N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice
Abstract
1. Introduction
2. Results
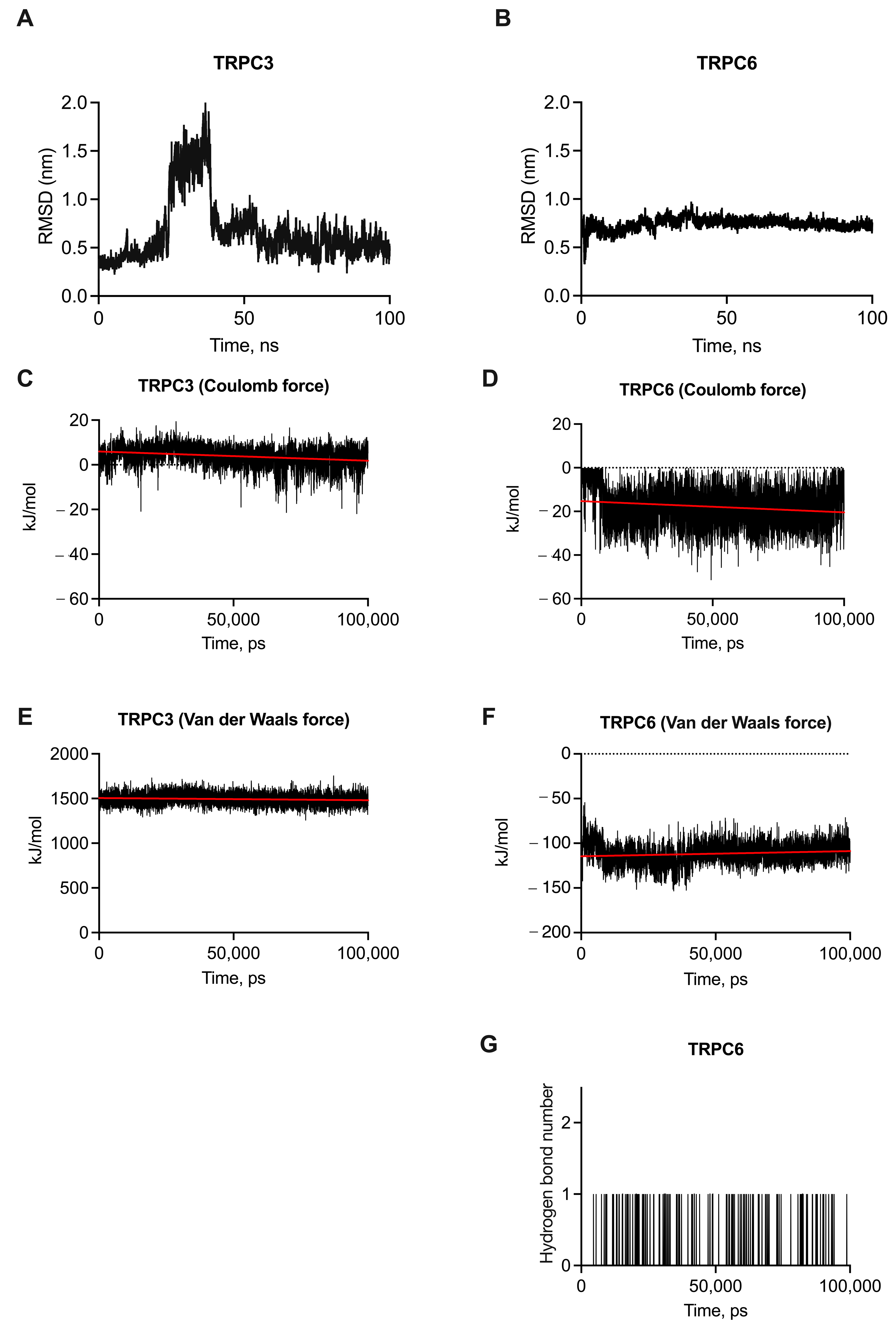
2.1. Molecular Dynamic Simulation of Cmp2 with Tetrameric TRPC3 and TRPC6
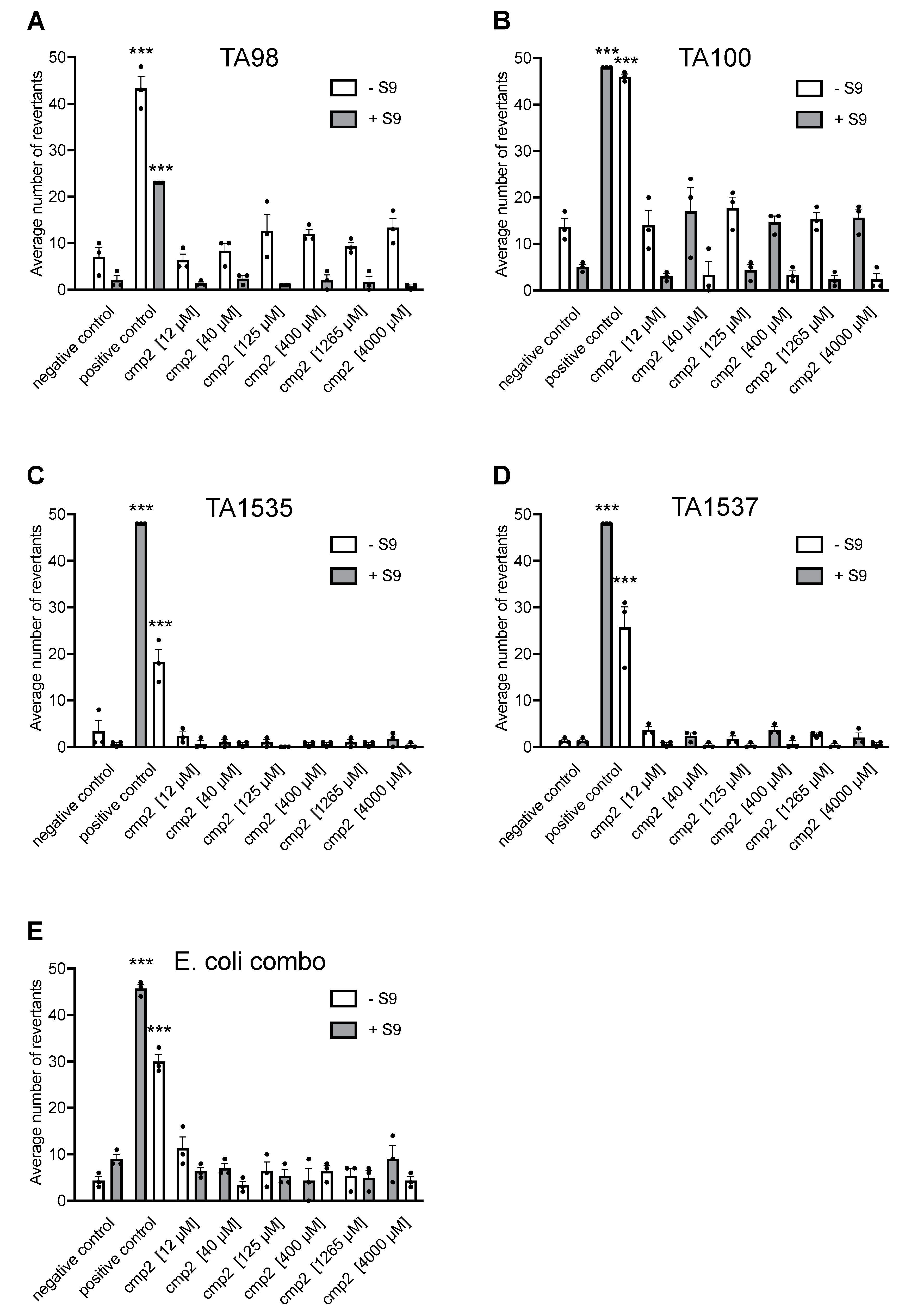
2.2. Cmp2 Is Not Mutagenic upon AMES Testing
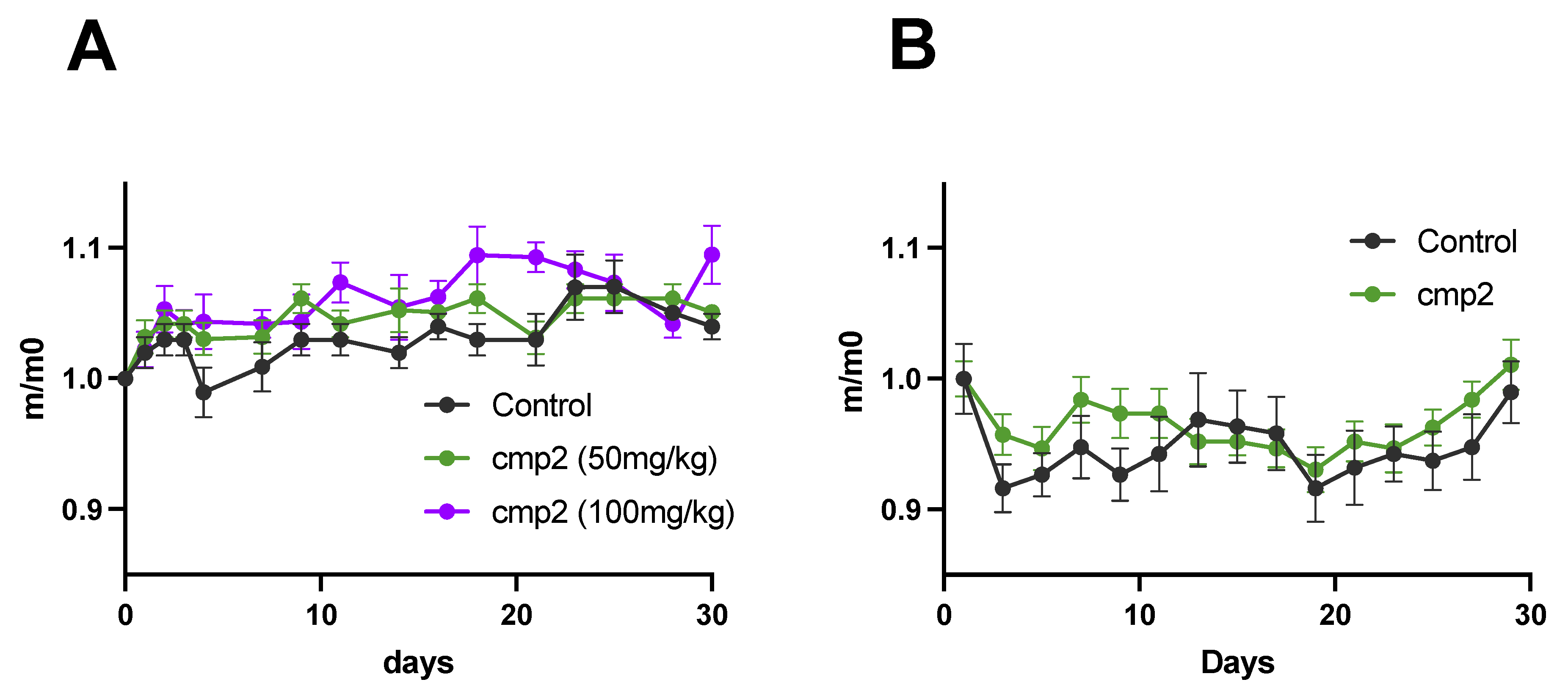
