First-Principles Investigation of Structural, Electronic, and Magnetic Properties of BiFeO3 and Bi2Fe4O9 Nanostructures
Abstract
1. Introduction
2. Results and Discussion
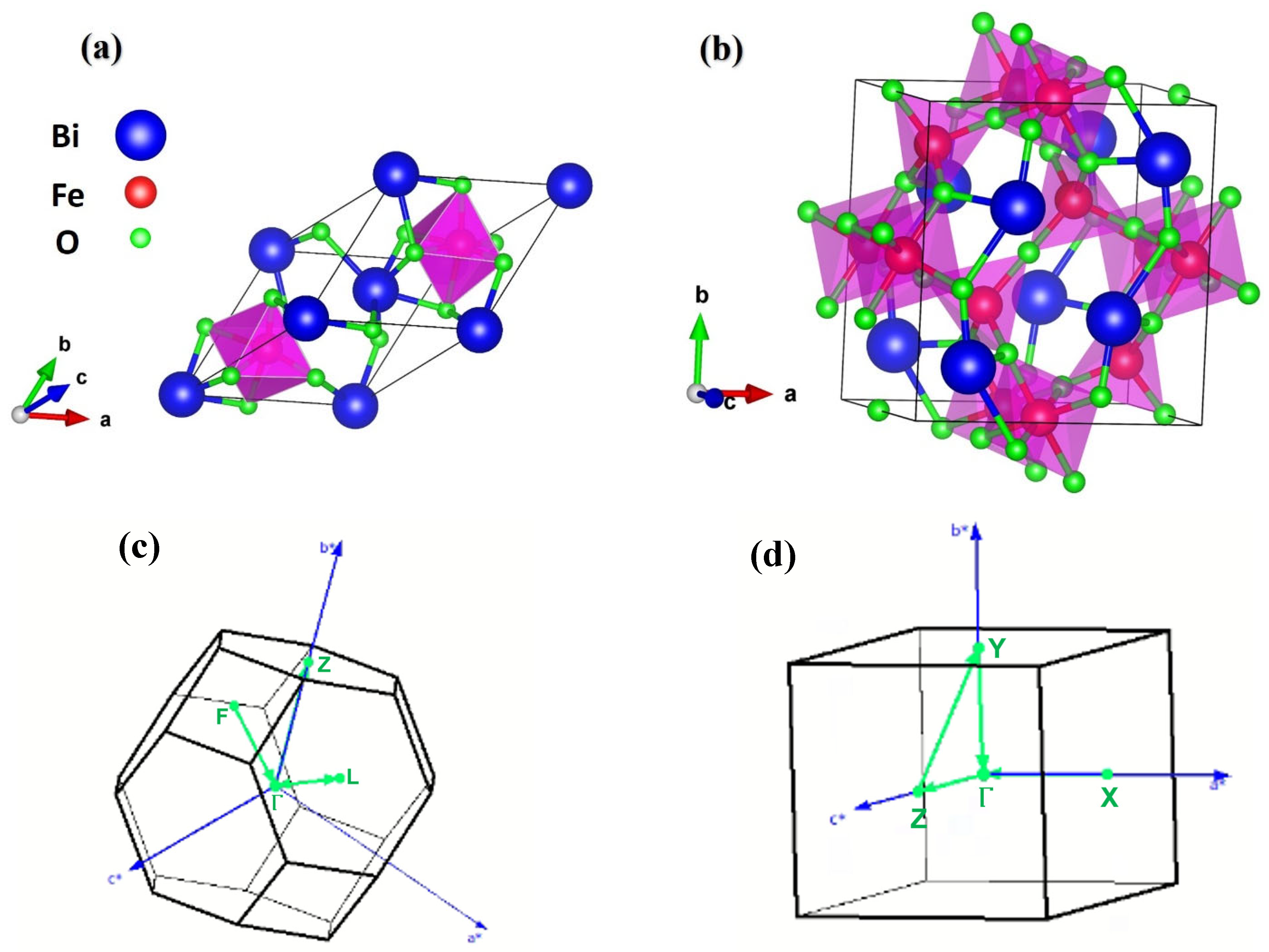
2.1. Structural Properties
| This Work | Experimental Result [13] | Literature | |
|---|---|---|---|
| BiFeO3 | |||
| a = b = c (Å) | 5.6360(1) | 5.5827(2) | 5.59–5.69 [18,20,21,22] |
| 59.28 | 60 | 59–60 [20,21,22,23] | |
| Eg (eV) | 2.4 | 2.2 | 1–2.7 [20,21,23,24] |
| Bi2Fe4O9 | |||
| a (Å) | 7.9740(1) | 7.9431(4) | 7.905–7.96 [19,25] |
| b(Å) | 8.4240(1) | 8.5276(2) | 8.428–8.44 [19,25] |
| c (Å) | 5.8670(1) | 6.0291(2) | 5.99–6.005 [19,25] |
| 90 | 90 | 90 [19,25] | |
| Eg (eV) | 2.3 | 2.34 | 1–2.4 [19,25,26] |
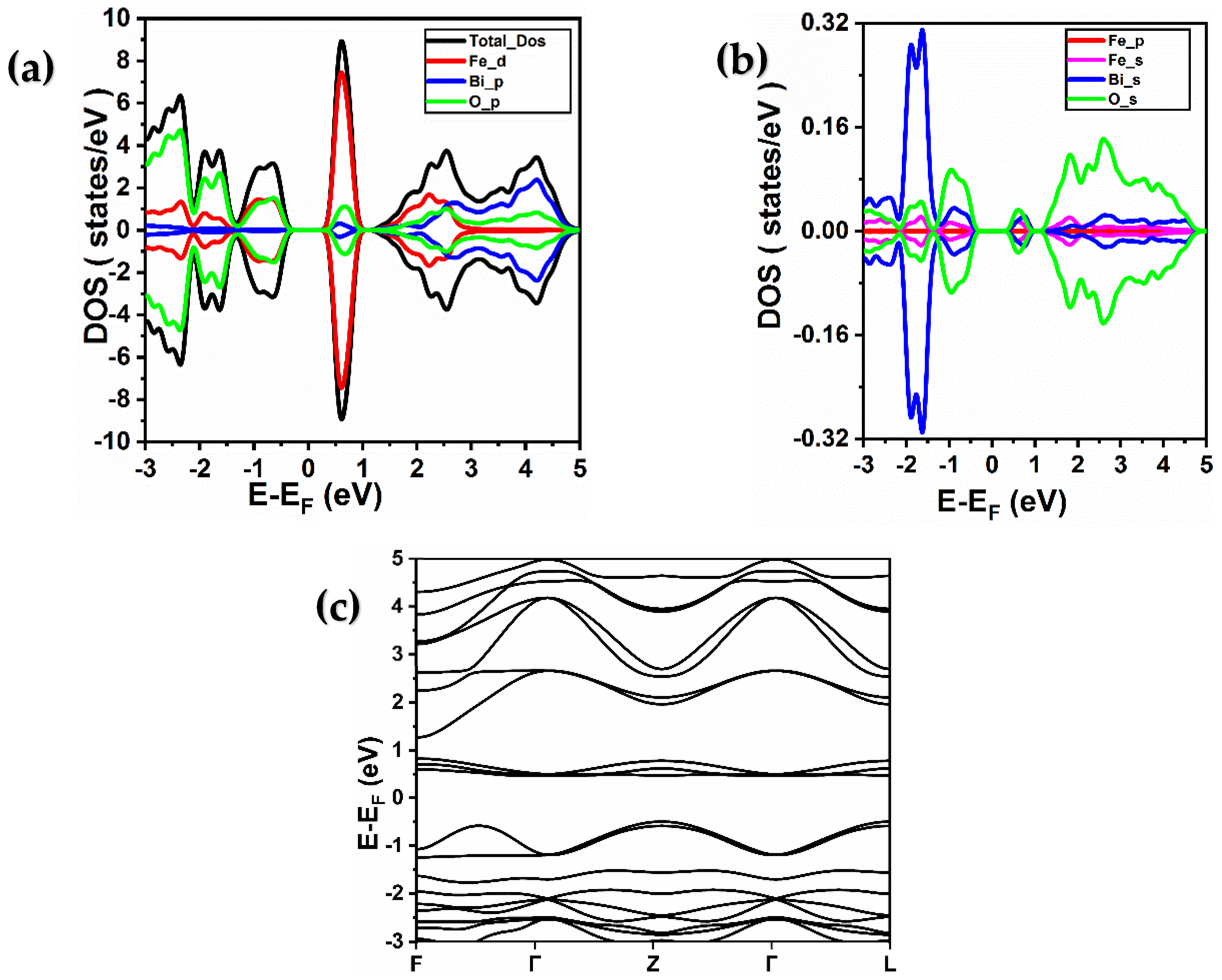
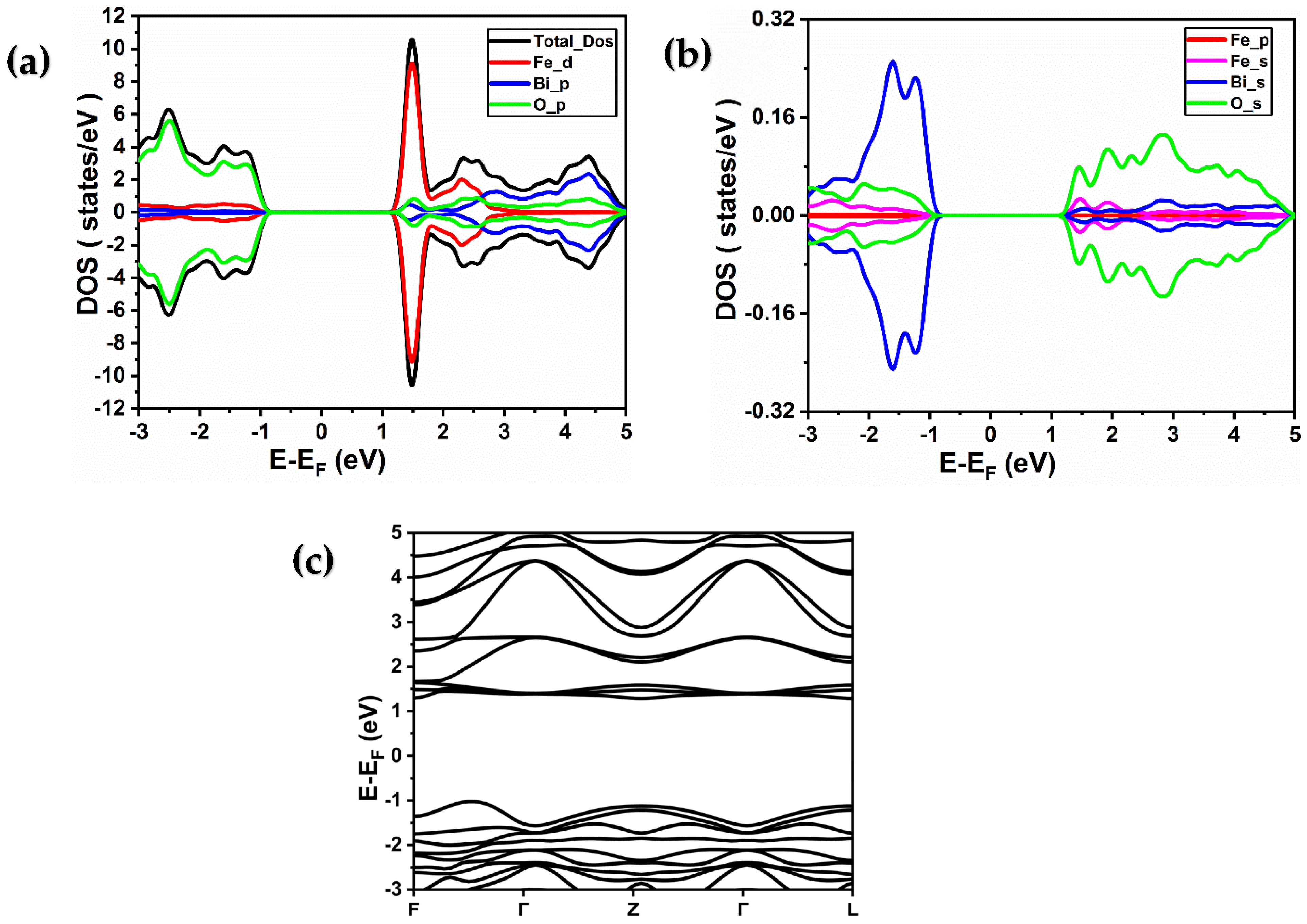
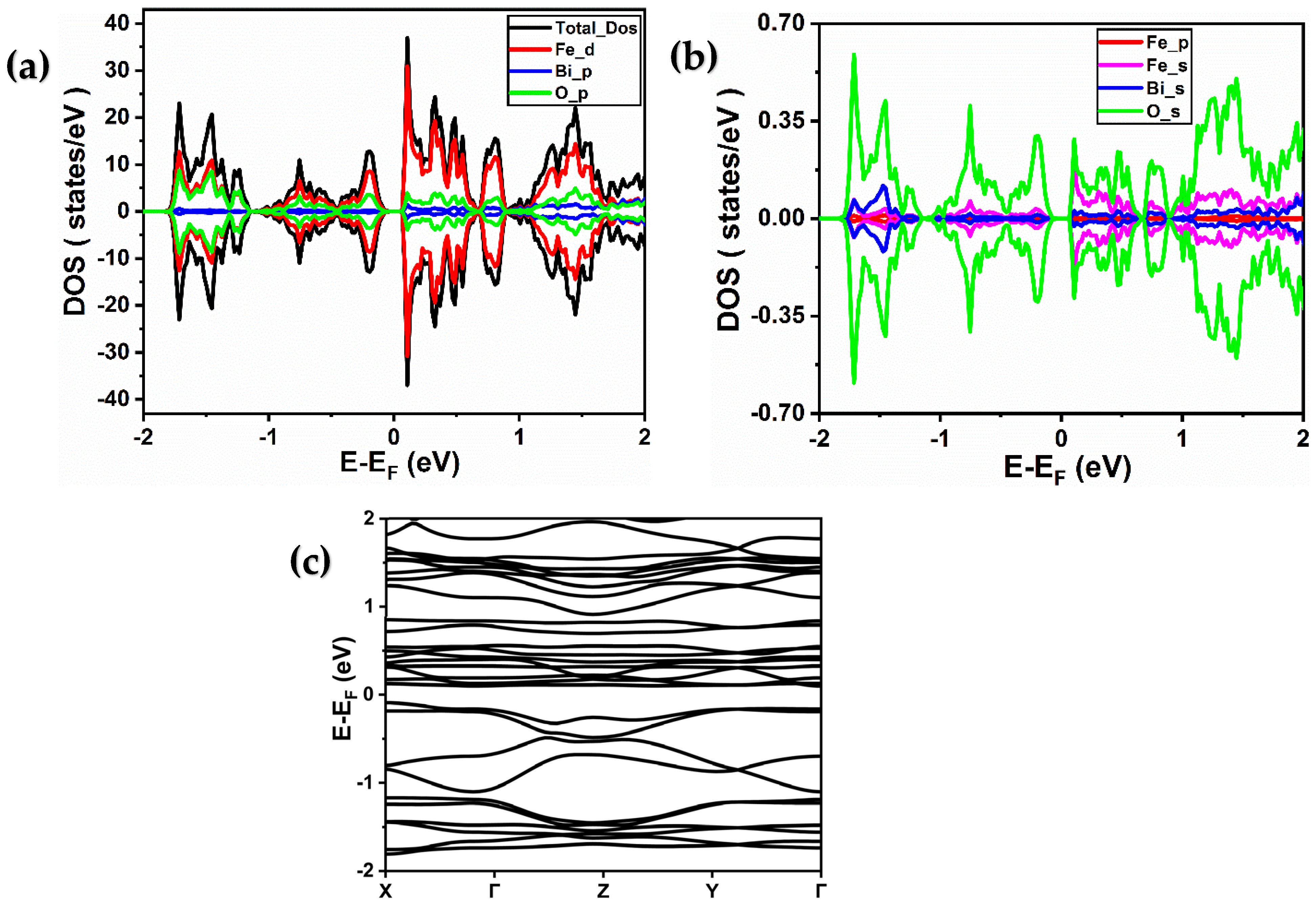
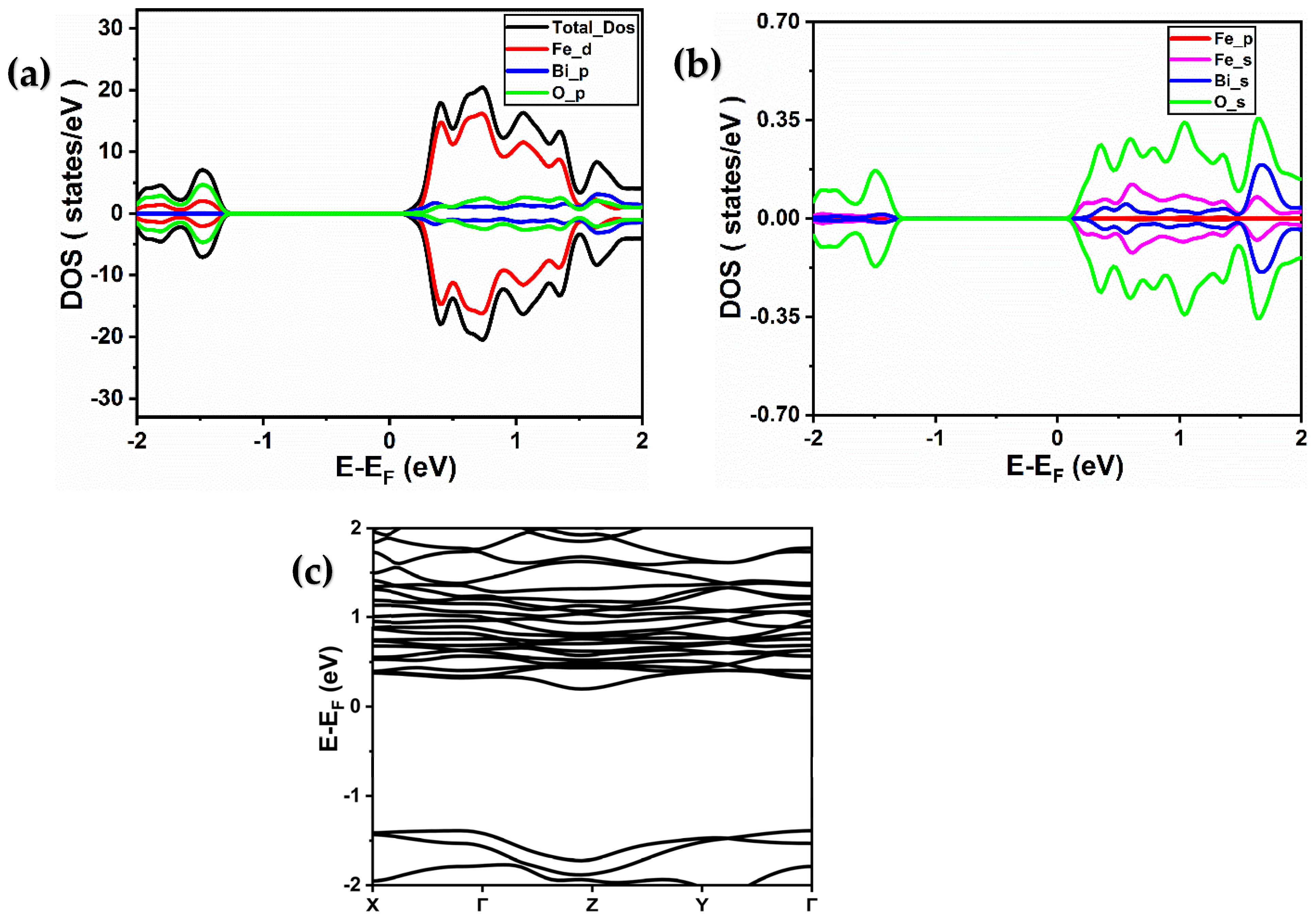
2.2. Electronic Properties
2.3. Magnetic Structure and Magnetic Anisotropy
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tokunaga, M.; Akaki, M.; Ito, T.; Miyahara, S.; Miyake, A.; Kuwahara, H.; Furukawa, N. Magnetic control of transverse electric polarization in BiFeO3. Nat. Commun. 2015, 6, 5878. [Google Scholar] [CrossRef]
- Fischer, P.; Polomska, M.; Sosnowska, I.; Szymanski, M.M. Temperature dependence of the crystal and magnetic structures of BiFeO3. J. Phys. C Solid State Phys. 1980, 13, 1931. [Google Scholar] [CrossRef]
