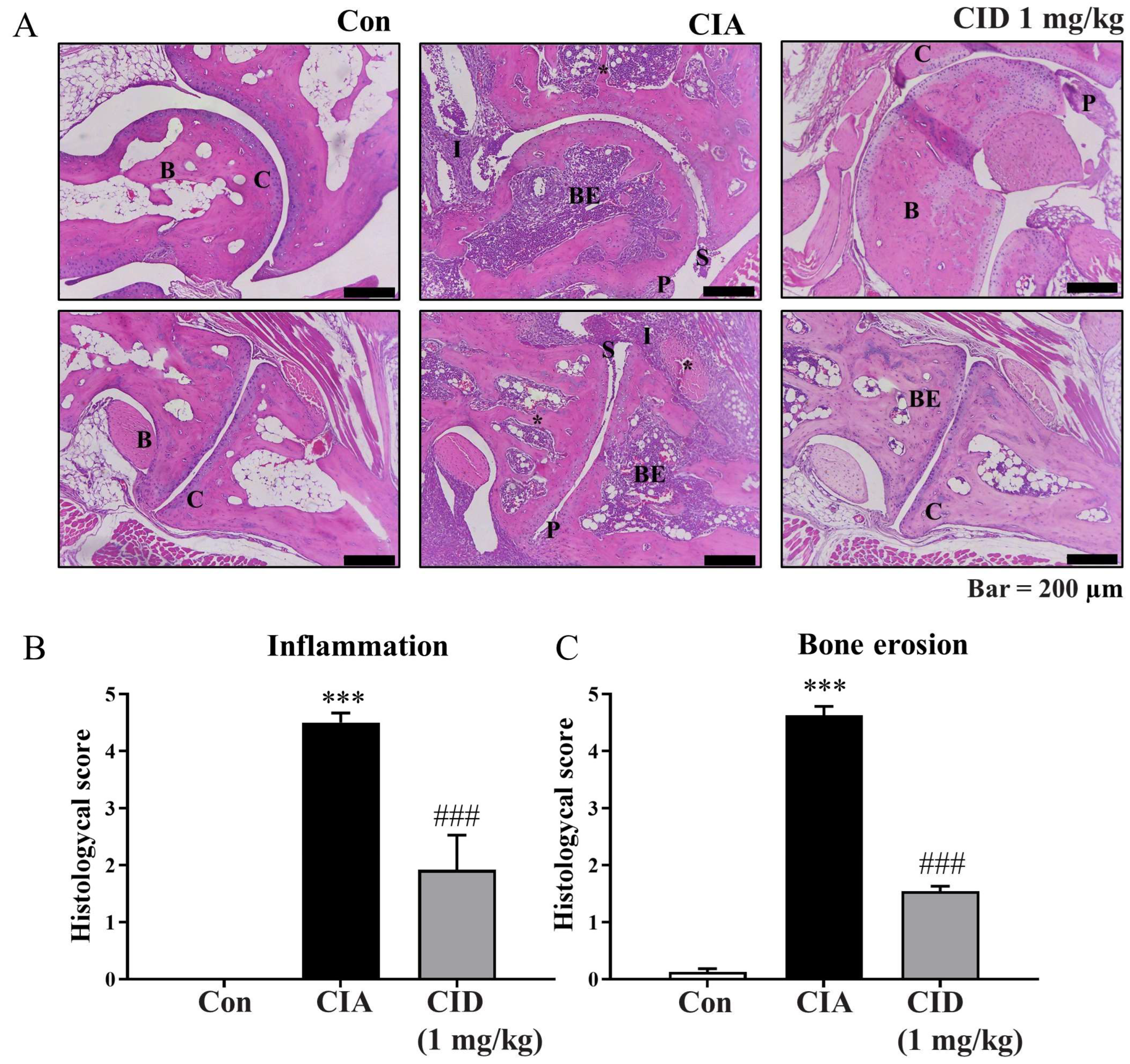
2.2. CID16020046 Treatment Reduced the Histological Alterations Associated with Rheumatoid Arthritis in DBA-1J Mice
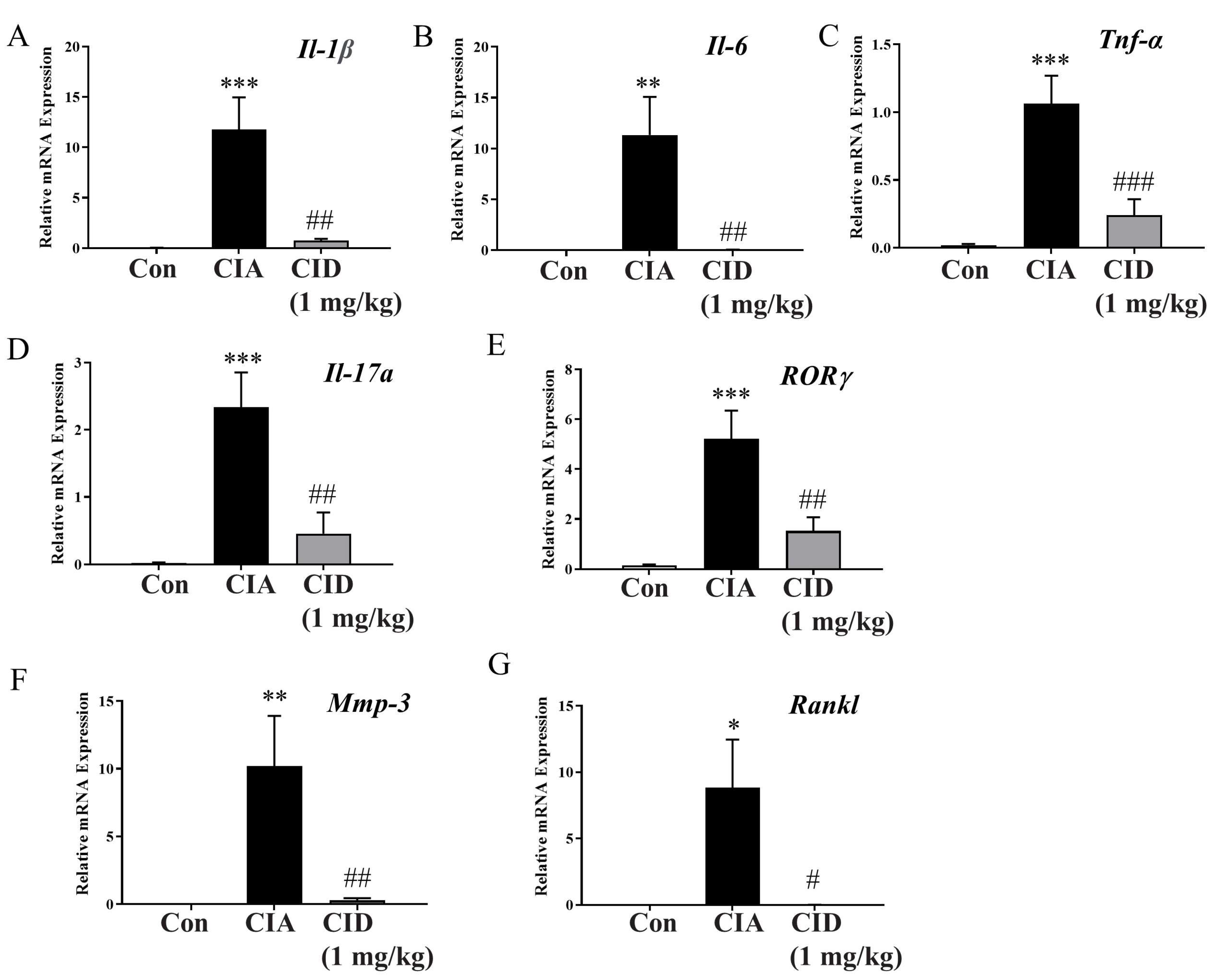
2.3. CID16020046 Treatment Reduced mRNA Expression Levels of Pro-Inflammatory Cytokines in Foot Tissues in DBA-1J Mice
2.4. CID16020046 Treatment Suppressed Serum IgG Levels in DBA-1J Mice
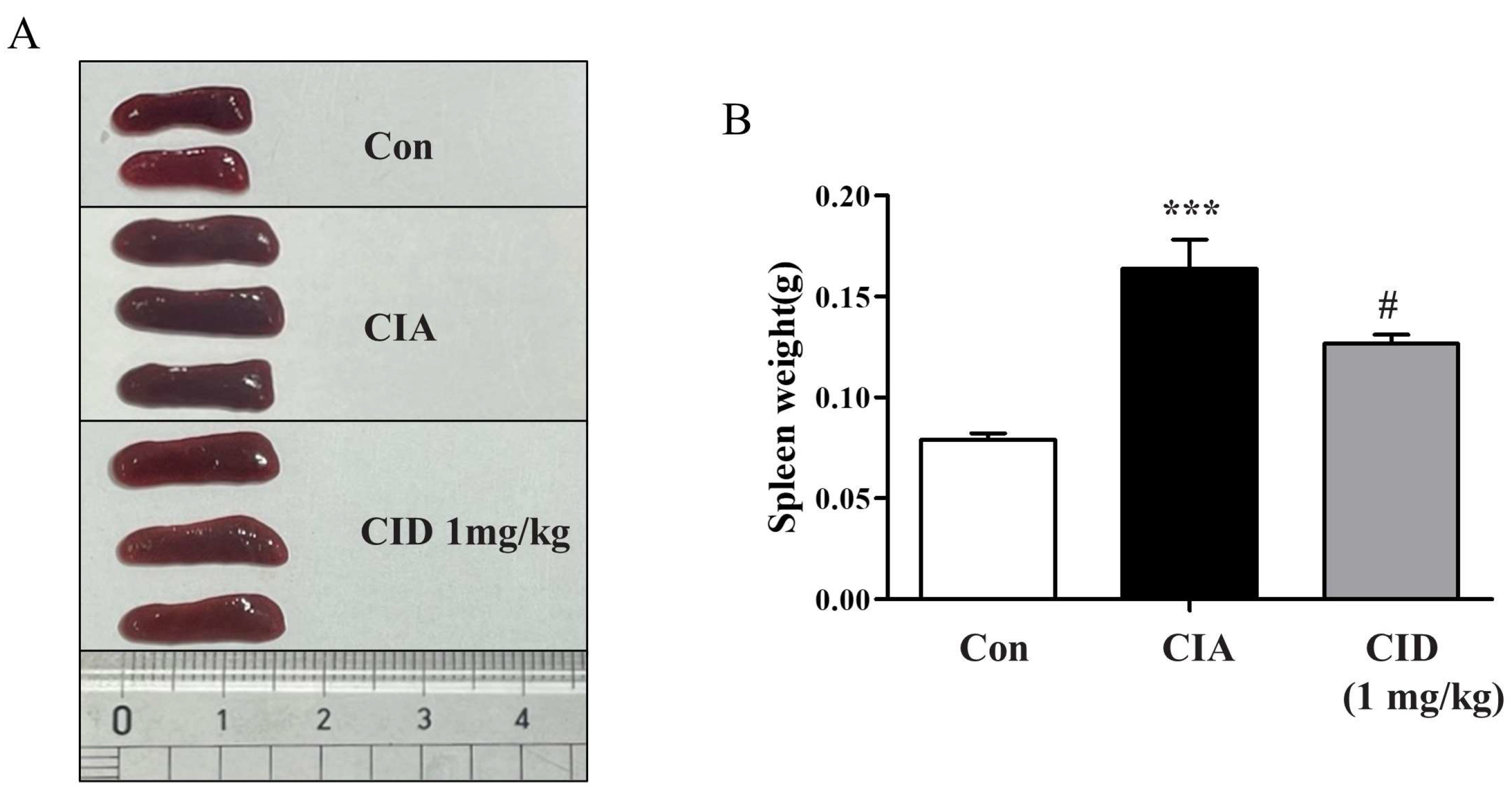
2.5. CID16020046 Treatment Reduced Spleen Enlargement in DBA-1J Mice
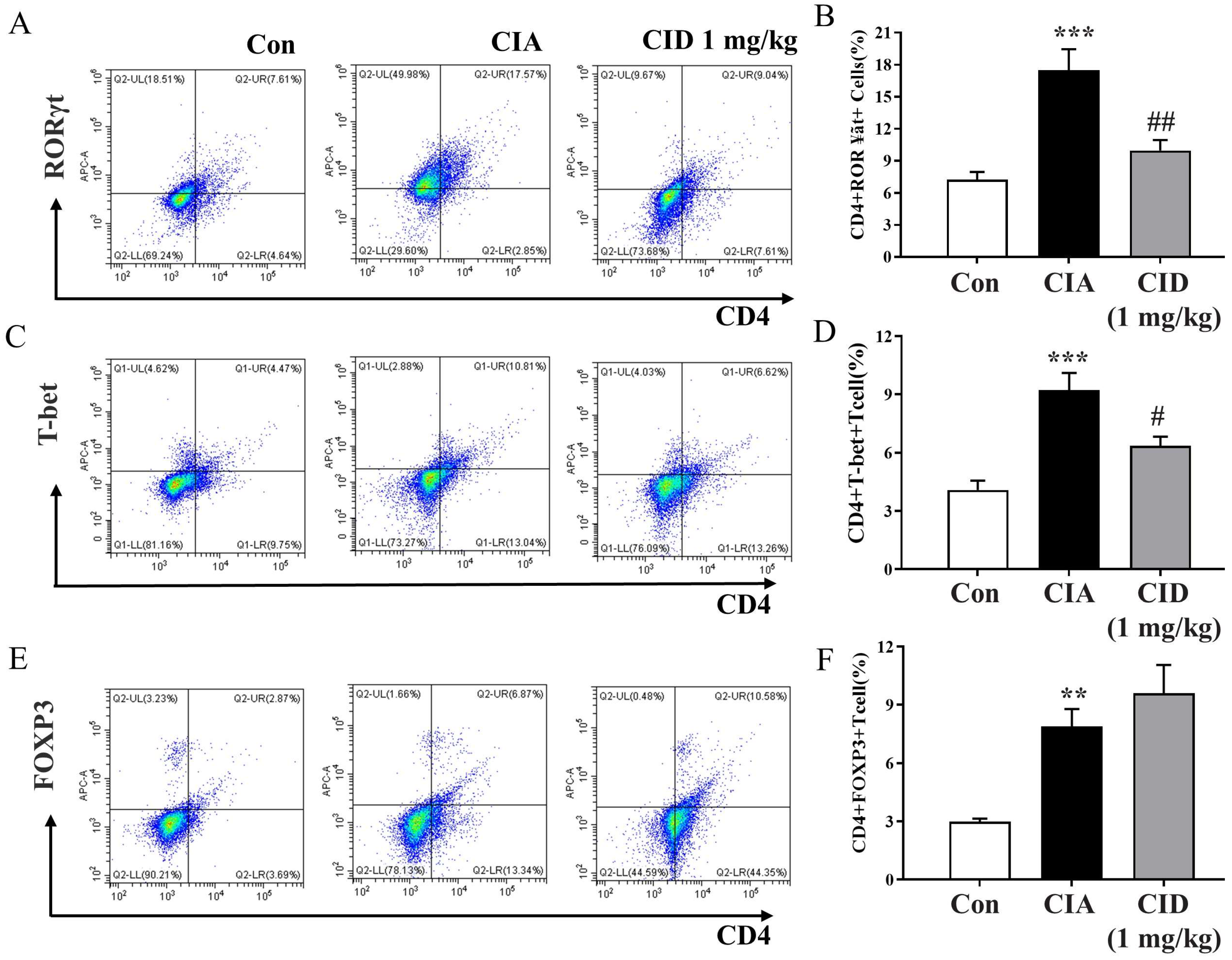
2.6. CID16020046 Treatment Diminished the CIA-Induced Rise in Populations of Th1 and Th17 Cells in the Spleens of DBA-1J Mice
2.7. CID16020046 Treatment Inhibited the Differentiation of Naïve T Cells into Th17 Cells
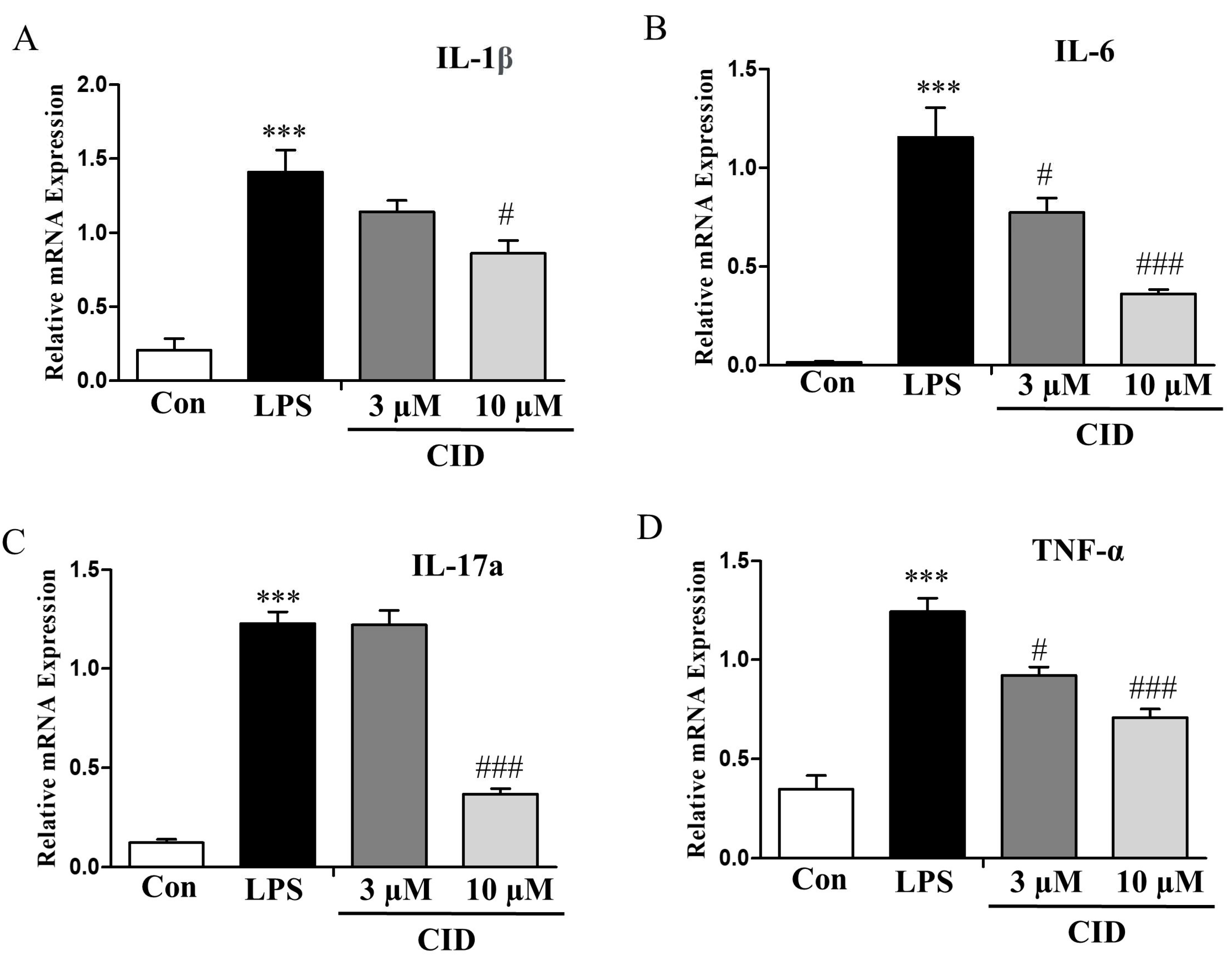
2.8. CID16020046 Treatment Reduced the mRNA Expression Levels of Inflammatory Cytokines in SW982 Human Synovial Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Animals
4.4. Human SW982 Synovial Cell Treatment
4.5. Induction of Rheumatoid Arthritis in DBA-1J Mice and CID16020046 Administration
4.6. Measurement of the Severity of Arthritis
4.7. Histological Assessment of Arthritis
