Impact of Type 1 Diabetes on Testicular Microtubule Dynamics, Sperm Physiology, and Male Reproductive Health in Rat †
Abstract
1. Introduction
2. Results
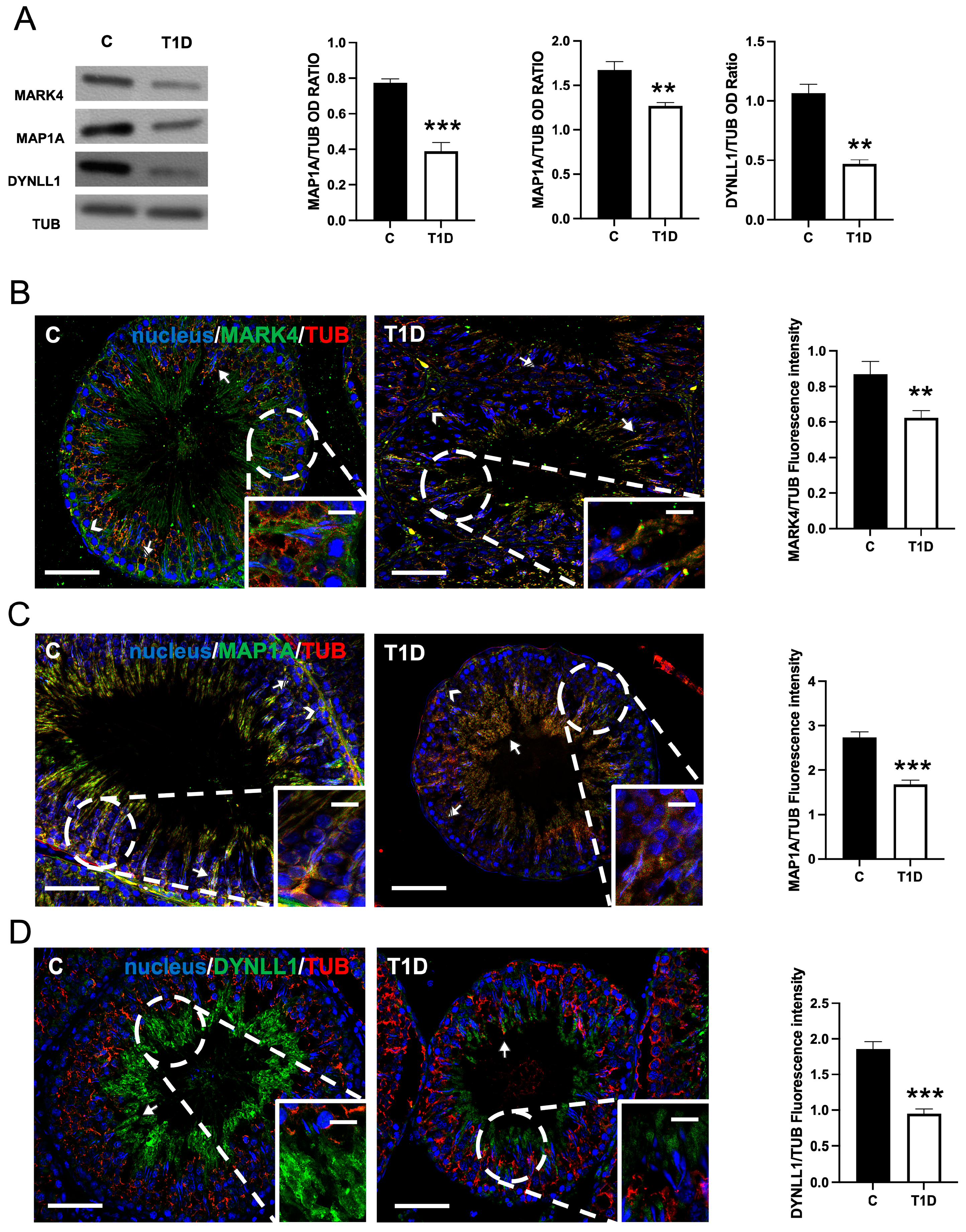
2.1. Effect of T1D on MARK4, MAP1A, and DYNLL1
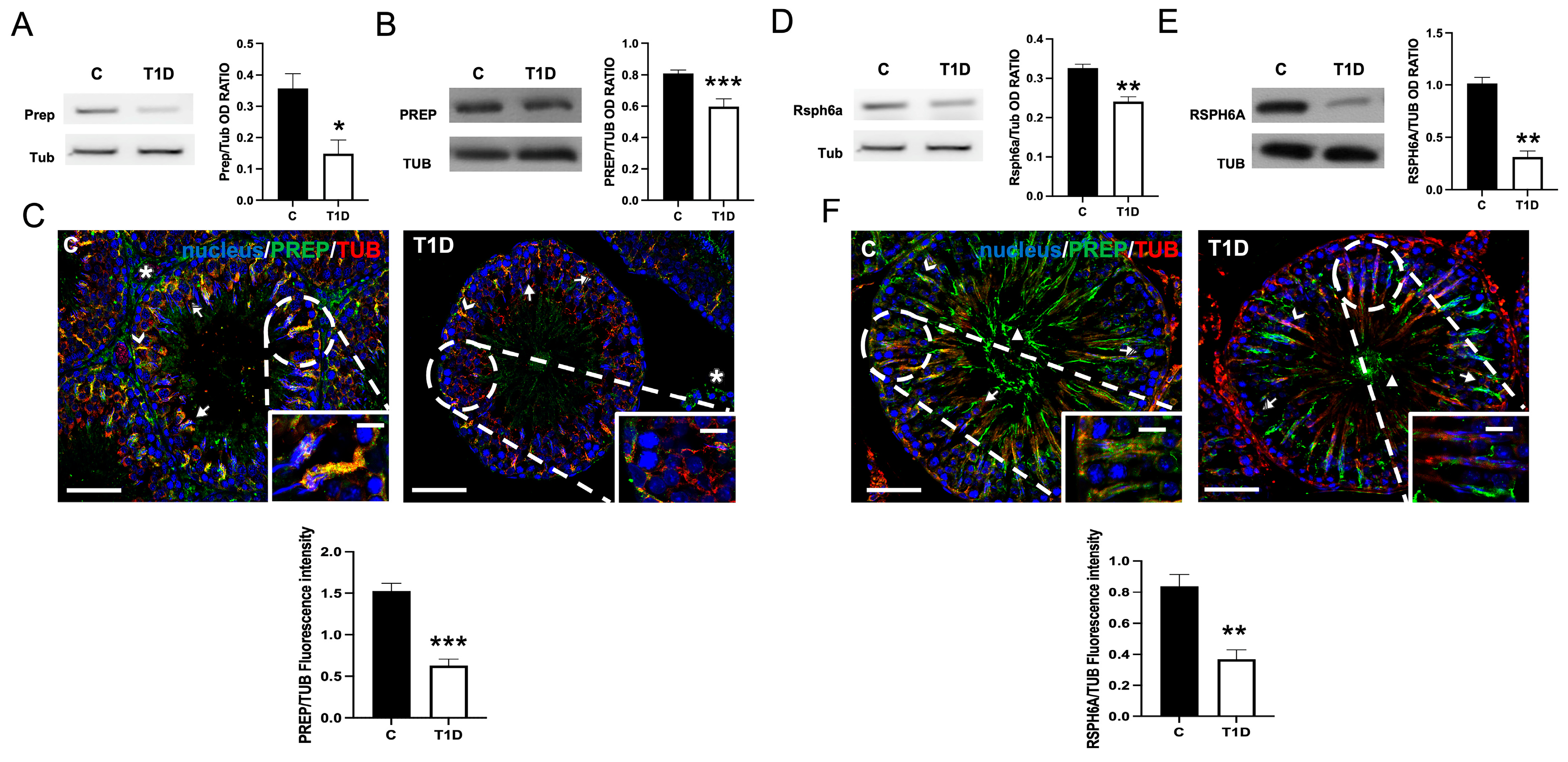
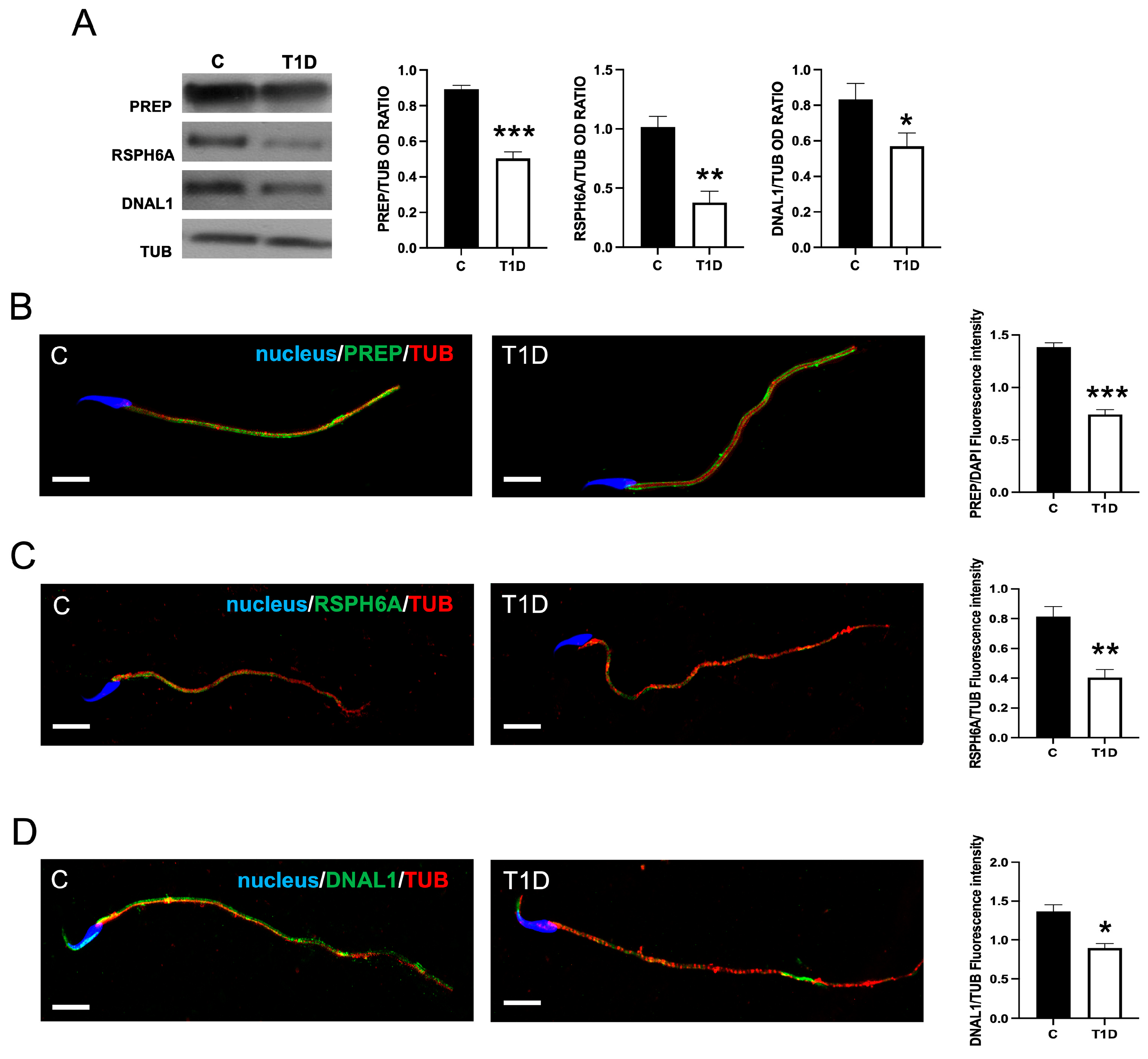
2.2. Effect of T1D on PREP and RSPH6A
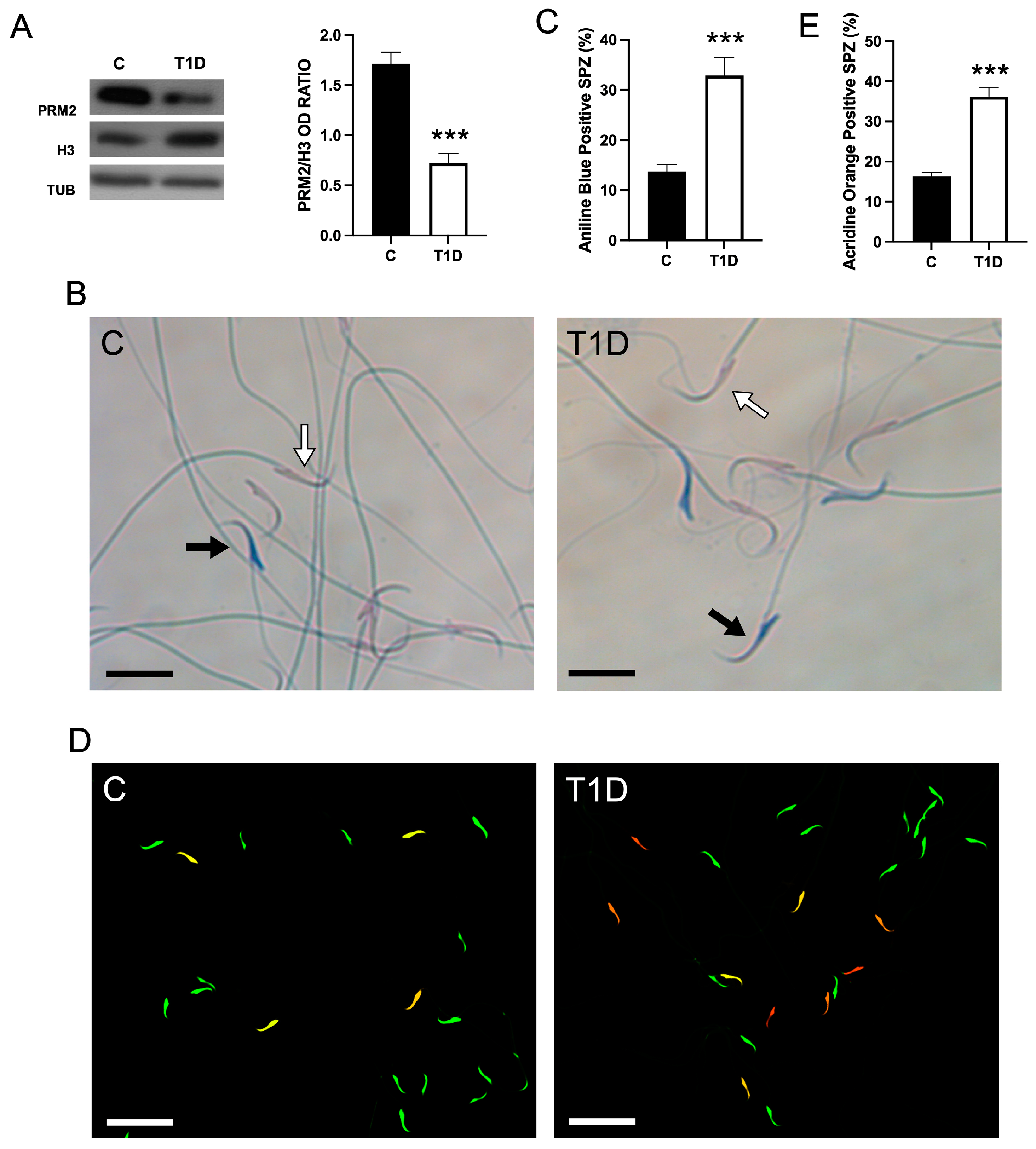
2.3. Effect of T1D on Sperm Parameters and Quality
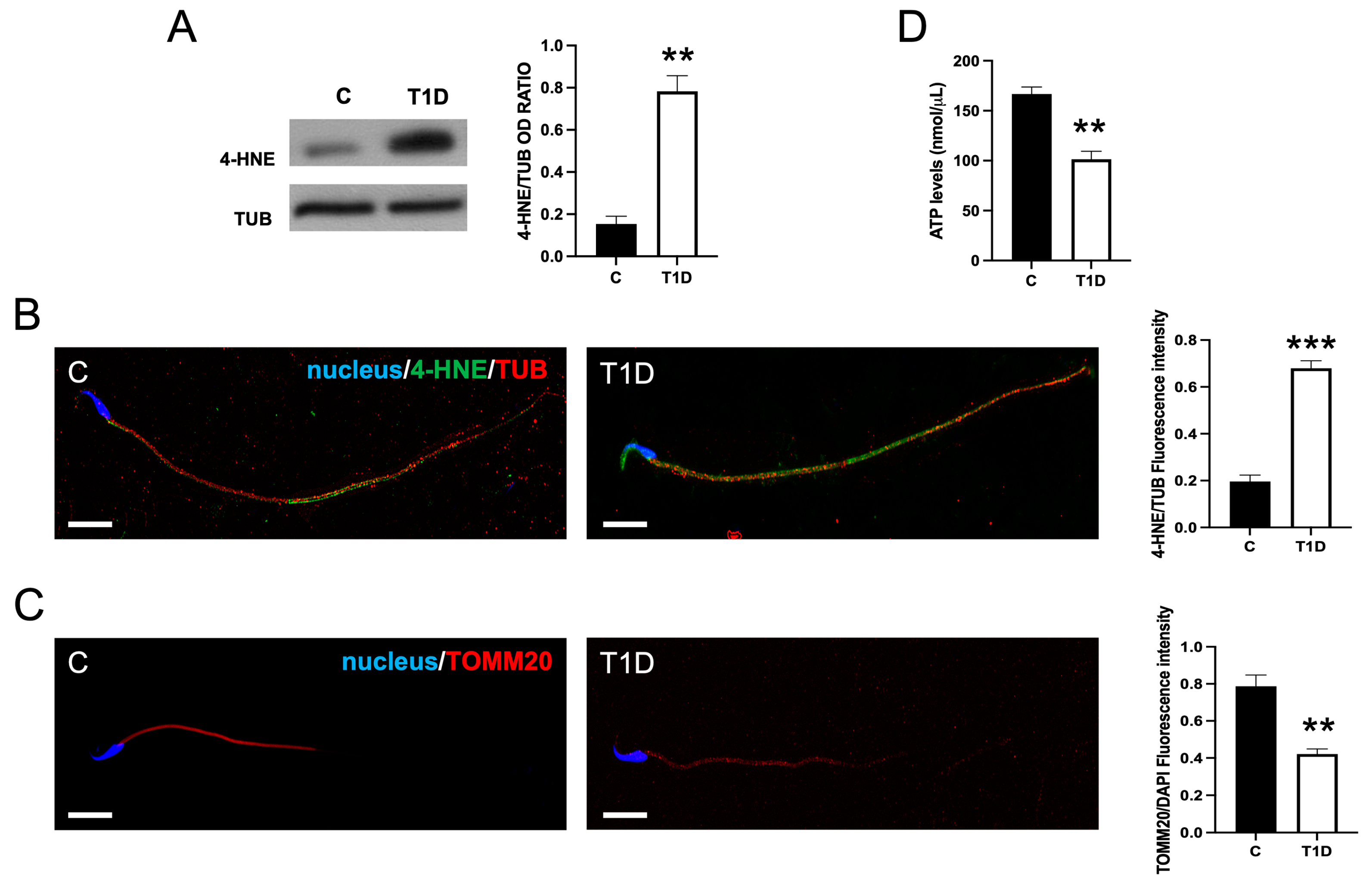