- Kirsch, A.; Murshed, M.M.; Gaczynski, P.; Becker, K.-D.; Gesing, T.M. Bi2Fe4O9: Structural changes from nano- to micro-crystalline state. Z. Für Naturforschung B 2016, 71, 447–455. [Google Scholar] [CrossRef]
- Vopson, M.M. Fundamentals of Multiferroic Materials and Their Possible Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 223. [Google Scholar] [CrossRef]
- Othman, M.; Mallek-Zouari, I.; Akrout, H.; Thabet-Mliki, N. Synthesis and properties of ultra-small BiFeO3 nanoparticles doped with cobalt. Ceram. Int. 2023, 49, 10580–10587. [Google Scholar] [CrossRef]
- Liu, K.; Fan, H.; Ren, P.; Yang, C. Structural, electronic and optical properties of BiFeO3 studied by first-principles. J. Alloys Compd. 2011, 509, 1901–1905. [Google Scholar] [CrossRef]
- Lavasani, S.; Mirzaee, O.; Shokrollahi, H.; Moghadam, A.; Salami, M. Magnetic and morphological characterization of Bi2Fe4O9 nanoparticles synthesized via a new reverse chemical co-precipitation method. Ceram. Int. 2017, 43, 12120–12125. [Google Scholar] [CrossRef]
- Lejman, M.; Paillard, C.; Juvé, V.; Vaudel, G.; Guiblin, N.; Bellaiche, L.; Viret, M.; Gusev, V.E.; Dkhil, B.; Ruello, P. Magnetoelastic and magnetoelectric couplings across the antiferromagnetic transition in multiferroic BiFeO3. Phys. Rev. B 2019, 99, 104103. [Google Scholar] [CrossRef]
