Developing Efficient Methods of Sperm Cryopreservation for Three Fish Species (Cyprinus carpio L., Schizothorax prenanti, Glyptosternum maculatum)
Abstract
1. Introduction
2. Results
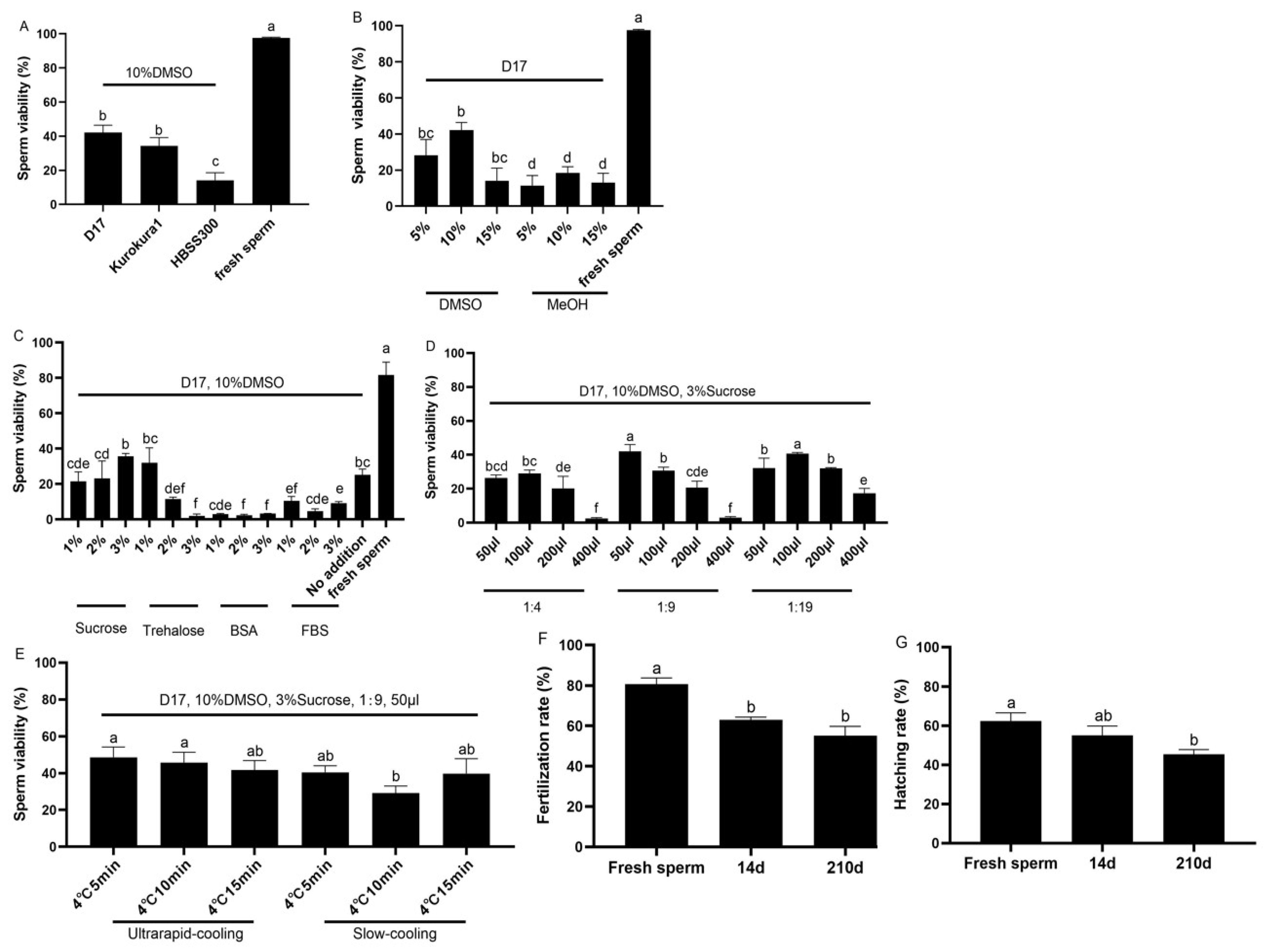
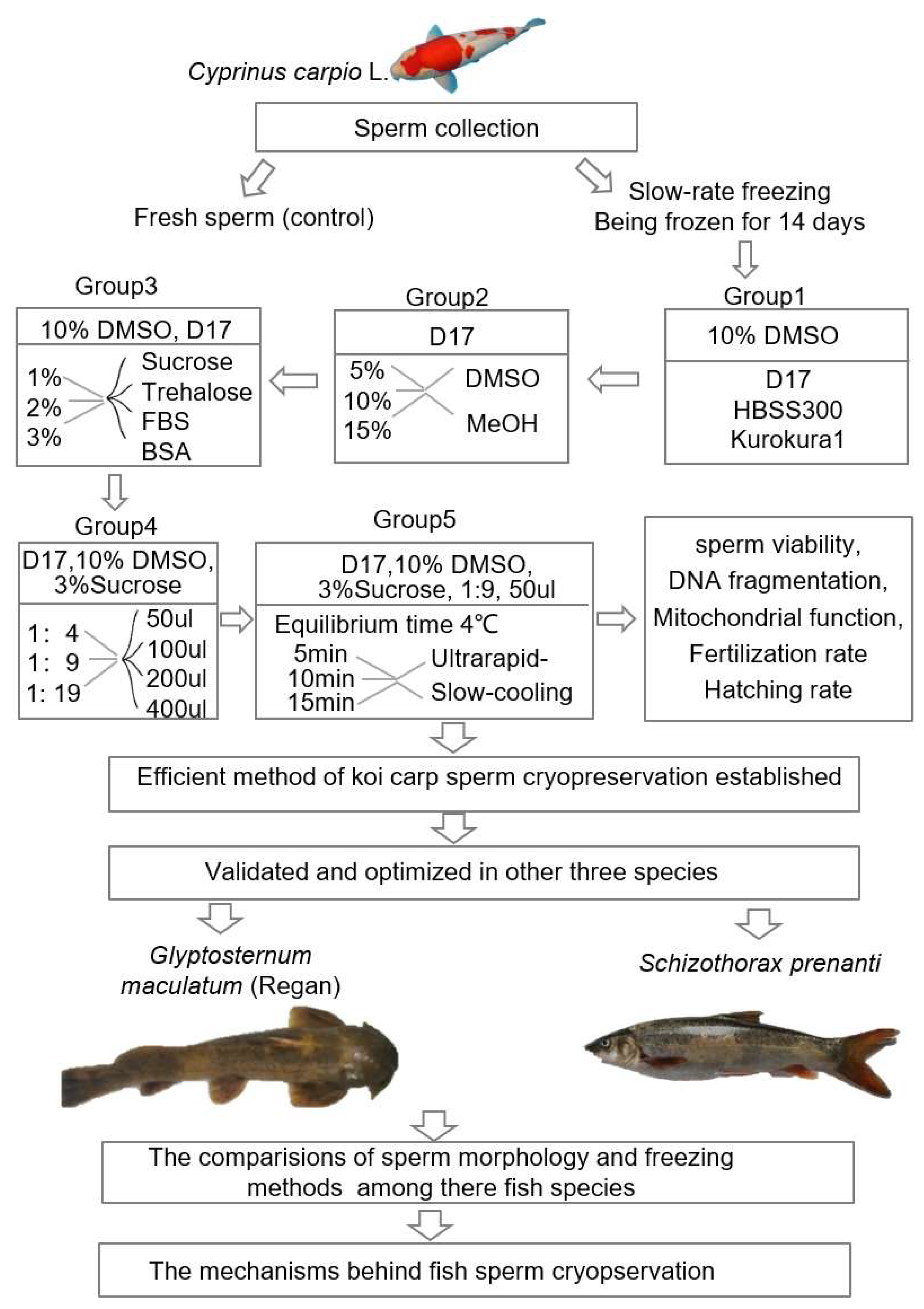
2.1. Effects of Different Sperm Cryopreservation Protocols on the Sperm Viability in Koi Carp
2.2. Effects of Equilibrium Time on Fertilization Rate and Hatching Rate of Frozen–Thawed Sperm in Koi Carp
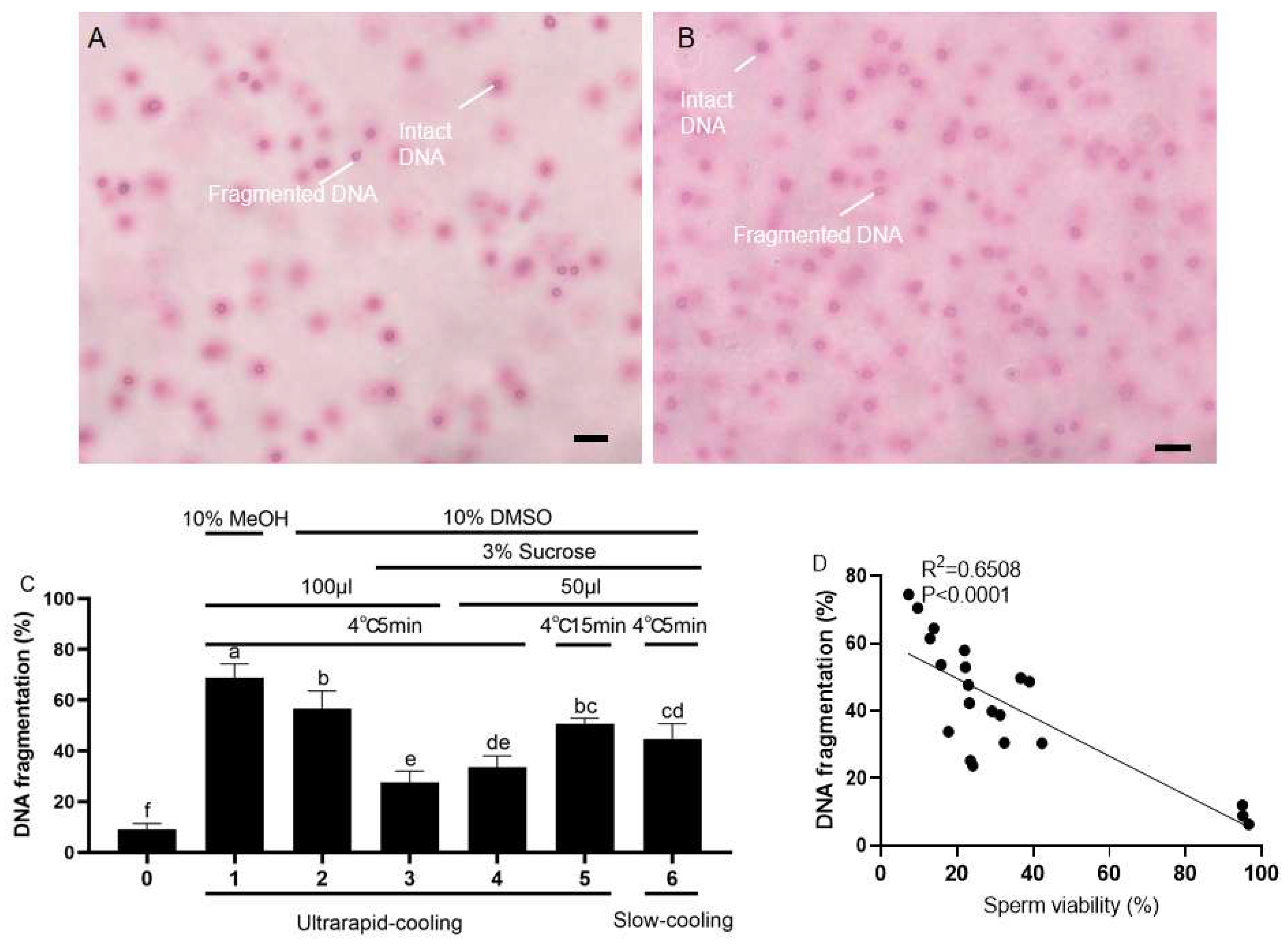
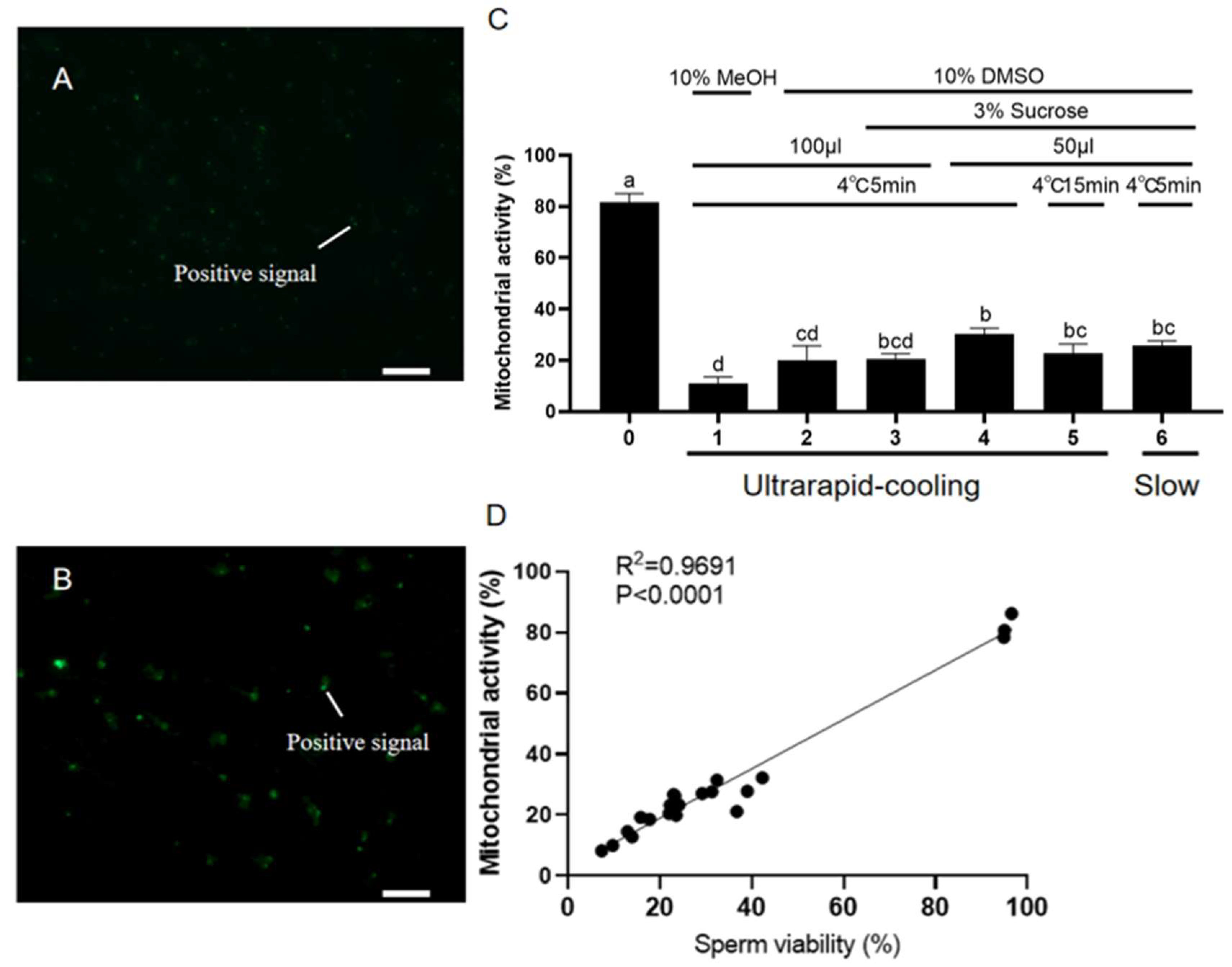
2.3. Effects of Different Cryomedia and Procedures on DNA Integrity and Mitochondrial Function of the Frozen–Thawed Sperm in Koi Carp
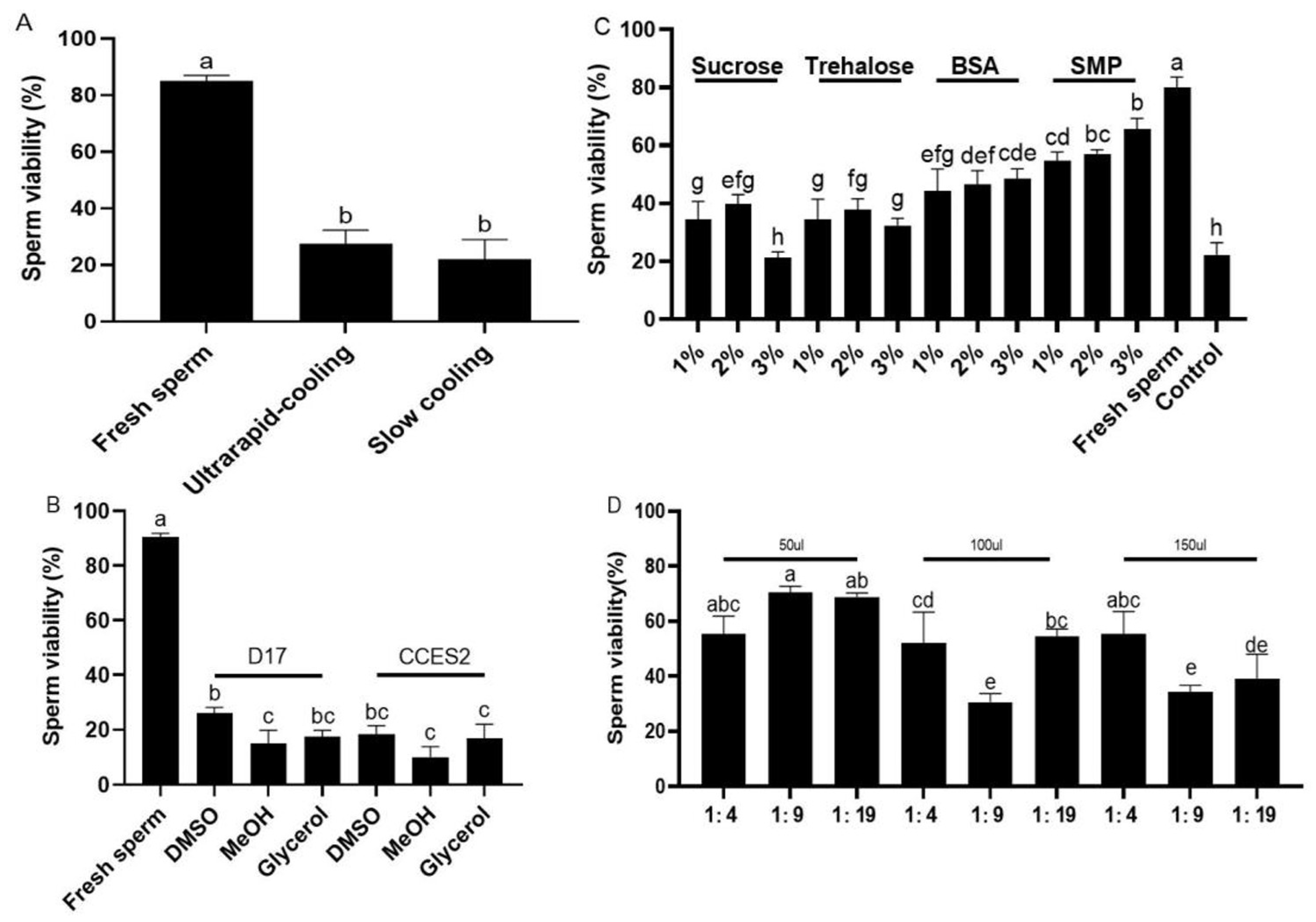
2.4. The Optimized Cryopreservation Protocol Validated in Another Batch of Sperm from Three Fish Species
2.5. Cryopreservation Protocol for Sperm Modified in Ya Fish
2.6. Effects of Cryopreservation on Sperm Ultrastructure
3. Discussion
3.1. An Effective Method Developed for Sperm Cryopreservation Using a Sucrose–DMSO Cryomedium in Koi Carp
3.2. The Dilution, Density, and Volume of Sperm Are Important for Cryopreservation
3.3. The Advantages of Ultrarapid Cooling in Cryopreserving Fish Sperm
3.4. The Main Injuries on Fish Sperm Caused by Cryopreservation
4. Materials and Methods
4.1. Fish and Samples
4.2. Freezing Sperm
4.3. The Assessment of Sper’ Viability
4.4. DNA Damage
4.5. Mitochondrial Membrane Potential
4.6. Fertilization and Hatching Rate
4.7. Electron Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AbdelHafez, F.; Bedaiwy, M.; El-Nashar, S.A.; Sabanegh, E.; Desai, N. Techniques for cryopreservation of individual or small numbers of human spermatozoa: A systematic review. Hum. Reprod. Update 2009, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Szell, A.Z.; Bierbaum, R.C.; Hazelrigg, W.B.; Chetkowski, R.J. Live births from frozen human semen stored for 40 years. J. Assist. Reprod. Genet. 2013, 30, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef] [PubMed]
- Aramli, M.S.; Golshahi, K.; Nazari, R.M.; Aramli, S. Effect of freezing rate on motility, adenosine triphosphate content and fertilizability in beluga sturgeon (Huso huso) spermatozoa. Cryobiology 2015, 70, 170–174. [Google Scholar] [CrossRef]
