Can Circulating MicroRNAs, Cytokines, and Adipokines Help to Differentiate Psoriatic Arthritis from Erosive Osteoarthritis of the Hand? A Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Study Participants
2.2. MiRNAs, Cytokines, and Adipokines
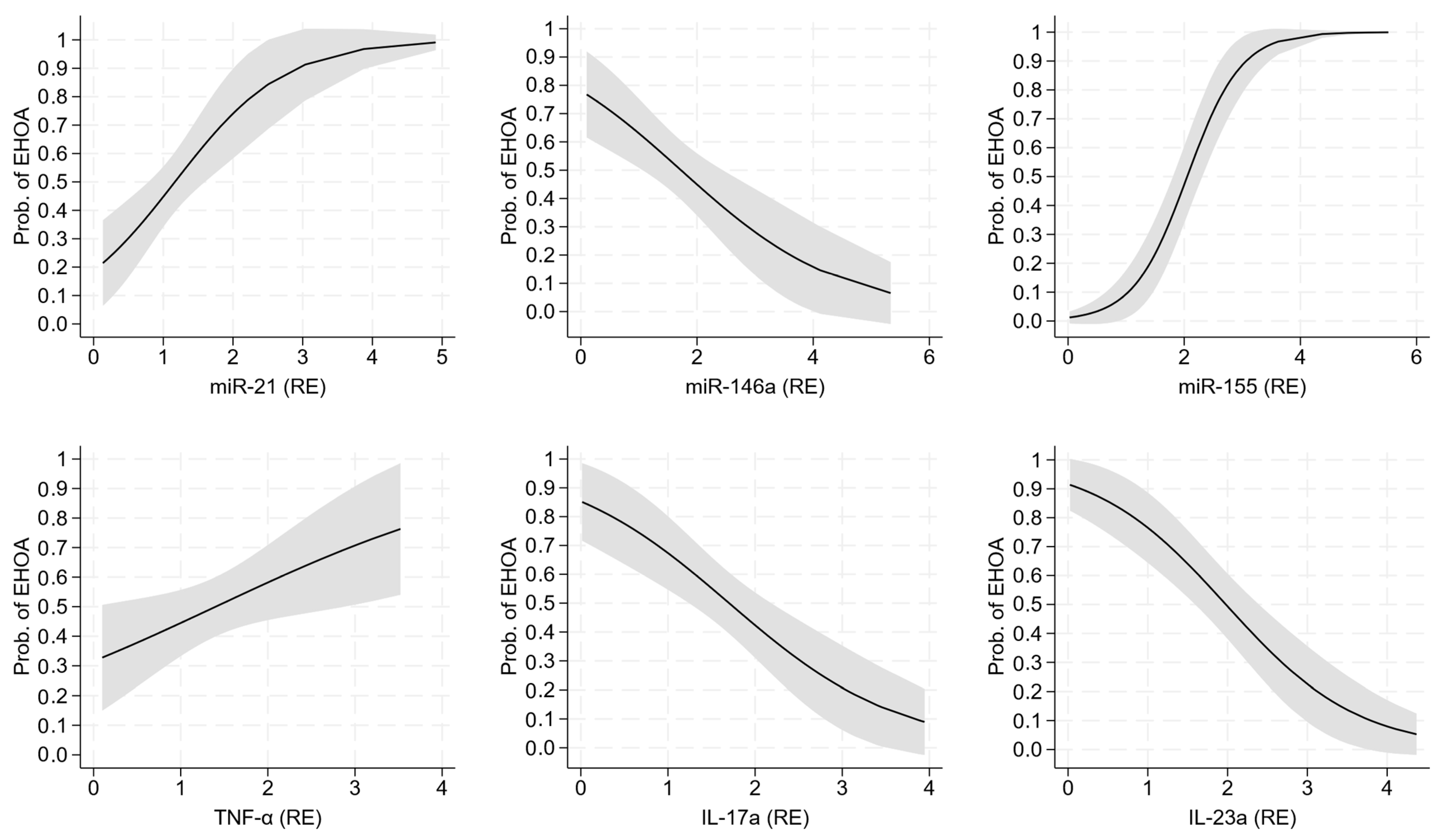
2.3. Diagnostic Performance of miRNA and Cytokine Expression
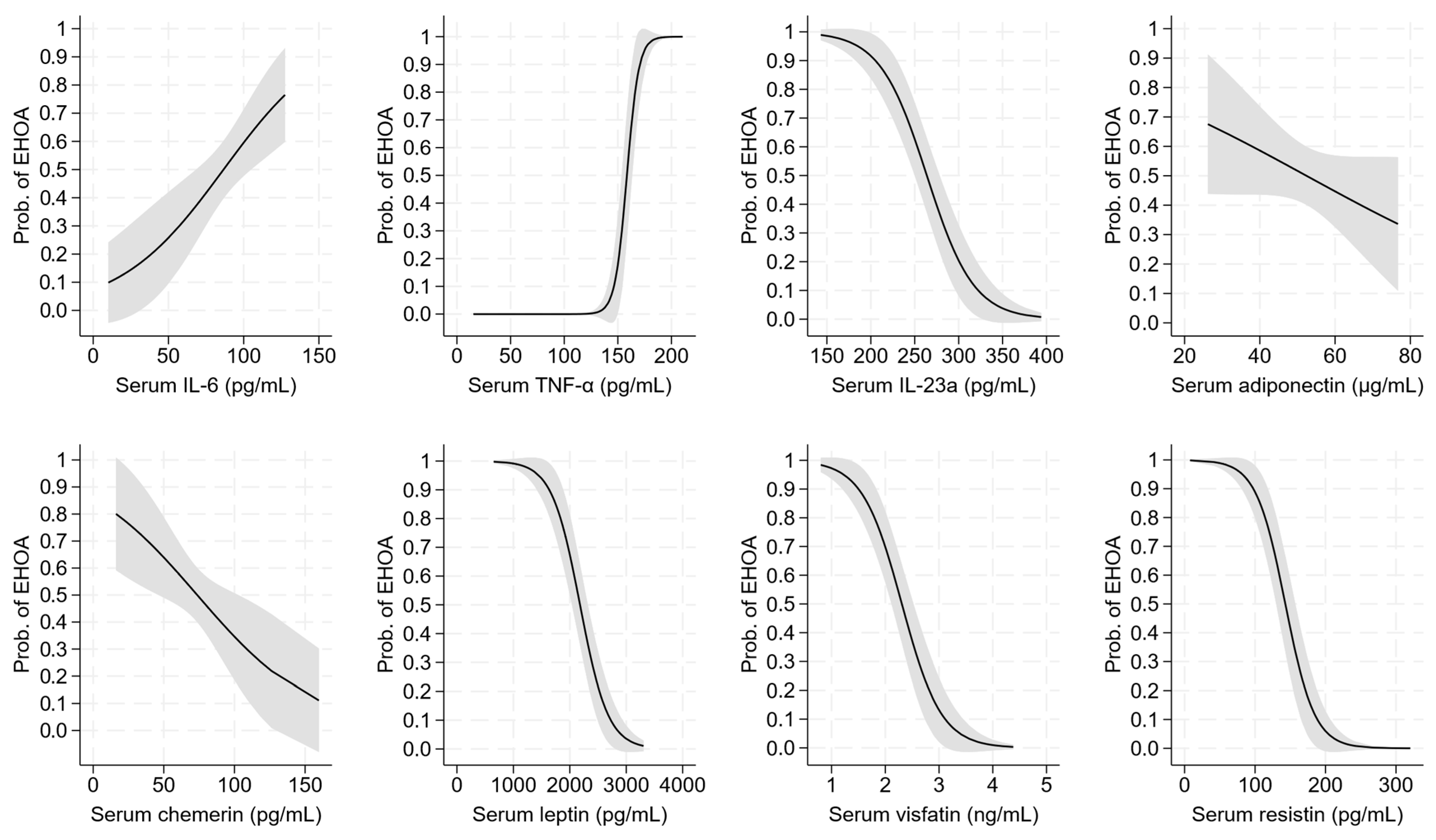
2.4. Diagnostic Performance of Serum Cytokines and Adipokines
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Participants
4.3. Clinical Examination
4.4. Questionnaires and Scales
4.5. Laboratory Analysis
4.6. MiRNA and Cytokine Expression Analysis
4.7. Serum Cytokines and Adipokines
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auroux, M.; Merle, B.; Fontanges, E.; Duvert, F.; Lespessailles, E.; Chapurlat, R. The disability associated with hand osteoarthritis is substantial in a cohort of post-menopausal women: The QUALYOR study. Osteoarthr. Cartil. 2022, 30, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Watt, F.E.; Vincent, T.L.; Dziedzic, K. Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 2018, 14, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Tenti, S.; Ferretti, F.; Gusinu, R.; Gallo, I.; Giannotti, S.; Pozza, A.; Fioravanti, A.; Coluccia, A. Impact of thumb osteoarthritis on pain, function, and quality of life: A comparative study between erosive and non-erosive hand osteoarthritis. Clin. Rheumatol. 2020, 39, 2195–2206. [Google Scholar] [CrossRef]
- Wan, J.; Qian, X.; He, Z.; Zhu, Z.; Cheng, P.; Chen, A. Epidemiological trends of hand osteoarthritis from 1990 to 2019: Estimates from the 2019 Global Burden of Disease study. Front. Med. 2022, 9, 922321. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study Group. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Addimanda, O.; Mancarella, L.; Dolzani, P.; Punzi, L.; Fioravanti, A.; Pignotti, E.; Meliconi, R. Clinical and radiographic distribution of structural damage in erosive and nonerosive hand osteoarthritis. Arthritis Care Res. 2012, 64, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Driban, J.B.; Roberts, M.B.; Duryea, J.; Haugen, I.K.; Schaefer, L.F.; Smith, S.E.; Mathiessen, A.; Eaton, C. Erosive hand osteoarthritis: Incidence and Predictive characteristics among participants in the Osteoarthritis Initiative. Arthritis Rheumatol. 2021, 73, 2015–2024. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Ortolan, A.; Lorenzin, M.; Oliviero, F.; Doria, A.; Scanzello, C.R.; Ramonda, R. Erosive hand osteoarthritis: Latest findings and outlook. Nat. Rev. Rheumatol. 2022, 18, 171–183. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Stefansdottir, L.; Thorleifsson, G.; Stefansson, O.A.; Saevarsdottir, S.; Lund, S.H.; Rafnar, T.; Hoshijima, K.; Novak, K.; Oreiro, N.; et al. Meta-analysis of erosive hand osteoarthritis identifies four common variants that associate with relatively large effect. Ann. Rheum. Dis. 2023, 82, 873–880. [Google Scholar] [CrossRef]
- Marshall, M.; Nicholls, E.; Kwok, W.Y.; Peat, G.; Kloppenburg, M.; van der Windt, D.; Myers, H.; Dziedzic, K. Erosive osteoarthritis: A more severe form of radiographic hand osteoarthritis rather than a distinct entity? Ann. Rheum. Dis. 2015, 74, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Poletto, E.; Tinazzi, I.; Marchetta, A.; Smania, N.; Rossato, E. Hand erosive osteoarthritis and distal interphalangeal involvement in Psoriatic Arthritis:the Place of conservative therapy. J. Clin. Med. 2021, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef]