- Petkov, V.; Shastri, S. Lattice symmetry breaking transition and critical size Lattice symmetry breaking transition and critical size limit for ferroic orders in nanophase BiFeO3. Phys. Rev. B 2021, 104, 054121. [Google Scholar] [CrossRef]
- Campetella, M.; Calandra, M. Polar magnetic metallic state in few-layer BiFeO3. Phys. Rev. B 2021, 104, 174111. [Google Scholar] [CrossRef]
- Yang, R.-P.; Lin, S.-X.; Fang, X.-G.; Liu, J.-M. Electronic and magnetic properties of BiFeO3 with intrinsic defects: First-principles prediction. Chin. Phys. B 2014, 23, 067102. [Google Scholar] [CrossRef]
- Shen, T.; Hu, C.; Dai, H.L.; Yang, W.L.; Liu, H.C.; Wei, X.L. First principles study of structural, electronic, and optical properties of BiFeO3 in ferroelectric and paraelectric phases. Mater. Res. Innov. 2015, 19, S5-684–S5-688. [Google Scholar] [CrossRef]
- Ben Taazayet, W.; Zouari, I.M.; Hosni, N.; Dkhil, B.; Mliki, N.T. Facile synthesis of pure BiFeO3 and Bi2Fe4O9 nanostructures with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2022, 33, 2518–2533. [Google Scholar] [CrossRef]
- Sahoo, A.; Bhattacharya, D.; Das, M.; Mandal, P. Mandal Shape dependent multiferroic behavior in Bi2Fe4O9 nanoparticles. Nanotechnology 2022, 33, 30. [Google Scholar] [CrossRef]