- Yavuz, U.; Bozkurt, Y. Protective Effect of Vitamin C (Ascorbic Acid) Supplementation on Post-Thaw Motility and Fertility of Cryopreserved Rainbow Trout (Oncorhynchus mykiss) Sperm. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1348–1352. [Google Scholar] [CrossRef]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirao, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.L.; Hu, J.L.; Zhou, W.J.; Huang, Z.H.; Luo, X.F.; Li, Z.; Zhu, W.B. Update on techniques for cryopreservation of human spermatozoa. Asian J. Androl. 2022, 24, 563–569. [Google Scholar] [CrossRef]
- Mazur, P.; Leibo, S.P.; Seidel, G.E. Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol. Reprod. 2008, 78, 2–12. [Google Scholar] [CrossRef]
- Ugur, M.R.; Abdelrahman, A.S.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Magnotti, C.; Cerqueira, V.; Lee-Estevez, M.; Farias, J.G.; Valdebenito, I.; Figueroa, E. Cryopreservation and vitrification of fish semen: A review with special emphasis on marine species. Rev. Aquac. 2018, 10, 15–25. [Google Scholar] [CrossRef]
- Alcay, S.; Aktar, A.; Koca, D.; Kilic, M.A.; Akkasoglu, M.; Yilmaz, M.M.; Sagirkaya, H. Autologous Platelet Rich Plasma Have Positive Effect on Ram Spermatozoa During Cryopreservation in Non-Breeding Season. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 229–234. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; García, B.M.; Samper, J.C.; Aparicio, I.M.; Tapia, J.A.; Ferrusola, C.O. Dissecting the molecular damage to stallion spermatozoa: The way to improve current cryopreservation protocols? Theriogenology 2011, 76, 1177–1186. [Google Scholar] [CrossRef]
- Talarczyk-Desole, J.; Berger, A.; Taszarek-Hauke, G.; Hauke, J.; Pawelczyk, L.; Jedrzejczak, P. Manual vs. computer-assisted sperm analysis: Can CASA replace manual assessment of human semen in clinical practice? Ginekol. Pol. 2017, 88, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Tempero, G.W.; Ling, N.; Hicks, B.J.; Osborne, M.W. Age composition, growth, and reproduction of koi carp (Cyprinus carpio) in the lower Waikato region, New Zealand. N. Z. J. Mar. Freshw. Res. 2006, 40, 571–583. [Google Scholar] [CrossRef]
- Chen, C.N.; Huang, Y.Y.; Li, H.; Long, Z.H.; Lai, J.S.; Liu, G.X.; Zhao, G. The complete mitochondrial genome of Schizothorax prenanti (Tchang) (Teleostei, Cyprinidae, Schizothoracinae). Mitochondrial DNA Part A 2016, 27, 253–254. [Google Scholar] [CrossRef]