2.3. Cmp2 Does Not Demonstrate Toxic Effects at 50 and 100 mg/kg Doses During Acute Administration
2.4. Cmp2 Does Not Demonstrate Toxic Effects at a 10 mg/kg Dose During Chronic Administration
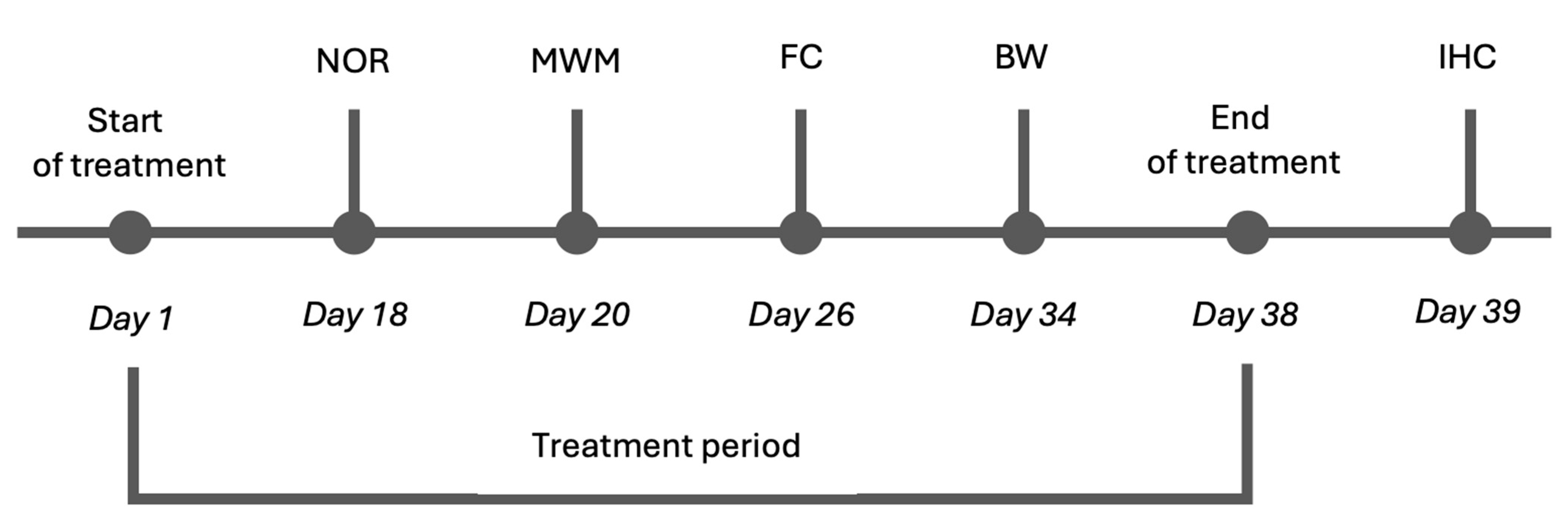
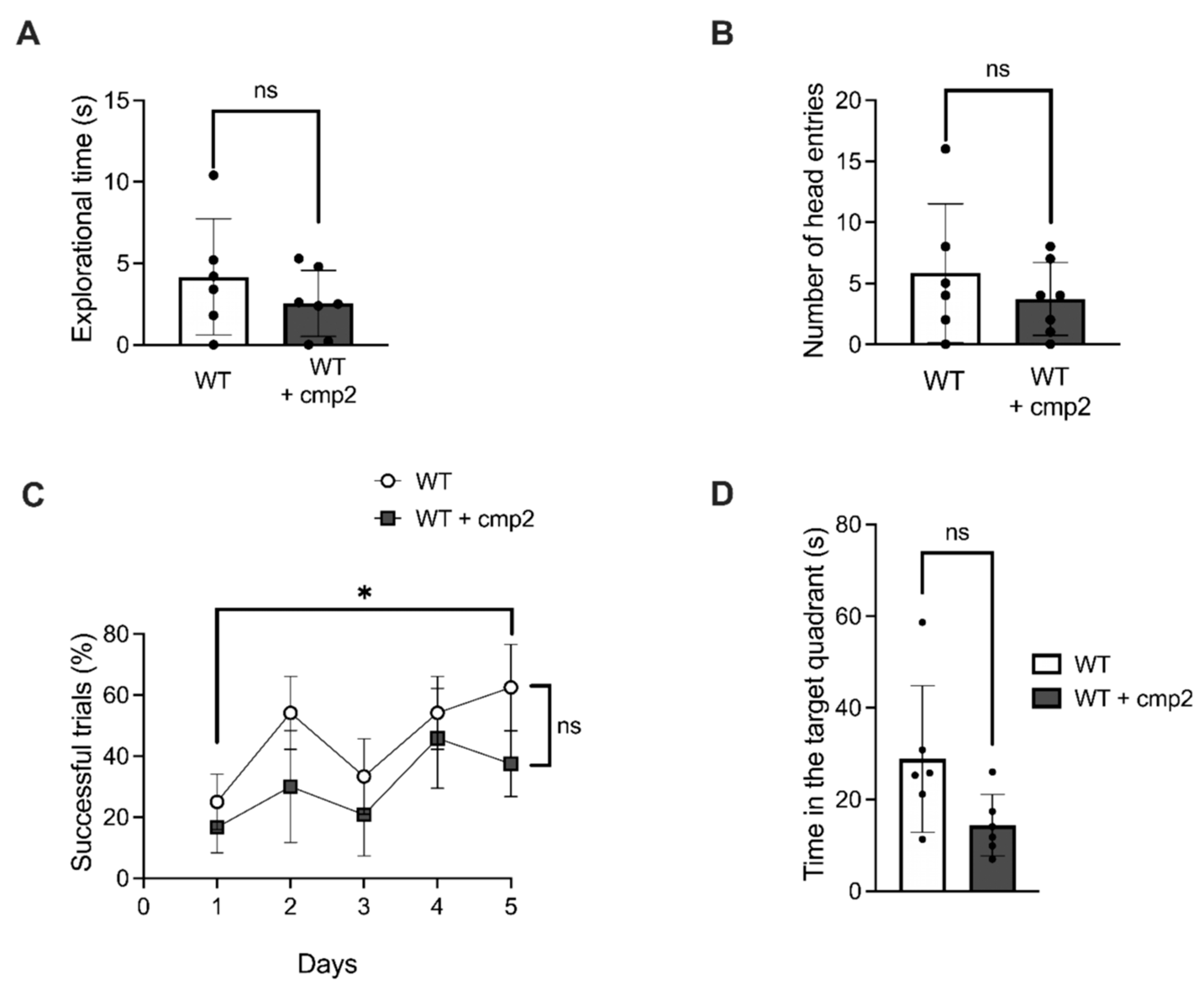
2.5. Effects of Cmp2 on the Cognitive and Motor Functions of WT Male Mice