- Mallek-Zouari, I.; Taazayet, W.B.; Grenèche, J.-M.; Bessais, L.; Dkhil, B.; Mliki, N. Field-induced spin cycloidal modulation to antiferromagnetic transition and possible flexomagnetic effect in BiFeO3 nanoparticles. J. Alloys Compd. 2023, 934, 167944. [Google Scholar] [CrossRef]
- Trujillo, D.; Ghosh, A.; Nakhmanson, S.M.; Alpay, S.P. Surface structure and energetics of low index facets of bismuth ferrite. Phys. Chem. Chem. Phys. 2020, 22, 16400–16406. [Google Scholar] [CrossRef]
- Burns, S.R.; Paull, O.; Juraszek, J.; Sando, D. The Experimentalist’s Guide to the Cycloid, or Noncollinear Antiferromagnetism in Epitaxial BiFeO3. Adv. Mater. 2020, 32, 2003711. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Nordblad, P.; Tellgren, R.; Ericsson, T.; Korchagina, S.K.; Rybakova, L.F.; Hewat, A. Influence of PbZrO3 doping on the structural and magnetic properties of BiFeO3. Solid State Sci. 2008, 10, 1875–1885. [Google Scholar] [CrossRef]
- Nobukazu, N.; Masatada, W. The crystal structures of Bi2Mn4O10, Bi2Al4O9 and Bi2Fe4O9. Z. Krist. Cryst. Mater. 1968, 127, 173–187. [Google Scholar] [CrossRef]
- Grover, S.; Butler, K.T.; Waghmare, U.V.; Grau-Crespo, R. Co Substituted BiFeO3: Electronic, Ferroelectric, and Thermodynamic Properties from First Principles. Adv. Theory Simul. 2023, 6, 2200673. [Google Scholar] [CrossRef]
- Goffinet, M.; Hermet, P.; Bilc, D.I.; Ghosez, P. Hybrid functional study of prototypical multiferroic bismuth ferrite. Phys. Rev. B 2009, 79, 014403. [Google Scholar] [CrossRef]