- Hioki, T.; Komine, M.; Ohtsuki, M. Diagnosis and intervention in early Psoriatic Arthritis. J. Clin. Med. 2022, 11, 2051. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Guldberg-Moller, J.; Mogensen, M.; Ellegaard, K.; Zavareh, A.; Wakefield, R.J.; Tan, A.L.; Boesen, M.; Dehmeshki, J.; Kubassova, O.; Dreyer, L.; et al. Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in psoriatic arthritis (MIDAS): A cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis. RMD Open 2022, 8, e002109. [Google Scholar] [CrossRef]
- Prajzlerova, K.; Senolt, L.; Filkova, M. Is there a potential of circulating miRNAs as biomarkers in rheumatic diseases? Genes Dis. 2023, 10, 1263–1278. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Ali, S.A.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I.; Kapoor, M. The non-coding RNA interactome in joint health and disease. Nat. Rev. Rheumatol. 2021, 17, 692–705. [Google Scholar] [CrossRef]
- Shaikh, F.S.; Siegel, R.J.; Srivastava, A.; Fox, D.A.; Ahmed, S. Challenges and promise of targeting miRNA in rheumatic diseases: A computational approach to identify miRNA association with cell types, cytokines, and disease mechanisms. Front. Immunol. 2023, 14, 1322806. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.M.; McGarry, T.; Wade, S.C.; Fearon, U.; Veale, D.J. Serum MicroRNA Signature as a diagnostic and therapeutic marker in patients with Psoriatic Arthritis. J. Rheumatol. 2020, 47, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Pederzani, A.; Carena, M.C.; Ceribelli, A.; Wordsworth, P.B.; De Santis, M.; Selmi, C.; Vecellio, M. MicroRNAs in Axial Spondylarthritis: An overview of the recent progresses in the field with a focus on Ankylosing Spondylitis and Psoriatic Arthritis. Curr. Rheumatol. Rep. 2021, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Bonek, K.; Kuca Warnawin, E.; Kornatka, A.; Plebanczyk, M.; Burakowski, T.; Maslinski, W.; Wislowska, M.; Gluszko, P.; Ciechomska, M. Circulating miRNA Correlates with lipid profile and disease activity in psoriatic arthritis, rheumatoid arthritis, and ankylosing spondylitis patients. Biomedicines 2022, 10, 893. [Google Scholar] [CrossRef]
- Haschka, J.; Simon, D.; Bayat, S.; Messner, Z.; Kampylafka, E.; Fagni, F.; Skalicky, S.; Hackl, M.; Resch, H.; Zwerina, J.; et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology 2023, 62, 3448–3458. [Google Scholar] [CrossRef]
- Baloun, J.; Pekacova, A.; Svec, X.; Kropackova, T.; Horvathova, V.; Hulejova, H.; Prajzlerova, K.; Ruzickova, O.; Sleglova, O.; Gatterova, J.; et al. Circulating miRNAs in hand osteoarthritis. Osteoarthr. Cartil. 2023, 31, 228–237. [Google Scholar] [CrossRef]
- Auroux, M.; Millet, M.; Merle, B.; Fontanges, E.; Duvert, F.; Gineyts, E.; Rousseau, J.C.; Borel, O.; Mercier-Guery, A.; Lespessailles, E.; et al. Evaluation of circulating microRNA signature in patients with erosive hand osteoarthritis: The HOAmiR study. Osteoarthr. Cartil. 2024, 32, 1452–1462. [Google Scholar] [CrossRef]
- Zapata-Linares, N.; Eymard, F.; Berenbaum, F.; Houard, X. Role of adipose tissues in osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 84–93. [Google Scholar] [CrossRef]
- Toussirot, E. The influence of adipokines on radiographic damage in inflammatory rheumatic diseases. Biomedicines 2023, 11, 536. [Google Scholar] [CrossRef]
- Giardullo, L.; Corrado, A.; Maruotti, N.; Cici, D.; Mansueto, N.; Cantatore, F.P. Adipokine role in physiopathology of inflammatory and degenerative musculoskeletal diseases. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015034. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Cheleschi, S.; De Palma, A.; Addimanda, O.; Mancarella, L.; Pignotti, E.; Pulsatelli, L.; Galeazzi, M.; Meliconi, R. Can adipokines serum levels be used as biomarkers of hand osteoarthritis? Biomarkers 2018, 23, 265–270. [Google Scholar] [CrossRef]