- Li, H.J.; Xie, C.X.; Li, D.P.; Chai, Y.; Liu, H.Y.; Fan, Q.X.; Zhu, B.K. Exo-celiac liver in Glyptosternum maculatum. Prog. Nat. Sci.-Mater. Int. 2007, 17, 1109–1113. [Google Scholar]
- Coimbra, V.C.; Rodrigues, J.; dos Santos, R.S.; Rodrigues, R.B.; Streit, D., Jr.; Caldas, A.L.D.; Albuquerque, E.S.D.; Ferreira, E.J.D.; Maximino, C.; de Siqueira-Silva, D.H. Cryopreserved sperm does not affect larval ontogeny and quality in Rhamdia quelen. Fish Physiol. Biochem. 2025, 51, 42. [Google Scholar] [CrossRef]
- Shi, X.; Xu, J.Y.; Hou, Y.J.; Wei, Z.; Guo, L.F.; Ma, X.; Wu, L.M.; Ma, W.E.; Tian, X.; Waiho, K.; et al. Short-Term Low-Temperature Storage and Cryopreservation of Qihe Crucian Carp (Carassius auratus) Sperm. Animals 2025, 15, 698. [Google Scholar] [CrossRef]
- Ruan, Q.X.; Yang, S.; Hua, S.J.; Zhang, W.W.; Li, D.; Yang, Y.; Wang, X.; Wang, Q.H.; Meng, Z.N. Supplementation of Extender with Melatonin Improves the Motility, Mitochondrial Membrane Potential, and Fertilization Ability of Cryopreserved Brown-Marbled Grouper Sperm. Animals 2024, 14, 995. [Google Scholar] [CrossRef]
- Ding, X.Y.; Tian, Y.S.; Qiu, Y.S.; Duan, P.F.; Wang, X.Y.; Li, Z.T.; Li, L.L.; Liu, Y.; Wang, L.N. Effects of Long-Term Cryopreservation on the Transcriptomes of Giant Grouper Sperm. Genes 2024, 15, 523. [Google Scholar] [CrossRef]
- Villalobos, E.F.; Pereira, W.A.; Perez-Atehortúa, M.; Sandoval-Vargas, L.; Romero, J.; Oliveira, R.P.S.; Valdebenito, I.; Villasante, A. Influence of Dietary Fatty Acids on Fish Sperm Tolerance to Cryopreservation. Rev. Aquac. 2025, 17, e12968. [Google Scholar] [CrossRef]
- Chen, S.L. CRYOPRESERVATION OF SPERMATOZOA OF SILVER CARP, COMMON CARP, BLUNT SNOUT BREAM AND GRASS CARP. Curr. Zool. 1992, 38, 413–424. [Google Scholar]
- Liu, G.; Wu, X.; He, Y.; Deng, Z.; Yang, D.; Wang, X.; Yang, S.; Liu, H. Cryopreservation and its effects on spermatozoa quality of Coreius guichenoti. J. Fish. Sci. China 2020, 27, 44–52. [Google Scholar]
- Bustani, G.S.; Baiee, F.H. Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World 2021, 14, 1220–1233. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J. Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biol. Int. 2006, 30, 1–14. [Google Scholar] [CrossRef]
- Morisawa, M.; Suzuki, K.; Shimizu, H.; Morisawa, S.; Yasuda, K. EFFECTS OF OSMOLALITY AND POTASSIUM ON MOTILITY OF SPERMATOZOA FROM FRESH-WATER CYPRINID FISHES. J. Exp. Biol. 1983, 107, 95–103. [Google Scholar] [CrossRef]