4.8. Flowcytometric Analysis
4.9. Quantitative Real-Time PCR
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Th17 Cell Differentiation
4.12. Verification of Normality and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Lysophosphatidylinositols, from Cell Membrane Constituents to GPR55 Ligands. Trends Pharmacol. Sci. 2018, 39, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, A.Y.; Park, S.Y.; Liu, K.H.; Im, D.S. O-1602 Promotes Hepatic Steatosis through GPR55 and PI3 Kinase/Akt/SREBP-1c Signaling in Mice. Int. J. Mol. Sci. 2021, 22, 3091. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Catalán, V.; Whyte, L.; Díaz-Arteaga, A.; Vázquez-Martínez, R.; Rotellar, F.; Guzmán, R.; Gómez-Ambrosi, J.; Pulido, M.R.; Russell, W.R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 2012, 61, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Mundra, P.A.; Fan, F.; Galvin, A.; Weir, J.M.; Wong, G.; Chin-Dusting, J.; Cicuttini, F.; Meikle, P.; Dart, A.M. Plasma lipidomic profiling in patients with rheumatoid arthritis. Metabolomics 2016, 12, 136. [Google Scholar] [CrossRef]
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Cruz, S.L.; Sánchez-Miranda, E.; Castillo-Arellano, J.I.; Cervantes-Villagrana, R.D.; Ibarra-Sánchez, A.; González-Espinosa, C. Anandamide inhibits FcεRI-dependent degranulation and cytokine synthesis in mast cells through CB2 and GPR55 receptor activation. Possible involvement of CB2-GPR55 heteromers. Int. Immunopharmacol. 2018, 64, 298–307. [Google Scholar] [CrossRef]
- Pietr, M.; Kozela, E.; Levy, R.; Rimmerman, N.; Lin, Y.H.; Stella, N.; Vogel, Z.; Juknat, A. Differential changes in GPR55 during microglial cell activation. FEBS Lett. 2009, 583, 2071–2076. [Google Scholar] [CrossRef]
- Stančić, A.; Jandl, K.; Hasenöhrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.; Schicho, R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445. [Google Scholar] [CrossRef]
- Chiocchetti, R.; Salamanca, G.; De Silva, M.; Gobbo, F.; Aspidi, F.; Cunha, R.Z.; Galiazzo, G.; Tagliavia, C.; Sarli, G.; Morini, M. Cannabinoid receptors in the inflammatory cells of canine atopic dermatitis. Front. Vet. Sci. 2022, 9, 987132. [Google Scholar] [CrossRef]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.D.; Le Maitre, C.L. Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage: Implications for Future Therapies. Cannabis Cannabinoid Res. 2016, 1, 3–15. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef]