- Feld, J.; Nissan, S.; Eder, L.; Rahat, M.A.; Elias, M.; Rimar, D.; Laor, A.; Bitterman, H.; Zisman, D. Increased prevalence of metabolic syndrome and adipocytokine levels in a psoriatic arthritis cohort. J. Clin. Rheumatol. 2018, 24, 302–307. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Bedogni, G.; Fioravanti, A. Circulating Mir-140 and leptin improve the accuracy of the differential diagnosis between psoriatic arthritis and rheumatoid arthritis: A case-control study. Transl. Res. 2022, 239, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Morozzi, G.; Bellisai, F.; Fioravanti, A.; Galeazzi, M. Absence of anti-cyclic citrullinated peptide antibodies in erosive osteoarthritis: Further serological evidence of the disease as a subset of osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1095–1096. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Simon, D.; Nas, K.; Zaiss, M.M.; Luo, Y.; Zhao, Y.; Rech, J.; Schett, G. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res. Ther. 2020, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Yoshitomi, H.; Tanida, S.; Ishikawa, M.; Nishitani, K.; Ito, H.; Nakamura, T. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010, 12, R86. [Google Scholar] [CrossRef]
- Rocha, F.A.C.; Ali, S.A. Soluble biomarkers in osteoarthritis in 2022: Year in review. Osteoarthr. Cartil. 2023, 31, 167–176. [Google Scholar] [CrossRef]
- Felekkis, K.; Pieri, M.; Papaneophytou, C. Exploring the feasibility of circulating miRNAs as diagnostic and prognostic biomarkers in osteoarthritis: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 13144. [Google Scholar] [CrossRef]
- Millet, M.; Auroux, M.; Beaudart, C.; Demonceau, C.; Ladang, A.; Cavalier, E.; Reginster, J.Y.; Bruyere, O.; Chapurlat, R.; Rousseau, J.C. Association of circulating hsa-miRNAs with sarcopenia: The SarcoPhAge study. Aging Clin. Exp. Res. 2024, 36, 70. [Google Scholar] [CrossRef]
- Ciancio, G.; Ferracin, M.; Saccenti, E.; Bagnari, V.; Farina, I.; Furini, F.; Galuppi, E.; Zagatti, B.; Trotta, F.; Negrini, M.; et al. Characterisation of peripheral blood mononuclear cell microRNA in early onset psoriatic arthritis. Clin. Exp. Rheumatol. 2017, 35, 113–121. [Google Scholar]
- Pelosi, A.; Lunardi, C.; Fiore, P.F.; Tinazzi, E.; Patuzzo, G.; Argentino, G.; Moretta, F.; Puccetti, A.; Dolcino, M. MicroRNA expression profiling in psoriatic arthritis. BioMed Res. Int. 2018, 2018, 7305380. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Ho, J.C.; Li, S.C.; Chen, J.F.; Hsiao, C.C.; Lee, C.H. MiR-146a-5p Expression in peripheral CD14(+) monocytes from patients with Psoriatic Arthritis induces osteoclast activation, bone resorption, and correlates with clinical response. J. Clin. Med. 2019, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.M.; Trenkmann, M.; McGarry, T.; Canavan, M.; Marzaioli, V.; Wade, S.C.; Veale, D.J.; Fearon, U. Altered expression of microRNA-23a in psoriatic arthritis modulates synovial fibroblast pro-inflammatory mechanisms via phosphodiesterase 4B. J. Autoimmun. 2019, 96, 86–93. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z. Role of miRNAs in the pathogenesis of psoriasis and psoriatic arthritis: A genome-wide Mendelian randomization study. Clin. Rheumatol. 2025, 44, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.D.; Feng, S.Y.; Huang, A.F. Role of miR-155 in inflammatory autoimmune diseases: A comprehensive review. Inflamm. Res. 2022, 71, 1501–1517. [Google Scholar] [CrossRef]