- Ricinschi, D.; Yun, K.-Y.; Okuyama, M. A mechanism for the 150 μC cm−2 polarization of BiFeO3 films based on first principles calculations and new structural data. J. Phys. Condens. Matter 2006, 18, L97–L105. [Google Scholar] [CrossRef]
- Srihari, N.V.; Nayak, S.; Poornesh, P.; Nagaraja, K.K. Variation in the electronic, mechanical, and structural properties among the polymorphs of bismuth ferrite: A first-principles approach. Eur. Phys. J. Plus 2023, 138, 465. [Google Scholar] [CrossRef]
- Youssef, M.B.; Saitzek, S.; Rajput, N.S.; El Marssia, M.; Jouiad, M. Effect of Sr and Ti substitutions on optical and photocatalytic properties of Bi1−xSrxFe1−xTixO3 nanomaterials. Nanoscale Adv. 2023, 5, 869. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Gong, W.; Wang, J.; Ning, X.; Wang, Z.; Zhao, X.; Ren, W.; Zhang, Z. Size-Dependent Magnetic, Photoabsorbing, and Photocatalytic Properties of Single-Crystalline Bi2Fe4O9 Semiconductor Nanocrystals. J. Phys. Chem. C 2011, 115, 25241–25246. [Google Scholar] [CrossRef]
- Irshad, Z.; Shah, S.H.; Rafiq, M.A.; Hasan, M.M. First principles study of structural, electronic and magnetic properties of ferromagnetic Bi2Fe4O9. J. Alloys Compd. 2015, 624, 131–136. [Google Scholar] [CrossRef]