2.6. Cmp2 Improves the Recognition Memory of 5xFAD Male Mice in the Novel Object Recognition Test
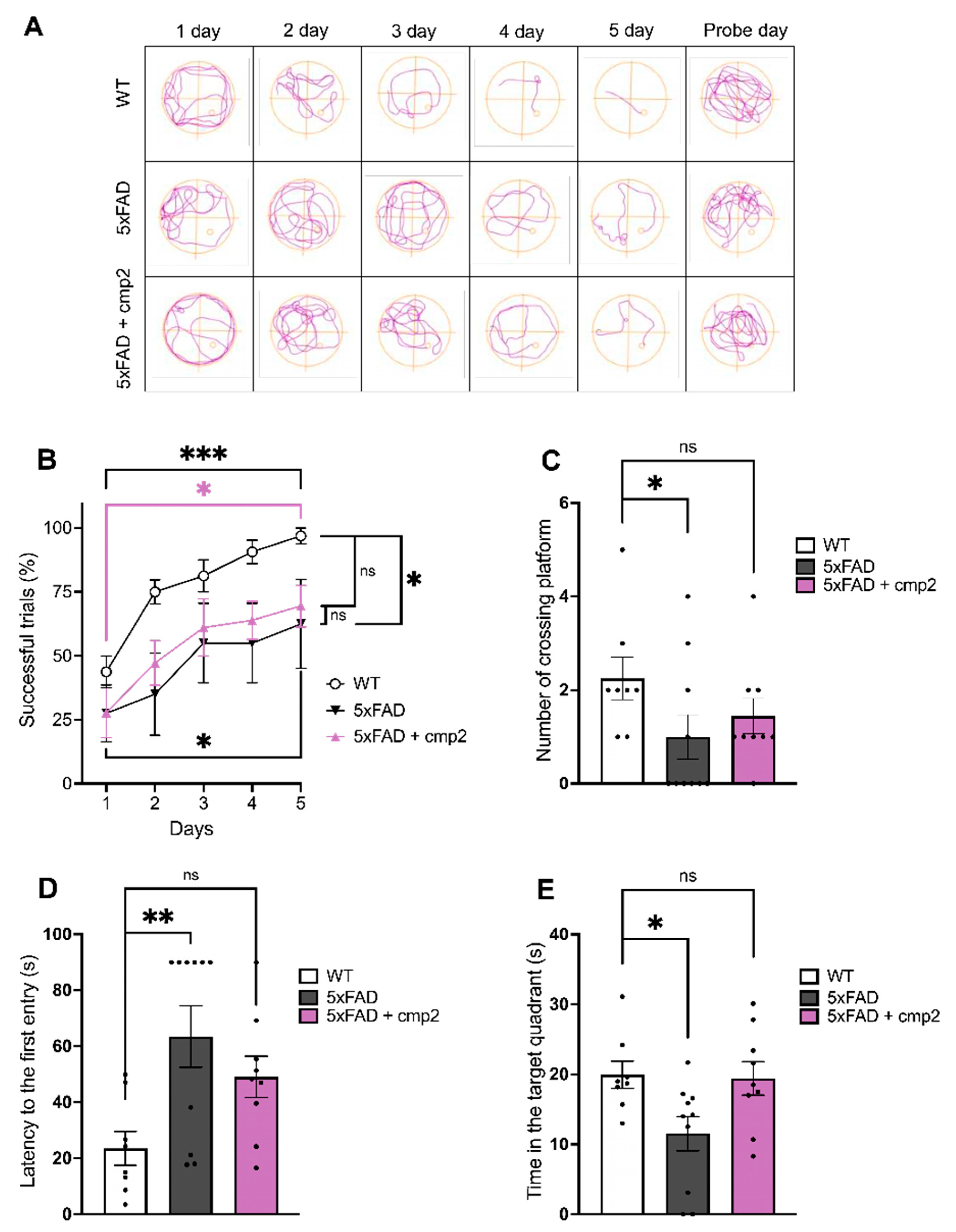
2.7. Cmp2 Improves the Spatial Memory of 5xFAD Mice in the Morris Water Maze Task
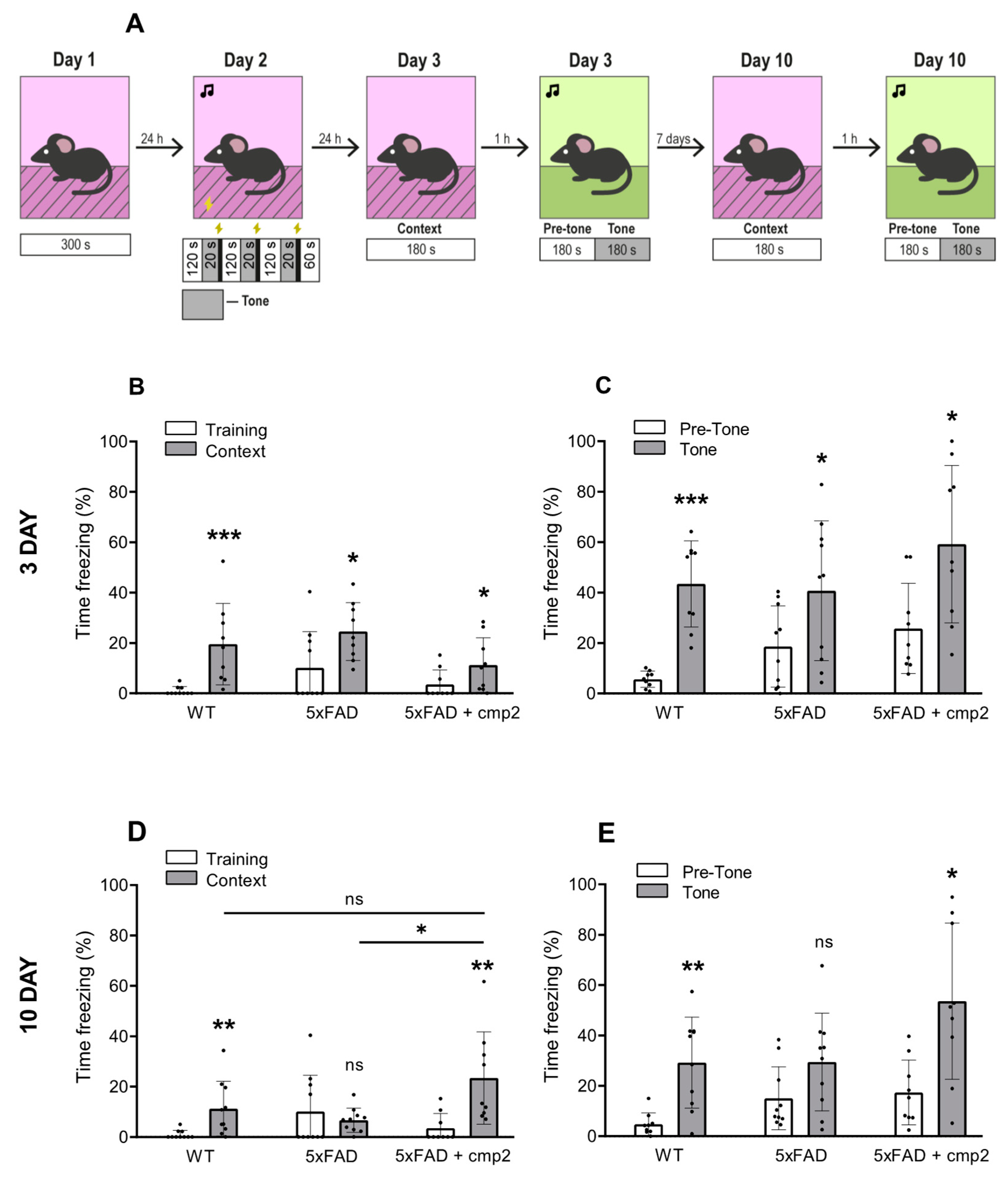
2.8. Cmp2 Recovers the Deficit of Contextual and Cued Memory in 8-Month-Old 5xFAD Mice
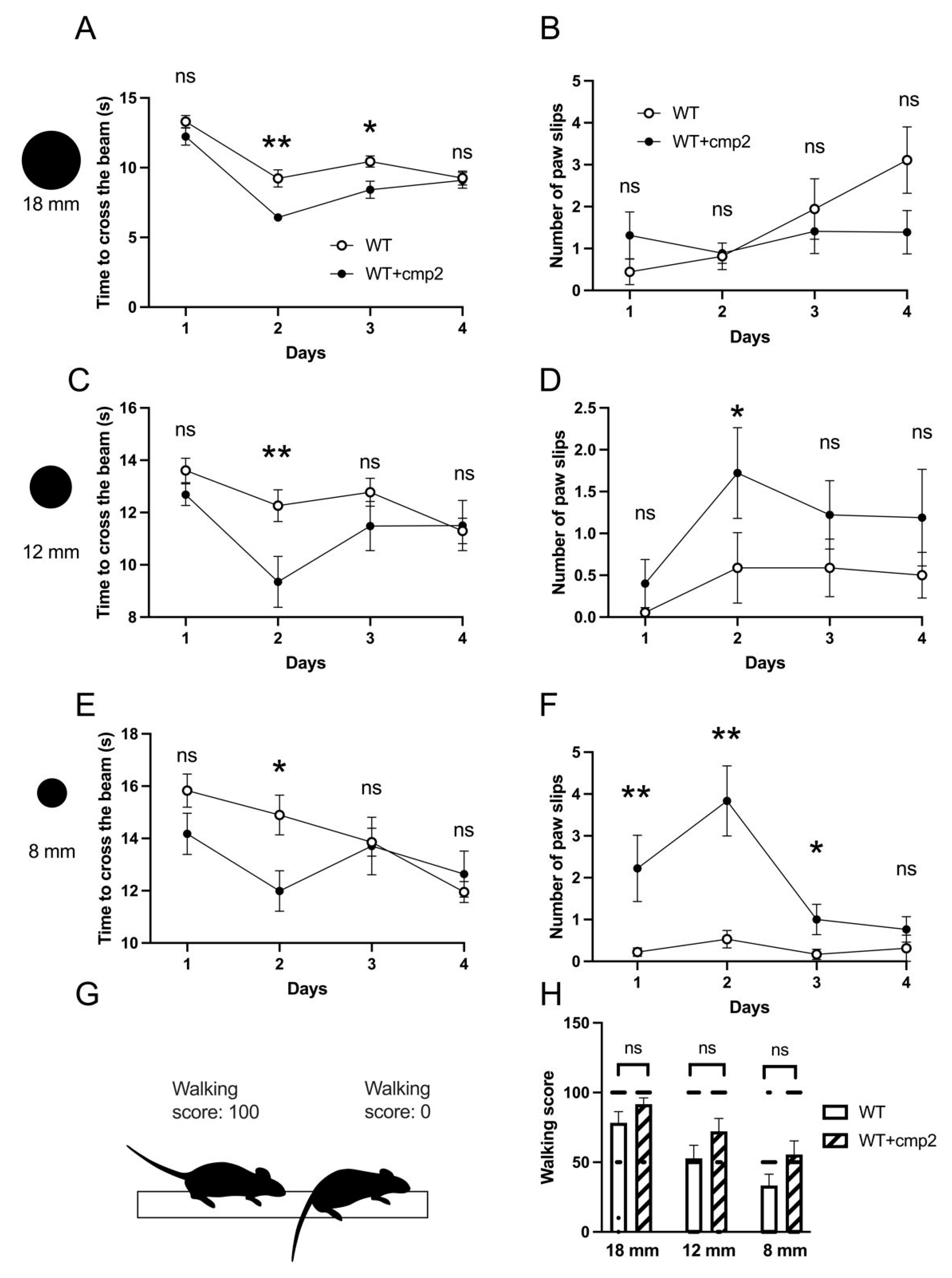