- Li, G.; Xiu, L.; Li, X.; Ma, L.; Zhou, J. miR-155 inhibits chondrocyte pyroptosis in knee osteoarthritis by targeting SMAD2 and inhibiting the NLRP3/Caspase-1 pathway. J. Orthop. Surg. Res. 2022, 17, 48. [Google Scholar] [CrossRef]
- D’Adamo, S.; Alvarez-Garcia, O.; Muramatsu, Y.; Flamigni, F.; Lotz, M.K. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr. Cartil. 2016, 24, 1082–1091. [Google Scholar] [CrossRef]
- Li, S.H.; Wu, Q.F. MicroRNAs target on cartilage extracellular matrix degradation of knee osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1185–1197. [Google Scholar] [CrossRef]
- Okuhara, A.; Nakasa, T.; Shibuya, H.; Niimoto, T.; Adachi, N.; Deie, M.; Ochi, M. Changes in microRNA expression in peripheral mononuclear cells according to the progression of osteoarthritis. Mod. Rheumatol. 2012, 22, 446–457. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Ozgen, M.; Turgut Cosan, D.; Colak, E.; Gunes, H.V. miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene 2017, 627, 207–211. [Google Scholar] [CrossRef]
- Giannitti, C.; De Palma, A.; Pascarelli, N.A.; Cheleschi, S.; Giordano, N.; Galeazzi, M.; Fioravanti, A. Can balneotherapy modify microRNA expression levels in osteoarthritis? A comparative study in patients with knee osteoarthritis. Int. J. Biometeorol. 2017, 61, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef]
- Li, X.; Tian, F.; Wang, F. Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-alpha and IL-1beta in PBMCs. Int. J. Mol. Sci. 2013, 14, 23910–23921. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.M.; Fu, B.L.; Xu, L.L.; Wang, B. MicroRNA-21: An Emerging Player in Bone Diseases. Front. Pharmacol. 2021, 12, 722804. [Google Scholar] [CrossRef]
- Mohebbi, M.; Atabaki, M.; Tavakkol-Afshari, J.; Shariati-Sarabi, Z.; Poursamimi, J.; Mohajeri, S.A.; Mohammadi, M. Significant Effect of Crocin on the Gene Expression of MicroRNA-21 and MicroRNA-155 in Patients with Osteoarthritis. Iran. J. Allergy Asthma Immunol. 2022, 21, 322–331. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhao, F.C.; Yi, L.H.; Tang, J.L.; Zhu, Z.Y.; Pang, Y.; Chen, Y.S.; Li, D.Y.; Guo, K.J.; Zheng, X. MicroRNA-21-5p as a novel therapeutic target for osteoarthritis. Rheumatology 2019, 58, 1485–1497. [Google Scholar] [CrossRef]
- Hoshikawa, N.; Sakai, A.; Takai, S.; Suzuki, H. Targeting Extracellular miR-21-TLR7 Signaling Provides Long-Lasting Analgesia in Osteoarthritis. Mol. Ther. Nucleic Acids 2020, 19, 199–207. [Google Scholar] [CrossRef]
- Konteles, V.; Papathanasiou, I.; Tzetis, M.; Kriebardis, A.; Tsezou, A. Synovial Fibroblast Extracellular Vesicles Induce Inflammation via Delivering miR-21-5p in Osteoarthritis. Cells 2025, 14, 519. [Google Scholar] [CrossRef]
- Fioravanti, A.; Tenti, S.; McAllister, M.; Chemaly, M.; Eakin, A.; McLaughlin, J.; Bjourson, A.J.; Frati, E.; McGilligan, V.; Cheleschi, S.; et al. Exploring the involvement of NLRP3 and IL-1beta in osteoarthritis of the hand: Results from a pilot study. Mediat. Inflamm. 2019, 2019, 2363460. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Ramonda, R.; Scanu, A.; Galozzi, P.; Favero, M.; Punzi, L. Levels of inflammatory cytokines and metalloproteinases are increased in knee synovial fluid of patients with concomitant erosive hand osteoarthritis. Clin. Exp. Rheumatol. 2020, 38, 800. [Google Scholar] [PubMed]
- McAlindon, T.E.; Hunnicutt, J.L.; Roberts, M.B.; Haugen, I.K.; Schaefer, L.F.; Driban, J.B.; Lu, B.; Duryea, J.; Smith, S.E.; Booth, S.L.; et al. Associations of inflammatory and metabolic biomarkers with incident erosive hand osteoarthritis in the osteoarthritis initiative cohort. Osteoarthr. Cartil. 2024, 32, 592–600. [Google Scholar] [CrossRef]