- Kawamura, W.; Yazawa, R.; Tani, R.; Takeuchi, Y.; Morita, T.; Yoshikawa, H.; Yoshizaki, G. Development of a simple method for sperm cryopreservation of Scombridae fishes in outdoor environments. Aquac. Res. 2020, 51, 3376–3383. [Google Scholar] [CrossRef]
- Kutluyer, F.; Kayim, M.; Ögretmen, F.; Büyükleblebici, S.; Tuncer, P.B. Cryopreservation of rainbow trout Oncorhynchus mykiss spermatozoa: Effects of extender supplemented with different antioxidants on sperm motility, velocity and fertility. Cryobiology 2014, 69, 462–466. [Google Scholar] [CrossRef]
- Liu, Y.; Torres, L.; Tiersch, T.R. Cryopreservation of sperm bundles (spermatozeugmata) from endangered livebearing goodeids. Cryobiology 2018, 82, 49–56. [Google Scholar] [CrossRef]
- Pereira, F.A.; Corcini, C.D.; da Silva, A.C.; Pires, D.M.; Pereira, J.R.; Gheller, S.M.M.; Streit, D.P.; Maria, A.N.; Varela, A.S. REDUCED GLUTATHIONE AND ATP IN THE SEMINAL CRYOPRESERVATION OF TAMBAQUI. Cryoletters 2018, 39, 371–379. [Google Scholar]
- Psenicka, M.; Dietrich, G.J.; Wojtczak, M.; Nynca, J.; Rodina, M.; Linhart, O.; Cosson, J.; Ciereszko, A. Acrosome staining and motility characteristics of sterlet spermatozoa after cryopreservation with use of methanol and DMSO. Cryobiology 2008, 56, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, S.I.; Kowalska, A.; Morita, M.; Kowalski, R.K. Application of Glucose-Methanol Extender to Cryopreservation of Mozambique Tilapia (Oreochromis mossambicus) Sperm. Turk. J. Fish. Aquat. Sci. 2019, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, M.; Mariola, S.; Sylwia, J.; Halina, K.; Andrzej, C.; Dietrich, M.A. Identification of oxidatively modified proteins due to cryopreservation of carp semen. J. Anim. Sci. 2018, 96, 1453–1465. [Google Scholar] [CrossRef]
- Horokhovatskyi, Y.; Sampels, S.; Cosson, J.; Linhart, O.; Rodina, M.; Fedorov, P.; Blecha, M.; Dzyuba, B. Lipid composition in common carp (Cyprinus carpio) sperm possessing different cryoresistance. Cryobiology 2016, 73, 282–285. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Cryopreservation-induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Mutlu, A.G. Increase in Mitochondrial DNA Copy Number in Response to Ochratoxin A and Methanol-Induced Mitochondrial DNA Damage in Drosophila. Bull. Environ. Contam. Toxicol. 2012, 89, 1129–1132. [Google Scholar] [CrossRef]