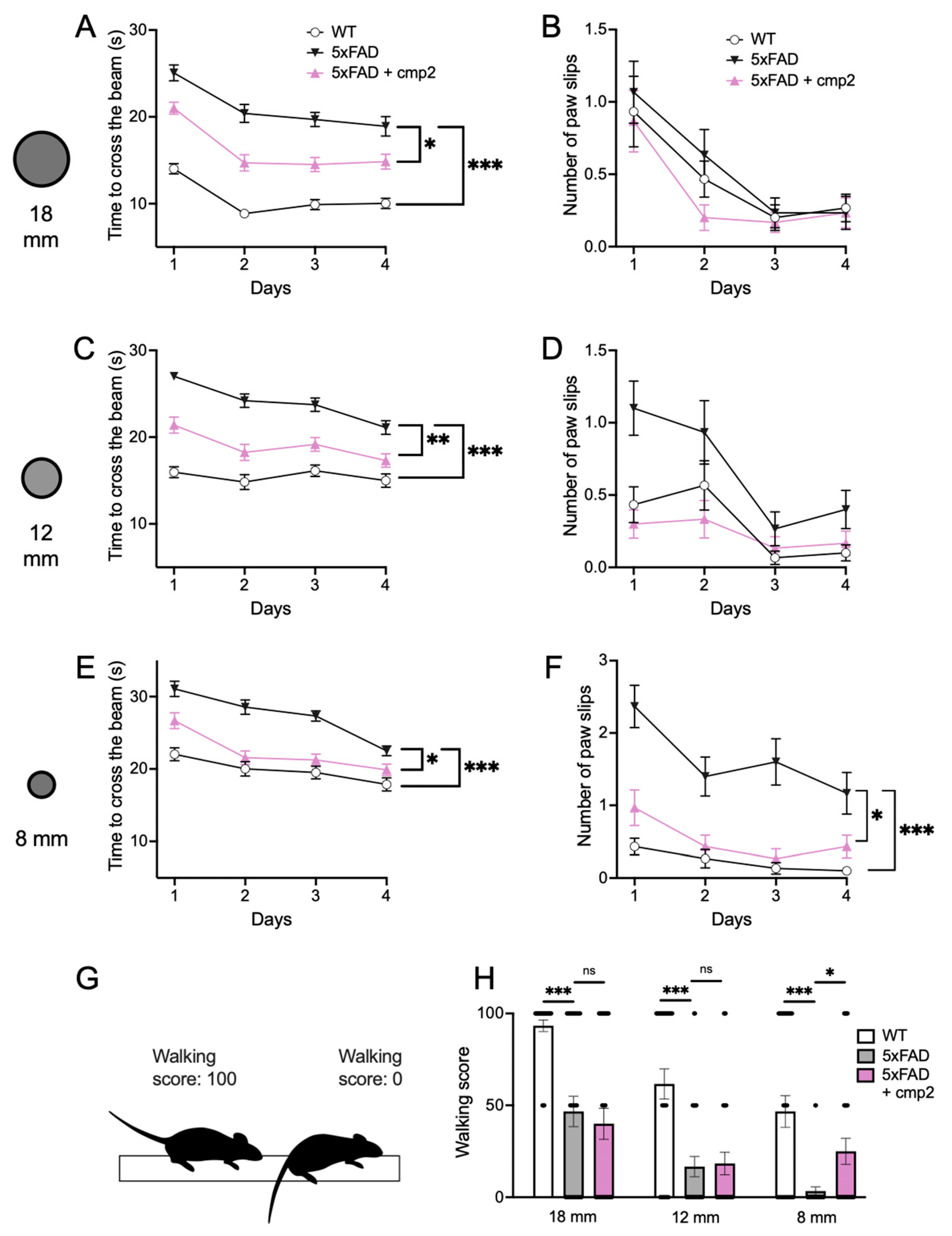
2.9. Cmp2 Recovers the Motor Performance of 8-Month-Old 5xFAD Mice
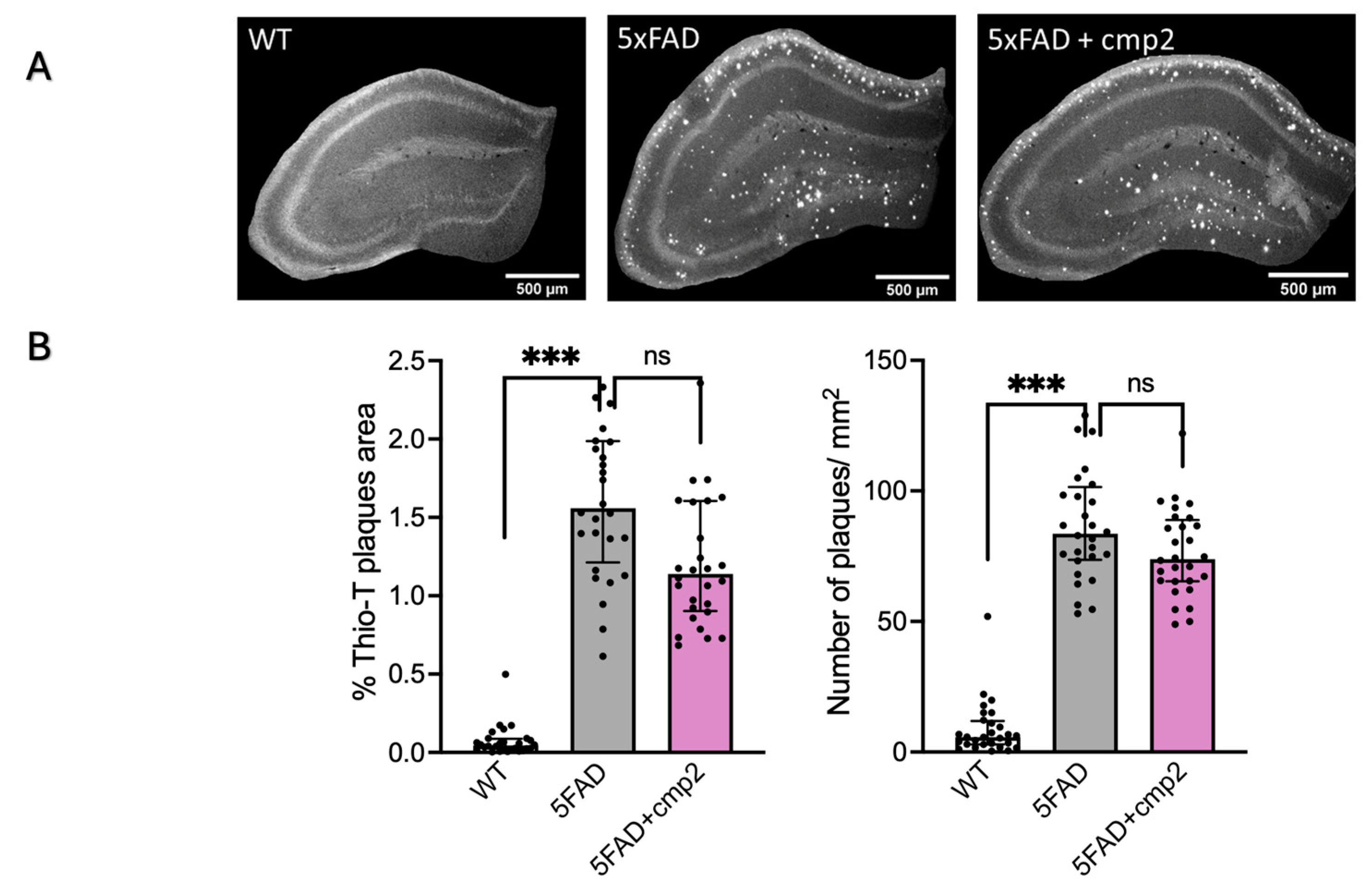
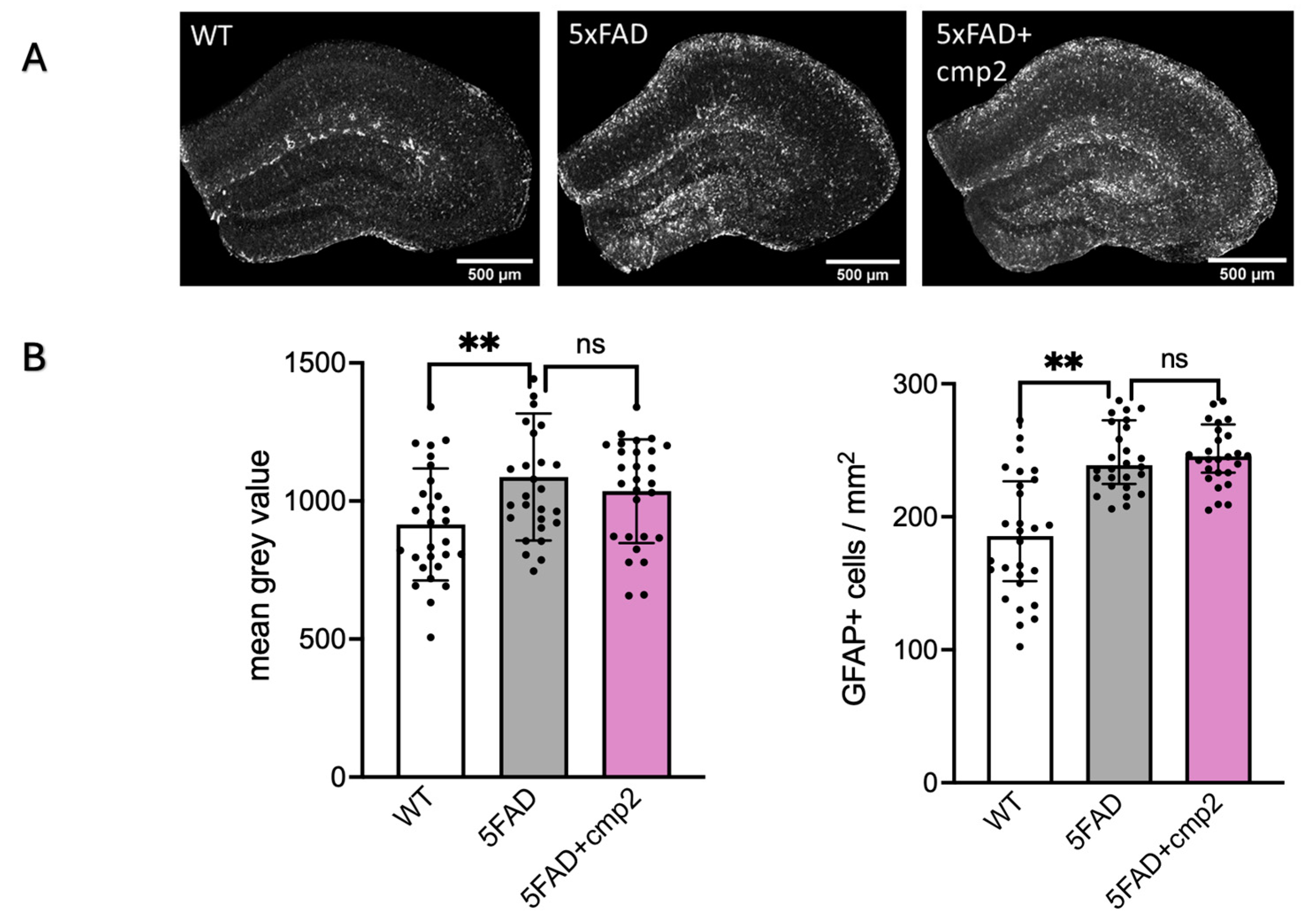
2.10. Cmp2 Intraperitoneal Injections for 38 Days Do Not Influence Amyloidosis or Astrogliosis in the Mouse Hippocampus
3. Discussion
Limitations of the Study
- (A)
- Testing only a single dose (10 mg/kg) represents a limitation of the current study. Comprehensive PK profiling and dose–response studies would provide valuable insights into the compound’s efficacy window and target specificity. To establish optimal dosing parameters, future PK/pharmacodynamic (PD) studies with multiple dose levels and using both sexes of mice are needed.
- (B)
- Potential sex-dependent toxicity was not assessed in this study; future work should evaluate both male and female mice to identify any sex-biased effects.
- (C)
- While our study identified statistically significant differences in behavioral tests between groups, we acknowledge that the statistical power for some comparisons depicted in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 did not reach the conventional threshold of 80% (0.8), particularly for effects of smaller magnitude. This limitation stems from the moderate sample sizes, which may have been insufficient to reliably detect subtle behavioral changes. Future studies with larger cohorts would help clarify the robustness of both positive and negative findings.