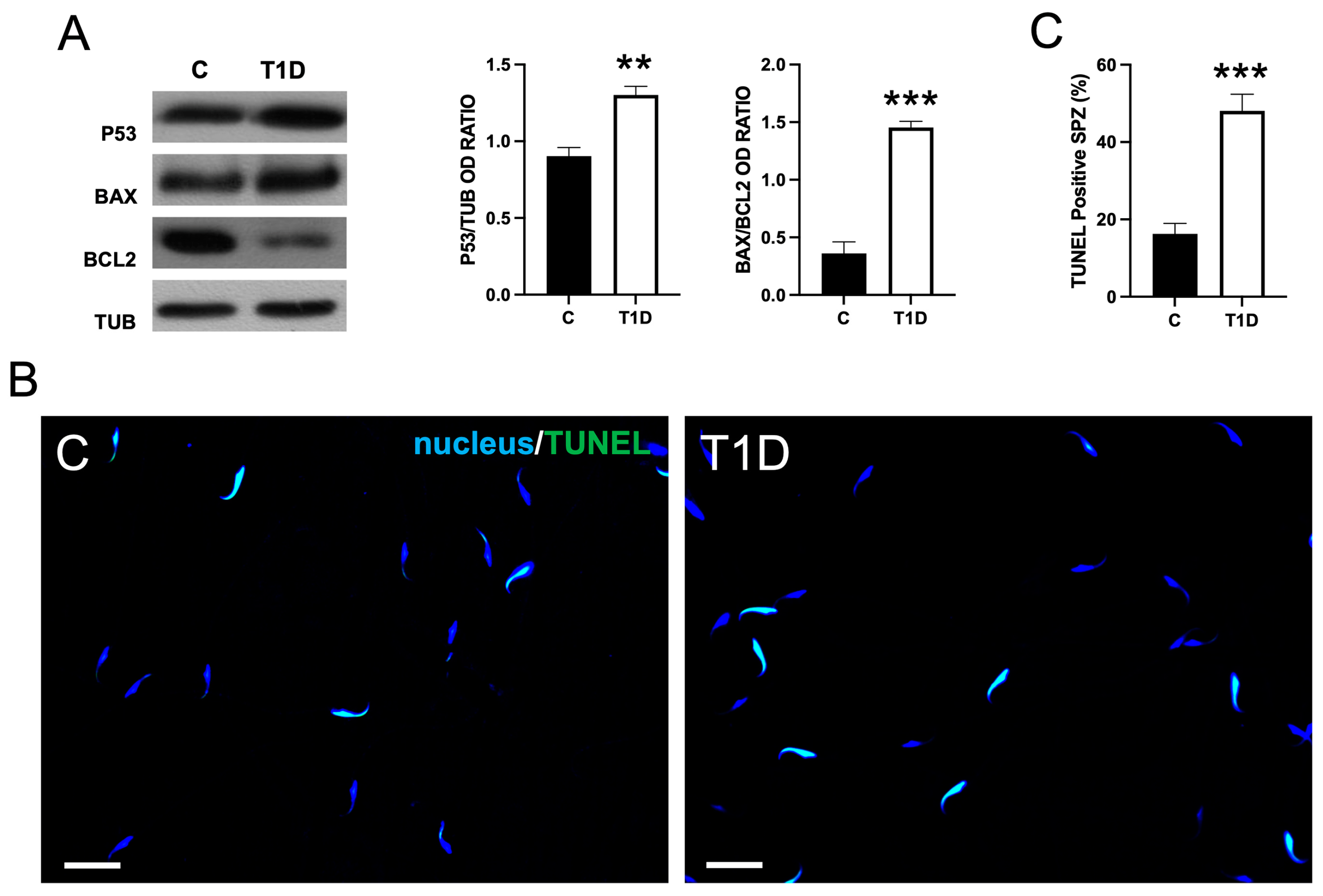
2.4. Effect of T1D on Sperm OS, Mitochondrial Mass, and Apoptotic Rate
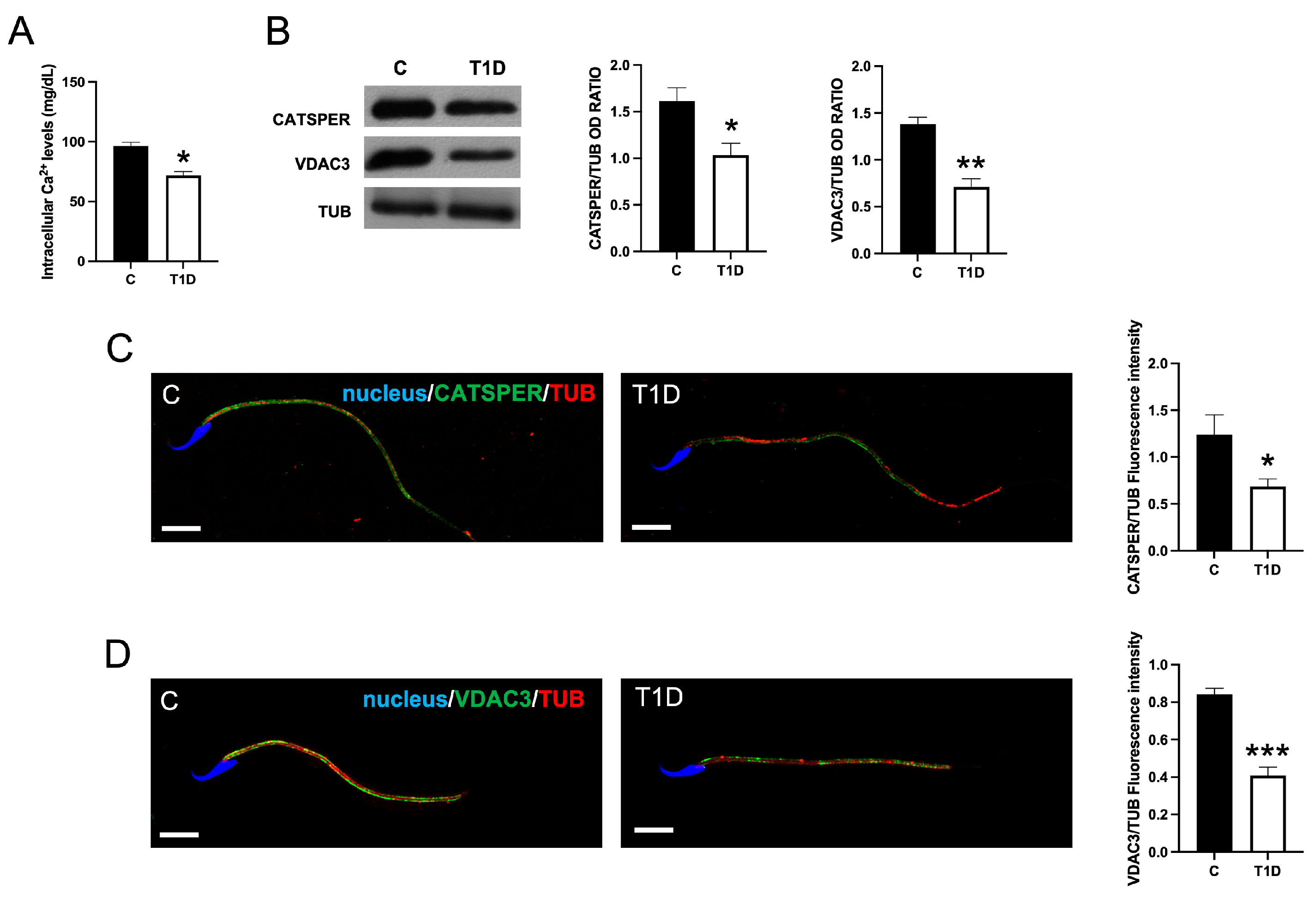
2.5. Effect of T1D on Sperm Ca2+ Level
2.6. Effect of T1D on Sperm Motility-Associated Factors
2.7. Effect of T1D on MAPs in SPZ
3. Discussion
4. Materials and Methods
4.1. Animals, Treatments, and Sample Collection
4.2. Sperm Parameter Evaluation
4.2.1. Epididymal Sperm Count, Motility, Viability, and Morphology
4.2.2. AB Staining