- Haugen, I.K.; Mathiessen, A.; Slatkowsky-Christensen, B.; Magnusson, K.; Boyesen, P.; Sesseng, S.; van der Heijde, D.; Kvien, T.K.; Hammer, H.B. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: Is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthr. Cartil. 2016, 24, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Tenti, S.; Bruyère, O.; Cheleschi, S.; Reginster, J.Y.; Veronese, N.; Fioravanti, A. An update on the use of conventional and biological disease-modifying anti-rheumatic drugs in hand osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231158618. [Google Scholar] [CrossRef]
- Neurath, L.; Sticherling, M.; Schett, G.; Fagni, F. Targeting cytokines in psoriatic arthritis. Cytokine Growth Factor Rev. 2024, 78, 1–13. [Google Scholar] [CrossRef]
- Dikbas, O.; Tosun, M.; Bes, C.; Tonuk, S.B.; Aksehirli, O.Y.; Soy, M. Serum levels of visfatin, resistin and adiponectin in patients with psoriatic arthritis and associations with disease severity. Int. J. Rheum. Dis. 2016, 19, 672–677. [Google Scholar] [CrossRef]
- Toussirot, E.; Aubin, F.; Dumoulin, G. Relationships between adipose tissue and psoriasis, with or without arthritis. Front. Immunol. 2014, 5, 368. [Google Scholar] [CrossRef]
- Economou, A.; Mallia, I.; Fioravanti, A.; Gentileschi, S.; Nacci, F.; Bellando Randone, S.; Lepri, G.; Guiducci, S. The role of adipokines between genders in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2024, 25, 10865. [Google Scholar] [CrossRef]
- Houttekiet, C.; de Vlam, K.; Neerinckx, B.; Lories, R. Systematic review of the use of CRP in clinical trials for psoriatic arthritis: A concern for clinical practice? RMD Open 2022, 8, e001756. [Google Scholar] [CrossRef]
- Punzi, L.; Ramonda, R.; Oliviero, F.; Sfriso, P.; Mussap, M.; Plebani, M.; Podswiadek, M.; Todesco, S. Value of C reactive protein in the assessment of erosive osteoarthritis of the hand. Ann. Rheum. Dis. 2005, 64, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Filkova, M.; Senolt, L.; Braun, M.; Hulejova, H.; Pavelkova, A.; Sleglova, O.; Kupka, K.; Gatterova, J.; Pavelka, K. Serum hyaluronic acid as a potential marker with a predictive value for further radiographic progression of hand osteoarthritis. Osteoarthr. Cartil. 2009, 17, 1615–1619. [Google Scholar] [CrossRef]
- Sackett, D.L.; Haynes, R.B. The architecture of diagnostic research. BMJ 2002, 324, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef]
- Tenti, S.; Veronese, N.; Cheleschi, S.; Seccafico, I.; Bruyere, O.; Reginster, J.Y.; Fioravanti, A. Prescription-grade crystalline glucosamine sulfate as an add-on therapy to conventional treatments in erosive osteoarthritis of the hand: Results from a 6-month observational retrospective study. Aging Clin. Exp. Res. 2022, 34, 1613–1625. [Google Scholar] [CrossRef]
- Kallman, D.A.; Wigley, F.M.; Scott, W.W.J.; Hochberg, M.C.; Tobin, J.D. New radiographic grading scales for osteoarthritis of the hand. Reliability for determining prevalence and progression. Arthritis Rheum. 1989, 32, 1584–1591. [Google Scholar] [CrossRef]
- Saviola, G.; Abdi-Ali, L.; Povino, M.R.; Campostrini, L.; Sacco, S.; Dalle Carbonare, L.; Carbonare, L.D. Intramuscular clodronate in erosive osteoarthritis of the hand is effective on pain and reduces serum COMP: A randomized pilot trial-The ER.O.D.E. study (ERosive Osteoarthritis and Disodium-clodronate Evaluation). Clin. Rheumatol. 2017, 36, 2343–2350. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1991. [Google Scholar]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S.