4. Materials and Methods
4.1. Three-Dimensional Molecular Models of Target Proteins
4.2. Molecular Dynamics
4.3. Animals
4.4. Chemical Compounds
4.5. Ames Assay
4.6. Chronic Toxicity Test
4.7. The Novel Object Recognition Test
4.8. Morris Water Maze
4.9. Fear Conditioning Test
4.10. Beam Walking Test
4.11. Immunohistochemistry and Thioflavin Staining
4.12. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karran, E.; Mercken, M.; Strooper, B.D. The Amyloid Cascade Hypothesis for Alzheimer’s Disease: An Appraisal for the Development of Therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Widera, E.W.; Brangman, S.A.; Chin, N.A. Ushering in a New Era of Alzheimer Disease Therapy. JAMA 2023, 330, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.; Gandy, S.; Saini, V.; George, D.R.; Larson, E.B.; Alexander, G.C.; Avorn, J.; Brownlee, S.; Camp, C.; Chertkow, H.; et al. Making the Case for Accelerated Withdrawal of Aducanumab. J. Alzheimer’s Dis. 2022, 87, 1003–1007. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Orzeł-Sajdłowska, A. Aducanumab—Hope or Disappointment for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4367. [Google Scholar] [CrossRef]
- Kim, A.Y.; Al Jerdi, S.; MacDonald, R.; Triggle, C.R. Alzheimer’s Disease and Its Treatment–Yesterday, Today, and Tomorrow. Front. Pharmacol. 2024, 15, 1399121. [Google Scholar] [CrossRef]
- Zernov, N.; Popugaeva, E. Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior. Int. J. Mol. Sci. 2023, 24, 15415. [Google Scholar] [CrossRef]
- Zernov, N.; Veselovsky, A.V.; Poroikov, V.V.; Melentieva, D.; Bolshakova, A.; Popugaeva, E. New Positive TRPC6 Modulator Penetrates Blood–Brain Barrier, Eliminates Synaptic Deficiency and Restores Memory Deficit in 5xFAD Mice. Int. J. Mol. Sci. 2022, 23, 13552. [Google Scholar] [CrossRef]
- Prikhodko, V.; Chernyuk, D.; Sysoev, Y.; Zernov, N.; Okovityi, S.; Popugaeva, E. Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia. Cells 2020, 9, 2351. [Google Scholar] [CrossRef]
- Hunanyan, L.; Ghamaryan, V.; Makichyan, A.; Popugaeva, E.; Seredenin, B.; Gudasheva, T.A.; Vakhitova, Y.V. Computer-Based Drug Design of Positive Modulators of Store-Operated Calcium Channels to Prevent Synaptic Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13618. [Google Scholar] [CrossRef]
- Laakmann, G.; Dienel, A.; Kieser, M. Clinical Significance of Hyperforin for the Efficacy of Hypericum Extracts on Depressive Disorders of Different Severities. Phytomedicine 1998, 5, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical Use of Hypericum Perforatum (St John’s Wort) in Depression: A Meta-Analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological Production of Hyperforin for Pharmaceutical Formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef]
- Sell, T.S.; Belkacemi, T.; Flockerzi, V.; Beck, A. Protonophore Properties of Hyperforin are Essential for Its Pharmacological Activity. Sci. Rep. 2014, 4, 7500. [Google Scholar] [CrossRef]
- Zernov, N.; Ghamaryan, V.; Melenteva, D.; Makichyan, A.; Hunanyan, L.; Popugaeva, E. Discovery of a Novel Piperazine Derivative, Cmp2: A Selective TRPC6 Activator Suitable for Treatment of Synaptic Deficiency in Alzheimer’s Disease Hippocampal Neurons. Sci. Rep. 2024, 14, 23512. [Google Scholar] [CrossRef] [PubMed]
- Karaytuğ, M.O.; Balcı, N.; Türkan, F.; Gürbüz, M.; Demirkol, M.E.; Namlı, Z.; Tamam, L.; Gülçin, İ. Piperazine derivatives with potent drug moiety as efficient acetylcholinesterase, butyrylcholinesterase, and glutathione S-transferase inhibitors. J. Biochem. Mol. Toxicol. 2023, 37, 23259. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and memory. Chem.-Biol. Interact. 2010, 187, 403–408. [Google Scholar] [CrossRef]
- Dong, H.; Csernansky, C.A.; Martin, M.V.; Bertchume, A.; Vallera, D.; Csernansky, J.G. Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology 2005, 181, 145–152. [Google Scholar] [CrossRef]
- Janas, A.M.; Cunningham, S.C.; Duffy, K.B.; Devan, B.D.; Greig, N.H.; Holloway, H.W.; Yu, Q.S.; Markowska, A.L.; Ingram, D.K.; Spangler, E.L. The cholinesterase inhibitor, phenserine, improves Morris water maze performance of scopolamine-treated rats. Life Sci. 2005, 76, 1073–1081. [Google Scholar] [CrossRef]
- Ghamaryan, V.S.; Hunanyan, L.S. Molecular docking and dynamic simulations of n-n-disubstituted piperasins with AChE and BuChE. Electron. J. Nat. Sci. 2024, 43, 4–11. [Google Scholar]
- Pádua, M.S.; Guil-Guerrero, J.L.; Lopes, P.A. Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. Int. J. Mol. Sci. 2024, 25, 6766. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Bayer, T.A. Motor impairment in Alzheimer’s disease and transgenic Alzheimer’s disease mouse models. Genes Brain Behav. 2008, 7, 1–5. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Du, W.; Jia, C.; Yao, H.; Wang, Y. TRPC6 inhibited NMDA receptor activities and protected neurons from ischemic excitotoxicity. J. Neurochem. 2012, 123, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Kong, J.; Ma, S.; Ban, Y.; Li, J.; Fan, Z. Upregulation of TRPC6 inhibits astrocyte activation and proliferation after spinal cord injury in rats by suppressing AQP4 expression. Brain Res. Bull. 2022, 190, 12–21. [Google Scholar] [CrossRef]
- Bezprozvanny, I. Alzheimer’s disease—Where do we go from here? Biochem. Biophys. Res. Commun. 2022, 633, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, W.; Zhou, K.; Tai, Y.; Yao, H.; Jia, Y.; Ding, Y.; Wang, Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat. Neurosci. 2008, 11, 741–743. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, J.; Tai, Y.; Wang, Y. TRPC channels promote cerebellar granule neuron survival. Nat. Neurosci. 2007, 10, 559–567. [Google Scholar] [CrossRef]
- Dasari, S.; Abramowitz, J.; Birnbaumer, L.; Gulledge, A.T. Do canonical transient receptor potential channels mediate cholinergic excitation of cortical pyramidal neurons? Neuroreport 2013, 24, 550–554. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zernov, N.; Melenteva, D.; Ghamaryan, V.; Makichyan, A.; Hunanyan, L.; Popugaeva, E. N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice. Int. J. Mol. Sci. 2025, 26, 4591. https://doi.org/10.3390/ijms26104591
Zernov N, Melenteva D, Ghamaryan V, Makichyan A, Hunanyan L, Popugaeva E. N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice. International Journal of Molecular Sciences. 2025; 26(10):4591. https://doi.org/10.3390/ijms26104591
Chicago/Turabian StyleZernov, Nikita, Daria Melenteva, Viktor Ghamaryan, Ani Makichyan, Lernik Hunanyan, and Elena Popugaeva. 2025. "N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice" International Journal of Molecular Sciences 26, no. 10: 4591. https://doi.org/10.3390/ijms26104591
APA StyleZernov, N., Melenteva, D., Ghamaryan, V., Makichyan, A., Hunanyan, L., & Popugaeva, E. (2025). N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice. International Journal of Molecular Sciences, 26(10), 4591. https://doi.org/10.3390/ijms26104591