- Kozakov, A.T.; Guglev, K.A.; Ilyasov, V.V.; Ershov, I.V.; Nikol’skii, A.V.; Smotrakov, V.G.; Eremkin, V.V. Electronic Structure of Bismuth Ferrite and Hematite Single Crystals: X-Ray Photoelectron Study and Calculation. Phys. Solid State 2011, 53, 41–47. [Google Scholar] [CrossRef]
- Farid, G.; Murtaza, G.; Flemban, T.H.; Althib, H.; AlObaid, A.A.; Al-Muhimeed, T.I.; Mera, A.; Haq, B.U.; Mahmood, Q. Structural, Magnetic, and Dielectric Properties of Sn-Doped BiFeO3: Experiment and DFT Analysis. J. Supercond. Nov. Magn. 2021, 34, 2179–2188. [Google Scholar] [CrossRef]
- Shamir, N.; Gurewitz, E.; Shaked, H. The Magnetic Structure of Bi2Fe4O9—Analysis of Neutron Diffraction Measurements. Acta Crystallogr. Sect. A 1978, 34, 662. [Google Scholar] [CrossRef]
- Mallek-Zouari, I.; Ben Taazayet, W.; Grenèche, J.-M.; Bessais, L.; Mliki, N.T. Mössbauer spectrometry for investigating the magnetic properties of Bi2Fe4O9 bismuth ferrite nanostructures. Mater. Chem. Phys. 2025, 332, 130266. [Google Scholar] [CrossRef]
- Kawachi, S.; Miyake, A.; Ito, T.; Dissanayake, S.E.; Matsuda, M.; Ratcliff, W.; Xu, Z.; Zhao, Y.; Miyahara, S.; Furukawa, N.; et al. Successive field-induced transitions in BiFeO3 around room temperature. Phys. Rev. Mater. 2017, 1, 024408. [Google Scholar] [CrossRef]
- Allen, M.; Aupiais, I.; Cazayous, M.; de Sousa, R. Size-dependent bistability in multiferroic nanoparticles. Phys. Rev. Mater. 2019, 3, 084402. [Google Scholar] [CrossRef]