- Ranza, R.; Marchesoni, A.; Calori, G.; Bianchi, G.; Braga, M.; Canazza, S.; Canesi, B.; Fumagalli, M.; Mastaglio, C.; Mathieu, A.; et al. The Italian version of the Functional Disability Index of the Health Assessment Questionnaire. A reliable instrument for multicenter studies on rheumatoid arthritis. Clin. Exp. Rheumatol. 1993, 11, 123–128. [Google Scholar]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef]
- Gandini, F.; Giannitti, C.; Fattore, G.; Giordano, N.; Galeazzi, M.; Fioravanti, A. Validation of an Italian version of the functional index for hand osteoarthritis (FIHOA). Mod. Rheumatol. 2012, 2, 758–765. [Google Scholar] [CrossRef]
- Acosta Felquer, M.L.; Ferreyra Garrott, L.; Marin, J.; Catay, E.; Scolnik, M.; Scaglioni, V.; Ruta, S.; Rosa, J.; Soriano, E.R. Remission criteria and activity indices in psoriatic arthritis. Clin. Rheumatol. 2014, 33, 1323–1330. [Google Scholar] [CrossRef]
- Feldman, S.R.; Krueger, G.G. Psoriasis assessment tools in clinical trials. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii65–ii68; discussion ii69. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Corkum, C.P.; Ings, D.P.; Burgess, C.; Karwowska, S.; Kroll, W.; Michalak, T.I. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT™) and standard density gradient. BMC Immunol. 2015, 16, 48. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034.1. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Royston, P.; Sauerbrei, W. Multivariable Model—Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables; Wiley: Chichester, UK, 2008. [Google Scholar]
| HS (n = 50) | EHOA (n = 50) | PsA (n = 50) | p-Value * HS vs. EHOA | p-Value * EHOA vs. PsA | p-Value * HS vs. PsA | |
|---|---|---|---|---|---|---|
| Female sex | 31 (62%) | 40 (80%) | 28 (56%) | 0.047 | 0.010 | 0.54 |
| Age (years) | 48 (40;59) | 68 (65;73) | 58 (55;63) | <0.001 | <0.001 | <0.001 |
| Disease (months) | — | 96 (60;120) | 72 (48;96) | — | 0.014 | — |
| BMI (kg/m2) | 23.8 (21.9;25.2) | 24.8 (22.5;26.3) | 25.0 (23.2;26.8) | 0.042 | 0.45 | 0.004 |
| Smoking | 13 (26%) | 4 (8%) | 19 (38%) | 0.017 | <0.001 | 0.20 |
| Hypertension | 12 (24%) | 24 (48%) | 21 (42%) | 0.012 | 0.55 | 0.056 |
| Cardiovascular disease | 10 (20%) | 9 (18%) | 18 (36%) | 0.80 | 0.043 | 0.075 |
| Type 2 diabetes mellitus | 0 (0%) | 7 (14%) | 12 (24%) | 0.006 | 0.20 | <0.001 |
| Glucose (mg/dL) | 87 (78;95) | 95 (88;101) | 90 (85;97) | 0.001 | 0.016 | 0.15 |
| Total cholesterol (mg/dL) | 180 (165;195) | 210 (183;233) | 182 (175;195) | <0.001 | <0.001 | 0.38 |
| HDL cholesterol (mg/dL) | 60 (52;65) | 60 (51;69) | 50 (42;57) | 0.84 | <0.001 | <0.001 |
| LDL cholesterol | 99 (85;111) | 140 (117;151) | 105 (98;120) | <0.001 | <0.001 | 0.005 |
| Triglycerides (mg/dL) | 110 (88;134) | 130 (91;156) | 120 (98;138) | 0.047 | 0.41 | 0.14 |
| ESR (mm/h) | 12 (8;18) | 15 (9;24) | 35 (25;42) | 0.062 | <0.001 | <0.001 |
| CRP (mg/dL) | 0.1 (0.0;0.1) | 0.2 (0.1;0.5) | 0.9 (0.7;1.4) | <0.001 | <0.001 | <0.001 |
| VAS pain (0–100) | — | 50 (20;70) | 38 (25;60) | — | 0.079 | |
| HAQ | 0 (0;0) | 1 (0;1) | 1 (0;1) | <0.001 | 0.41 | <0.001 |
| Tender joints (number) | — | 7 (5;8) | 8 (4;12) | — | 0.12 | — |
| Swollen joints (number) | — | 4 (2;6) | 2 (1;3) | — | <0.001 | — |
| Kallman score | — | 122 (98;150) | — | — | — | — |
| DAPSA-CRP | — | — | 18 (14;29) | — | — | — |
| DAS28-ESR | — | — | 5 (4;6) | — | — | — |