- Spikings, E.; Zarnpolla, T.; Rawson, D.; Wang, Y.; Zhang, T. Effect of methanol on mitochondrial organization in zebrafish (Danio rerio) ovarian follicles. Theriogenology 2012, 77, 28–38. [Google Scholar] [CrossRef]
- Thananurak, P.; Chuaychu-noo, N.; Thélie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019, 98, 4161–4171. [Google Scholar] [CrossRef]
- Hu, H.H.; Ji, G.J.; Shi, X.W.; Liu, R.; Zhang, J.; Zhang, H.; Yuan, X.Z.; Zhang, G.Y.; Yuan, W.Z.; Li, M.W. Comparison of rapid freezing versus vitrification for human sperm cryopreservation using sucrose in closed straw systems. Cell Tissue Bank. 2020, 21, 667–673. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, Z.L.; Liu, H.; Li, Q.W. Effect of Salvia miltiorrhiza polysaccharides on boar spermatozoa during freezing-thawing. Anim. Reprod. Sci. 2015, 159, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.B.; Uczay, M.; Brito, V.B.; Fossati, A.A.N.; Godoy, A.C.; Moura, D.J.; Vogel, C.I.G.; Vasconcelos, A.C.N.; Streit, D.P. Skim milk powder used as a non-permeable cryoprotectant reduces oxidative and DNA damage in cryopreserved zebrafish sperm. Cryobiology 2020, 97, 76–84. [Google Scholar] [CrossRef]
- Leclercq, E.; Antoni, L.; Bardon-Albaret, A.; Anderson, C.R.; Somerset, C.R.; Saillant, E.A. Spectrophotometric determination of sperm concentration and short-term cold-storage of sperm in Atlantic croaker Micropogonias undulatus L. broodstock. Aquac. Res. 2014, 45, 1283–1294. [Google Scholar] [CrossRef]
- DeGraaf, J.D.; Berlinsky, D.L. Cryogenic and refrigerated storage of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) spermatozoa. Aquaculture 2004, 234, 527–540. [Google Scholar] [CrossRef]
- Dorsey, K.M.; Guthrie, H.D.; Welch, G.R.; Mohler, J.; Theisen, D.D.; Siewerdt, F.; Vinyard, B.T.; Woods, L.C. Quality Assessment of Wild Atlantic Sturgeon Semen under Conditions of Short-Term Storage. N. Am. J. Aquac. 2011, 73, 418–425. [Google Scholar] [CrossRef]
- Orfao, L.H.; Maria, A.N.; Nascimento, A.F.; Isaú, Z.A.; Viveiros, A.T.M. Sperm fertility of the subtropical freshwater streaked prochilod Prochilodus lineatus (Characiformes) improved after dilution and cold storage. Aquac. Res. 2010, 41, e679–e687. [Google Scholar] [CrossRef]