- Zamith Cunha, R.; Zannoni, A.; Salamanca, G.; De Silva, M.; Rinnovati, R.; Gramenzi, A.; Forni, M.; Chiocchetti, R. Expression of cannabinoid (CB1 and CB2) and cannabinoid-related receptors (TRPV1, GPR55, and PPARα) in the synovial membrane of the horse metacarpophalangeal joint. Front. Vet. Sci. 2023, 10, 1045030. [Google Scholar] [CrossRef] [PubMed]
- Zamith Cunha, R.; Salamanca, G.; Mille, F.; Delprete, C.; Franciosi, C.; Piva, G.; Gramenzi, A.; Chiocchetti, R. Endocannabinoid System Receptors at the Hip and Stifle Joints of Middle-Aged Dogs: A Novel Target for the Therapeutic Use of Cannabis sativa Extract in Canine Arthropathies. Animals 2023, 13, 2833. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Balenga, N.A.; Kargl, J.; Andradas, C.; Brown, A.J.; Irving, A.; Sanchez, C.; Waldhoer, M. Minireview: Recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol. Endocrinol. 2011, 25, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Schuelert, N.; McDougall, J.J. The abnormal cannabidiol analogue O-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor GPR55. Neurosci. Lett. 2011, 500, 72–76. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Nadkarni, S.; Mauri, C.; Ehrenstein, M.R. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J. Exp. Med. 2007, 204, 33–39. [Google Scholar] [CrossRef]
- Behrens, F.; Himsel, A.; Rehart, S.; Stanczyk, J.; Beutel, B.; Zimmermann, S.Y.; Koehl, U.; Möller, B.; Gay, S.; Kaltwasser, J.P.; et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 1151–1156. [Google Scholar] [CrossRef]
- Lee, S.J.; Im, D.S. GPR55 Antagonist CID16020046 Protects against Atherosclerosis Development in Mice by Inhibiting Monocyte Adhesion and Mac-1 Expression. Int. J. Mol. Sci. 2021, 22, 13084. [Google Scholar] [CrossRef]
- Son, S.E.; Lee, Y.J.; Shin, Y.J.; Kim, D.H.; Im, D.S. GPR55 Antagonist CID16020046 Attenuates Obesity-Induced Airway Inflammation by Suppressing Chronic Low-Grade Inflammation in the Lungs. Int. J. Mol. Sci. 2024, 25, 7358. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Im, D.S. GPR55 antagonist CID16020046 suppresses DNCB-induced atopic dermatitis-like symptoms by suppressing Th1/Th2/Th17 populations in mice. Eur. J. Pharmacol. 2024, 985, 177088. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, M.; Talamonti, E.; Maccarrone, M.; Chiurchiù, V. Activation of GPR55 Receptors Exacerbates oxLDL-Induced Lipid Accumulation and Inflammatory Responses, while Reducing Cholesterol Efflux from Human Macrophages. PLoS ONE 2015, 10, e0126839. [Google Scholar]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflammation 2018, 15, 322. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, H.; Lehmann, C. Inhibition of GPR 55 improves dysregulated immune response in experimental sepsis. Clin. Hemorheol. Microcirc. 2018, 70, 553–561. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, H.; Shu, J.; Shu, H.; Lu, C.; Zhao, N.; Geng, Y.; He, X.; Lu, A. Pien Tze Huang alleviate the joint inflammation in collagen-induced arthritis mice. Chin. Med. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.; Vervoordeldonk, M.J.; Denis, M.C.; Armaka, M.; Hoffmann, M.; Bäcklund, J.; Nandakumar, K.S.; Niederreiter, B.; Geka, C.; Fischer, A.; et al. ‘SMASH’ recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models. Ann. Rheum. Dis. 2021, 80, 714–726. [Google Scholar] [CrossRef]
- Douni, E.; Sfikakis, P.P.; Haralambous, S.; Fernandes, P.; Kollias, G. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: Comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis. Res. Ther. 2004, 6, R65–R72. [Google Scholar] [CrossRef]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Im, D.-S. GPR55 Antagonist CID16020046 Suppresses Collagen-Induced Rheumatoid Arthritis by Suppressing Th1/Th17 Cells in Mice. Int. J. Mol. Sci. 2025, 26, 4680. https://doi.org/10.3390/ijms26104680
Lee J-E, Im D-S. GPR55 Antagonist CID16020046 Suppresses Collagen-Induced Rheumatoid Arthritis by Suppressing Th1/Th17 Cells in Mice. International Journal of Molecular Sciences. 2025; 26(10):4680. https://doi.org/10.3390/ijms26104680
Chicago/Turabian StyleLee, Jung-Eun, and Dong-Soon Im. 2025. "GPR55 Antagonist CID16020046 Suppresses Collagen-Induced Rheumatoid Arthritis by Suppressing Th1/Th17 Cells in Mice" International Journal of Molecular Sciences 26, no. 10: 4680. https://doi.org/10.3390/ijms26104680
APA StyleLee, J.-E., & Im, D.-S. (2025). GPR55 Antagonist CID16020046 Suppresses Collagen-Induced Rheumatoid Arthritis by Suppressing Th1/Th17 Cells in Mice. International Journal of Molecular Sciences, 26(10), 4680. https://doi.org/10.3390/ijms26104680