4.2.3. AO Staining
4.2.4. ATP and Ca2+ Assays in SPZ
4.3. Protein Extraction and WB Analysis
4.4. RT-PCR Analysis
4.5. IF Analysis on Testis and SPZ
4.6. TUNEL Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Gao, Y.; Chen, H.; Jesus, T.; Tang, E.; Li, N.; Lian, Q.; Ge, R.S.; Cheng, C.Y. Cell polarity, cell adhesion, and spermatogenesis: Role of cytoskeletons. F1000Research 2017, 6, 1565. [Google Scholar] [CrossRef]
- Mao, B.P.; Ge, R.; Cheng, C.Y. Role of microtubule +TIPs and −TIPs in spermatogenesis—Insights from studies of toxicant models. Reprod. Toxicol. 2020, 91, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Garbers, D.L. Male germ cell specification and differentiation. Dev. Cell. 2002, 2, 537–547. [Google Scholar] [CrossRef]
- Chu, D.S.; Shakes, D.C. Spermatogenesis. Adv. Exp. Med. Biol. 2013, 757, 171–203. [Google Scholar] [CrossRef]
- Venditti, M.; Minucci, S. Prothymosin alpha expression in the vertebrate testis: A comparative review. Zygote 2017, 25, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Minucci, S. Subcellular Localization of Prolyl Endopeptidase During the First Wave of Rat Spermatogenesis and in Rat and Human Sperm. J. Histochem. Cytochem. 2019, 67, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Escalier, D. Impact of genetic engineering on the understanding of spermatogenesis. Hum. Reprod. Update 2001, 7, 191–210. [Google Scholar] [CrossRef]