- Verseils, M.; Litvinchuk, A.P.; Brubach, J.-B.; Roy, P.; Beauvois, K.; Ressouche, E.; Skumryev, V.; Gospodinov, M.; Simonet, V.; de Brion, S. Infrared phonon spectroscopy on the Cairo pentagonal antiferromagnet Bi2Fe4O9: A study through the pressure-induced structural transition. Phys. Rev. B 2021, 103, 174403. [Google Scholar] [CrossRef]
- Da Silva, K.L.; Trautwein, R.S.; Da Silva, R.B.; Fabián, M.; Čižmár, E.; Holub, M.; Skurikhina, O.; Harničárová, M.; Girman, V.; Menzel, D.; et al. Suppression of the Cycloidal Spin Arrangement in BiFeO3 Caused by the Mechanically Induced Structural Distortion and Its Effect on Magnetism. Front. Mater. 2021, 8, 717185. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; Gironcoli, S.D.; Delugas, P.; Ruffino, F.F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kaczkowski, J.; Pugaczowa-Michalska, M.; Płowaś-Korus, I. Comparative density functional studies of pristine and doped bismuth ferrite polymorphs by GGA+U and meta-GGA SCAN+U. Phys. Chem. Chem. Phys. 2021, 23, 857. [Google Scholar] [CrossRef]
| This Work | Literature | |
|---|---|---|
| BiFeO3 | ||
| Magnetic moments of Fe () in an octahedral | 3.8168 | 3.75 [28] |
| Bi2Fe4O9 | ||
| Magnetic moments of Fe in an octahedral () | 3.6777 | 3.9 [26] |