- Zietara, M.S.; Slominska, E.; Swierczynski, J.; Rurangwa, E.; Ollevier, F.; Skorkowski, E.F. ATP content and adenine nucleotide catabolism in African catfish spermatozoa stored in various energetic substrates. Fish Physiol. Biochem. 2004, 30, 119–127. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dietrich, G.J.; Nynca, J.; Liszewska, E.; Karol, H.; Dobosz, S. The use of concentrated extenders to improve the efficacy of cryopreservation in whitefish spermatozoa. Aquaculture 2013, 408, 30–33. [Google Scholar] [CrossRef]
- Viveiros, A.T.M.; Godinho, H.P. Sperm quality and cryopreservation of Brazilian freshwater fish species: A review. Fish Physiol. Biochem. 2009, 35, 137–150. [Google Scholar] [CrossRef]
- Anthony, A.C.; Kingsley, H.B. Nitrogen vapor immersion: An accessible alternative for honey bee (Apis mellifera L.) semen cryopreservation. Cryobiology 2021, 100, 12–18. [Google Scholar] [CrossRef]
- Oldenhof, H.; Wolkers, W.F.; Sieme, H. Cryopreservation of Semen from Domestic Livestock: Bovine, Equine, and Porcine Sperm. Methods Mol. Biol. 2021, 2180, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.S.; Ruhlmann, C.; Iaizzo, R.S.; Marcial Lopez, C.A.; Martinez, A.G. Comparative analysis between slow freezing and ultra-rapid freezing for human sperm cryopreservation. JBRA Assist. Reprod. 2018, 22, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.G.; Oliveira, I.R.; Serralheiro, P.C.S.; Cerqueira, V.R. Sperm cryopreservation of lane snapper Lutjanus synagris (Linnaeus, 1758). Braz. J. Biol. 2015, 75, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.G.; Oliveira, I.R.; Serralheiro, P.C.S.; Cerqueira, V.R. Cryopreservation of mutton snapper (Lutjanus analis) sperm. An. Acad. Bras. Cienc. 2013, 85, 1083–1091. [Google Scholar] [CrossRef][Green Version]
- Cuevas-Uribe, R.; Leibo, S.P.; Daly, J.; Tiersch, T.R. Production of channel catfish with sperm cryopreserved by rapid non-equilibrium cooling. Cryobiology 2011, 63, 186–197. [Google Scholar] [CrossRef]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.; Riesco, M.F.; Valcarce, D.G.; Sarasquete, C.; Herráez, M.P.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, S.C.; Liu, X.Z.; Xu, Y.Y.; Wang, C.L.; Sawant, M.S.; Li, J.; Chen, S.L. Cryopreservation of flounder (Paralichthys olivaceus) sperm with a practical methodology. Theriogenology 2003, 60, 989–996. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dietrich, G.J.; Nynca, J.; Dobosz, S.; Zalewski, T. Cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 2014, 420, 275–281. [Google Scholar] [CrossRef]