| PASI | — | — | 6 (4;10) | — | — | — |
| HS (n = 50) | EHOA (n = 50) | PsA (n = 50) | p-Value HS vs. EHOA * | p-Value EHOA vs. PsA * | p-Value HS vs. PsA * | |
|---|---|---|---|---|---|---|
| miR-21 (RE) | 0.43 (0.27;0.81) | 1.19 (0.89;1.84) | 0.92 (0.57;1.28) | <0.001 | 0.001 | <0.001 |
| miR-140 (RE) | 0.73 (0.48;1.12) | 1.75 (0.94;2.04) | 1.55 (0.95;2.10) | <0.001 | 0.58 | <0.001 |
| miR-146a (RE) | 0.74 (0.28;0.93) | 1.13 (0.75;1.98) | 1.86 (1.50;2.54) | <0.001 | <0.001 | <0.001 |
| miR-155 (RE) | 0.56 (0.30;0.75) | 2.76 (2.12;3.23) | 1.39 (0.92;1.84) | <0.001 | <0.001 | <0.001 |
| miR-181b (RE) | 0.80 (0.70;0.95) | 3.43 (2.54;4.07) | 2.97 (2.05;4.18) | <0.001 | 0.11 | <0.001 |
| miR-223 (RE) | 1.19 (0.82;1.48) | 2.30 (1.68;2.89) | 2.44 (1.82;3.04) | <0.001 | 0.67 | <0.001 |
| IL-1β (RE) | 0.82 (0.42;0.98) | 1.03 (0.83;1.84) | 1.31 (0.92;1.76) | <0.001 | 0.59 | <0.001 |
| IL-6 (RE) | 0.31 (0.22;0.52) | 1.10 (0.66;2.03) | 1.58 (0.93;2.02) | <0.001 | 0.32 | <0.001 |
| IL-17a (RE) | 0.27 (0.15;0.41) | 1.19 (0.89;1.93) | 2.03 (1.46;2.40) | <0.001 | <0.001 | <0.001 |
| IL-23a (RE) | 0.30 (0.18;0.52) | 1.21 (0.90;2.04) | 2.31 (1.80;3.24) | <0.001 | <0.001 | <0.001 |
| TNF-α (RE) | 0.57 (0.29;0.94) | 1.49 (0.90;2.02) | 1.03 (0.80;1.79) | <0.001 | 0.047 | <0.001 |
| IL1-β (pg/mL) | 16.6 (12.1;23.1) | 22.2 (18.4;26.9) | 21.9 (16.9;27.1) | <0.001 | 0.43 | 0.002 |
| IL-6 (pg/mL) | 23.4 (17.2;30.7) | 94.7 (71.1;110.3) | 79.6 (66.1;100.1) | <0.001 | 0.004 | <0.001 |
| IL-17a (pg/mL) | 46.3 (37.6;55.3) | 70.0 (58.4;79.2) | 71.8 (59.7;81.9) | <0.001 | 0.44 | <0.001 |
| IL-23a (pg/mL) | 188.5 (171.0;201.0) | 230.5 (195.0;266.0) | 308.0 (256.0;338.0) | <0.001 | <0.001 | <0.001 |
| TNF-α (pg/mL) | 26.4 (20.8;31.8) | 187.8 (172.3;196.9) | 130.3 (122.9;141.9) | <0.001 | <0.001 | <0.001 |
| Adiponectin (μg/mL) | 43.73 (36.13;50.54) | 49.49 (43.81;58.67) | 51.33 (44.58;61.17) | <0.001 | 0.30 | <0.001 |
| Chemerin (pg/mL) | 34.82 (29.29;44.55) | 61.40 (54.63;83.34) | 74.44 (67.44;85.96) | <0.001 | 0.002 | <0.001 |
| Leptin (pg/mL) | 1716.12 (1492.12;1834.22) | 1737.39 (1260.28;2033.62) | 2637.39 (2282.95;2836.6) | 0.35 | <0.001 | <0.001 |
| Resistin (pg/mL) | 74.87 (58.14;89.13) | 95.64 (79.61;112.37) | 198.08 (174.09;230.83) | <0.001 | <0.001 | <0.001 |
| Visfatin (ng/mL) | 1.88 (1.43;2.38) | 1.78 (1.60;2.11) | 2.95 (2.30;3.56) | 0.35 | <0.001 | <0.001 |
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| miR-21 (RE) | 1.26 ** [0.46, 2.06] | — | — | — | — | — | — | — | — | — | — |
| miR-140 (RE) | — | 0.23 [−0.36, 0.81] | — | — | — | — | — | — | — | — | — |
| miR-146a (RE) | — | — | −0.74 ** [−1.21, −0.26] | — | — | — | — | — | — | — | — |
| miR-155 (RE) | — | — | — | 2.16 *** [1.35, 2.97] | — | — | — | — | — | — | — |
| miR-181b (RE) | — | — | — | — | 0.27 [−0.10, 0.63] | — | — | — | — | — | — |
| miR-223 (RE) | — | — | — | — | — | −0.11 [−0.60, 0.37] | — | — | — | — | — |
| IL-1β (RE) | — | — | — | — | — | — | −0.14 [−0.77, 0.49] | — | — | — | — |
| IL-6 (RE) | — | — | — | — | — | — | — | −0.11 [−0.59, 0.36] | — | — | — |
| TNF-α (RE) | — | — | — | — | — | — | — | — | 0.55 * [0.01, 1.10] | — | — |
| IL-17a (RE) | — | — | — | — | — | — | — | — | — | −1.03 *** [−1.62, −0.45] | — |
| IL-23a (RE) | — | — | — | — | — | — | — | — | — | — | −1.21 *** [−1.76, −0.66] |