| Magnetic moments of Fe in an Tetrahedral () | 3.7854 | 4.95 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallek-Zouari, I.; Kaddar, Y.; Ben Taazayet, W.; Mounkachi, O.; Hlil, E.-K.; Thabet Mliki, N.; El Moutaouakil, A. First-Principles Investigation of Structural, Electronic, and Magnetic Properties of BiFeO3 and Bi2Fe4O9 Nanostructures. Int. J. Mol. Sci. 2025, 26, 4671. https://doi.org/10.3390/ijms26104671
Mallek-Zouari I, Kaddar Y, Ben Taazayet W, Mounkachi O, Hlil E-K, Thabet Mliki N, El Moutaouakil A. First-Principles Investigation of Structural, Electronic, and Magnetic Properties of BiFeO3 and Bi2Fe4O9 Nanostructures. International Journal of Molecular Sciences. 2025; 26(10):4671. https://doi.org/10.3390/ijms26104671
Chicago/Turabian StyleMallek-Zouari, Ikbel, Youness Kaddar, Wael Ben Taazayet, Omar Mounkachi, El-Kebir Hlil, Najeh Thabet Mliki, and Amine El Moutaouakil. 2025. "First-Principles Investigation of Structural, Electronic, and Magnetic Properties of BiFeO3 and Bi2Fe4O9 Nanostructures" International Journal of Molecular Sciences 26, no. 10: 4671. https://doi.org/10.3390/ijms26104671
APA StyleMallek-Zouari, I., Kaddar, Y., Ben Taazayet, W., Mounkachi, O., Hlil, E.-K., Thabet Mliki, N., & El Moutaouakil, A. (2025). First-Principles Investigation of Structural, Electronic, and Magnetic Properties of BiFeO3 and Bi2Fe4O9 Nanostructures. International Journal of Molecular Sciences, 26(10), 4671. https://doi.org/10.3390/ijms26104671