- Figueroa, E.; Farias, J.G.; Lee-Estevez, M.; Valdebenito, I.; Risopatrón, J.; Magnotti, C.; Romero, J.; Watanabe, I.; Oliveira, R.P.S. Sperm cryopreservation with supplementation of α-tocopherol and ascorbic acid in freezing media increase sperm function and fertility rate in Atlantic salmon (Salmo salar). Aquaculture 2018, 493, 1–8. [Google Scholar] [CrossRef]
- Figueroa, E.; Merino, O.; Risopatrón, J.; Isachenko, V.; Sánchez, R.; Effer, B.; Isachenko, E.; Farias, J.G.; Valdebenito, I. Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 2015, 83, 238–245. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Merino, O.; Ubilla, A.; Risopatrón, J.; Farias, J.G. Cryopreservation of Atlantic salmon Salmo salar sperm: Effects on sperm physiology. J. Fish Biol. 2016, 89, 1537–1550. [Google Scholar] [CrossRef]
- Xing, L.-M.; Xiao, W.; Li, L.-L.; Zhang, L.-P.; Sai, Q.-Y.; Yu, Z.-X.; Wu, X.-D.; Lian, Z.-Q. STUDY OF SPERM CRYOPRESERVATION IN SILURUS LANZHOUENSIS AND DETECTION OF CELL DAMAGES AFTER CRYOPRESERVATION. Acta Hydrobiol. Sin. 2021, 45, 547–556. [Google Scholar] [CrossRef]
- Erraud, A.; Bonnard, M.; Cornet, V.; Ben Ammar, I.; Antipine, S.; Peignot, Q.; Lambert, J.; Mandiki, S.N.M.; Kestemont, P. How age, captivity and cryopreservation affect sperm quality and reproductive efficiency in precocious Atlantic salmon (Salmo salar L. 1758). Aquaculture 2021, 544, 737047. [Google Scholar] [CrossRef]
- Kurta, K.; Jeuthe, H.; Naboulsi, R.; de Koning, D.J.; Palaiokostas, C. Seasonal and age-related changes in sperm quality of farmed arctic charr (Salvelinus alpinus). BMC Genom. 2023, 24, 519. [Google Scholar] [CrossRef]
- Xu, H.Y.; Hong, X.Y.; Zhong, C.Y.; Wu, X.L.; Zhu, X.P. Restoring Genetic Resource through In Vitro Culturing Testicular Cells from the Cryo-Preserved Tissue of the American Shad (Alosa sapidissima). Biology 2022, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vazquez, R.; Alvarez, J.G. The sperm chromatin dispersion test:: A simple method for the determination of sperm DNA fragmentation. J. Androl. 2003, 24, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, X.; Ding, S.; Xing, J. Evaluation of sperm mitochondrial function using rh123/PI dual fluorescent staining in asthenospermia and oligoasthenozoospermia. J. Biomed. Res. 2010, 24, 404–410. [Google Scholar] [CrossRef]