| Intercept | −1.47 ** [−2.46, −0.48] | −0.35 [−1.34, 0.64] | 1.27 ** [0.38, 2.16] | −4.44 *** [−6.15, −2.73] | −0.85 [−2.09, 0.38] | 0.27 [−0.94, 1.47] | 0.20 [−0.75, 1.14] | 0.16 [−0.61, 0.93] | −0.77 [−1.63, 0.08] | 1.76 ** [0.69, 2.83] | 2.39 *** [1.24, 3.54] |
| N | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| AIC | 130 | 142 | 131 | 89 | 141 | 142 | 142 | 142 | 138 | 128 | 117 |
| BIC | 135 | 147 | 137 | 94 | 146 | 148 | 148 | 148 | 144 | 133 | 122 |
| C-statistic | 0.69 | 0.53 | 0.72 | 0.89 | 0.59 | 0.52 | 0.53 | 0.56 | 0.62 | 0.74 | 0.79 |
| R2 | 0.16 | 0.01 | 0.14 | 0.55 | 0.03 | 0.00 | 0.00 | 0.00 | 0.05 | 0.18 | 0.30 |
| M12 | M13 | M14 | M15 | M16 | M17 | M18 | M19 | M20 | M21 | |
|---|---|---|---|---|---|---|---|---|---|---|
| IL1-β (pg/mL) | 0.01 [−0.05, 0.07] | |||||||||
| IL-6 (pg/mL) | 0.03 ** [0.01, 0.05] | |||||||||
| TNF-α (pg/mL) | 0.18 *** [0.09, 0.27] | |||||||||
| IL-17a (pg/mL) | −0.01 [−0.04, 0.01] | |||||||||
| IL-23a (pg/mL) | −0.04 *** [−0.05, −0.02] | |||||||||
| Adiponectin (μg/mL) | −0.03 [−0.07, 0.01] | |||||||||
| Chemerin (pg/mL) | −0.02 * [−0.05, −0.01] | |||||||||
| Leptin (pg/mL) | −0.004 *** [−0.006, −0.003] | |||||||||
| Visfatin (ng/mL) | −2.72 *** [−3.77, −1.68] | |||||||||
| Resistin (pg/mL) | −0.05 *** [−0.07, −0.03] | |||||||||
| Intercept | −0.25 [−1.61, 1.11] | −2.50 ** [−4.31, −0.70] | −28.72 *** [−42.49, −14.95] | 0.98 [−1.04, 3.00] | 9.90 *** [6.07, 13.72] | 1.47 [−0.59, 3.52] | 1.78 * [0.13, 3.43] | 8.78 *** [5.39, 12.17] | 6.29 *** [3.90, 8.68] | 6.91 *** [4.47, 9.34] |
| N | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| AIC | 142 | 134 | 34 | 142 | 92 | 141 | 137 | 81 | 86 | 63 |
| BIC | 148 | 139 | 39 | 147 | 97 | 146 | 143 | 86 | 91 | 68 |
| C-statistic | 0.55 | 0.67 | 0.98 | 0.54 | 0.87 | 0.56 | 0.68 | 0.91 | 0.89 | 0.95 |
| Nagelkerke R2 | 0.00 | 0.11 | 0.88 | 0.01 | 0.53 | 0.03 | 0.07 | 0.61 | 0.58 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioravanti, A.; Cheleschi, S.; Cavalier, E.; Reginster, J.-Y.; Alokail, M.; Ladang, A.; Tenti, S.; Bedogni, G. Can Circulating MicroRNAs, Cytokines, and Adipokines Help to Differentiate Psoriatic Arthritis from Erosive Osteoarthritis of the Hand? A Case–Control Study. Int. J. Mol. Sci. 2025, 26, 4621. https://doi.org/10.3390/ijms26104621
Fioravanti A, Cheleschi S, Cavalier E, Reginster J-Y, Alokail M, Ladang A, Tenti S, Bedogni G. Can Circulating MicroRNAs, Cytokines, and Adipokines Help to Differentiate Psoriatic Arthritis from Erosive Osteoarthritis of the Hand? A Case–Control Study. International Journal of Molecular Sciences. 2025; 26(10):4621. https://doi.org/10.3390/ijms26104621
Chicago/Turabian StyleFioravanti, Antonella, Sara Cheleschi, Etienne Cavalier, Jean-Yves Reginster, Majed Alokail, Aurélie Ladang, Sara Tenti, and Giorgio Bedogni. 2025. "Can Circulating MicroRNAs, Cytokines, and Adipokines Help to Differentiate Psoriatic Arthritis from Erosive Osteoarthritis of the Hand? A Case–Control Study" International Journal of Molecular Sciences 26, no. 10: 4621. https://doi.org/10.3390/ijms26104621
APA StyleFioravanti, A., Cheleschi, S., Cavalier, E., Reginster, J.-Y., Alokail, M., Ladang, A., Tenti, S., & Bedogni, G. (2025). Can Circulating MicroRNAs, Cytokines, and Adipokines Help to Differentiate Psoriatic Arthritis from Erosive Osteoarthritis of the Hand? A Case–Control Study. International Journal of Molecular Sciences, 26(10), 4621. https://doi.org/10.3390/ijms26104621