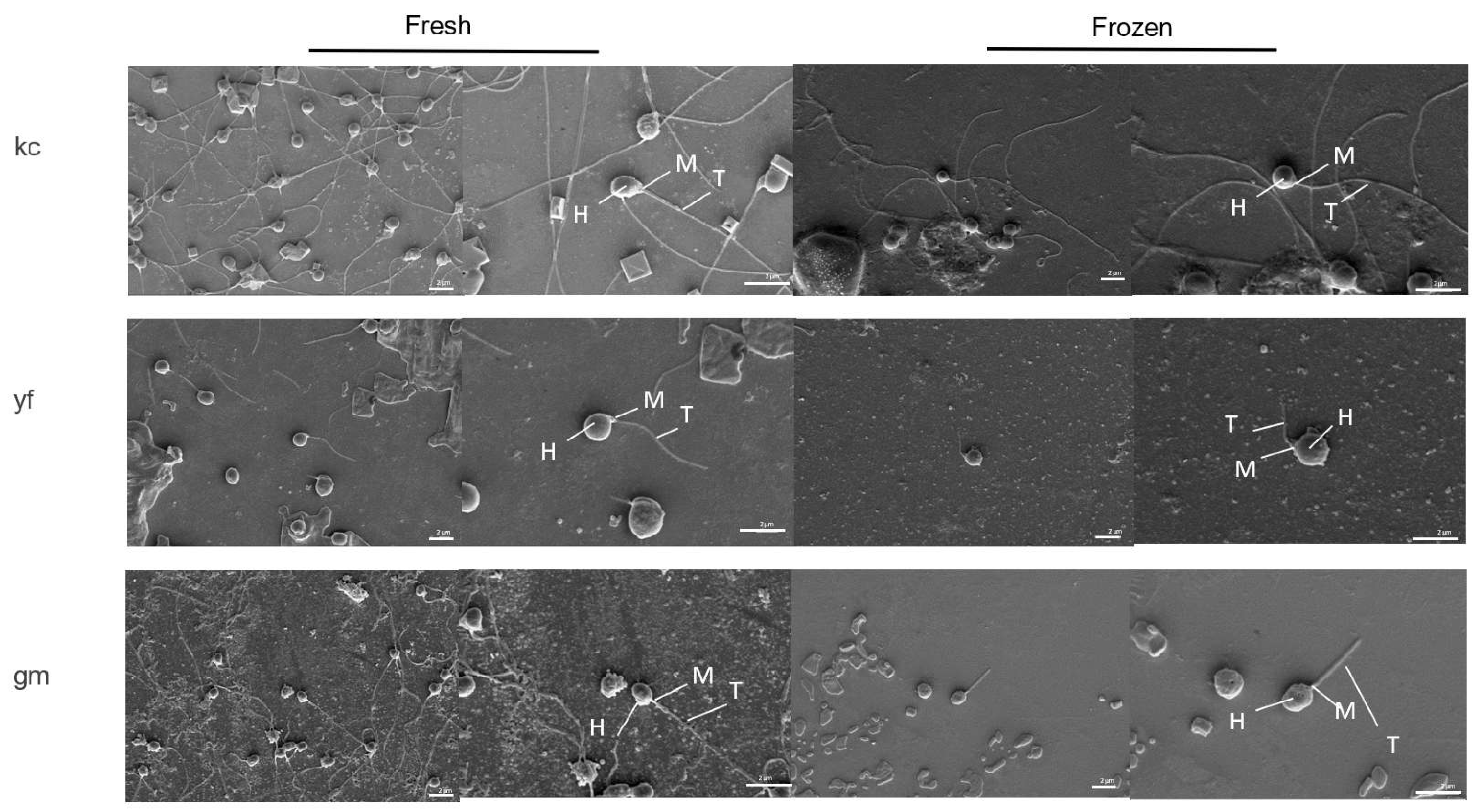
| Characteristics | Head Length (μm) | Head Width (μm) | Midpiece Length (μm) | Midpiece Width (μm) | Tail Length (μm) | Tail Width (μm) | |
|---|---|---|---|---|---|---|---|
| Sperm Specimens | |||||||
| Koi carp (Cyprinus carpio L.) | Fresh | 1.93 ± 0.027 | 1.62 ± 0.17 | 0.63 ± 0.14 | 1.19 ± 0.25 | 37.49 ± 3.58 | 0.36 ± 0.055 |
| Frozen | 2.25 ± 0.32 | 1.96 ± 0.36 | 0.59 ± 0.24 | 1.00 ± 0.20 | 28.48 ± 7.30 | 0.29 ± 0.029 | |
| Glyptosternum maculatum (Regan) | Fresh | 1.44 ± 0.16 | 1.57 ± 0.28 | 0.58 ± 0.12 | 1.56 ± 0.43 | 24.81 ± 2.75 | 0.28 ± 0.013 |
| Frozen | 1.89 | 2.00 | 0.63 | 1.15 | 5.56 | 0.36 | |
| Ya fish (Schizothorax prenanti) | Fresh | 2.33 ± 0.15 | 2.24 ± 0.13 | 0.58 ± 0.13 | 1.20 ± 0.28 | 8.19 ± 2.49 | 0.26 ± 0.042 |
| Frozen | 2.71 ± 0.054 | 2.65 ± 0.28 | 1.27 ± 0.58 | 1.60 ± 0.70 | 5.60 ± 1.77 | 0.35 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Yao, J.; Zeng, L.; Feng, K.; Zhou, C.; Liu, H.; Wang, W.; Zhou, J.; Xu, H. Developing Efficient Methods of Sperm Cryopreservation for Three Fish Species (Cyprinus carpio L., Schizothorax prenanti, Glyptosternum maculatum). Int. J. Mol. Sci. 2025, 26, 4648. https://doi.org/10.3390/ijms26104648
Zhu Z, Yao J, Zeng L, Feng K, Zhou C, Liu H, Wang W, Zhou J, Xu H. Developing Efficient Methods of Sperm Cryopreservation for Three Fish Species (Cyprinus carpio L., Schizothorax prenanti, Glyptosternum maculatum). International Journal of Molecular Sciences. 2025; 26(10):4648. https://doi.org/10.3390/ijms26104648
Chicago/Turabian StyleZhu, Zheng, Jingting Yao, Linghui Zeng, Ke Feng, Chaowei Zhou, Haiping Liu, Wanliang Wang, Jianshe Zhou, and Hongyan Xu. 2025. "Developing Efficient Methods of Sperm Cryopreservation for Three Fish Species (Cyprinus carpio L., Schizothorax prenanti, Glyptosternum maculatum)" International Journal of Molecular Sciences 26, no. 10: 4648. https://doi.org/10.3390/ijms26104648
APA StyleZhu, Z., Yao, J., Zeng, L., Feng, K., Zhou, C., Liu, H., Wang, W., Zhou, J., & Xu, H. (2025). Developing Efficient Methods of Sperm Cryopreservation for Three Fish Species (Cyprinus carpio L., Schizothorax prenanti, Glyptosternum maculatum). International Journal of Molecular Sciences, 26(10), 4648. https://doi.org/10.3390/ijms26104648