- Venditti, M.; Donizetti, A.; Aniello, F.; Minucci, S. EH domain binding protein 1-like 1 (EHBP1L1), a protein with calponin homology domain, is expressed in the rat testis. Zygote 2020, 28, 441–446. [Google Scholar] [CrossRef]
- Venditti, M.; Minucci, S. Differential Expression and Localization of EHBP1L1 during the First Wave of Rat Spermatogenesis Suggest Its Involvement in Acrosome Biogenesis. Biomedicines 2022, 10, 181. [Google Scholar] [CrossRef]
- O’Donnell, L.; O’Bryan, M.K. Microtubules and spermatogenesis. Semin. Cell. Dev. Biol. 2014, 30, 45–54. [Google Scholar] [CrossRef]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.S.; Sironen, A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016, 151, R43–R54. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell. Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef]
- Miyata, H.; Shimada, K.; Kaneda, Y.; Ikawa, M. Development of functional spermatozoa in mammalian spermiogenesis. Development 2024, 151, dev202838. [Google Scholar] [CrossRef]
- Amlani, S.; Vogl, A.W. Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat. Rec. 1988, 220, 143–160. [Google Scholar] [CrossRef]
- Guttman, J.A.; Kimel, G.H.; Vogl, A.W. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations). J. Cell. Sci. 2000, 113 Pt 12, 2167–2176. [Google Scholar] [CrossRef]
- Vaid, K.S.; Guttman, J.A.; Singaraja, R.R.; Vogl, A.W. A kinesin is present at unique sertoli/spermatid adherens junctions in rat and mouse testes. Biol. Reprod. 2007, 77, 1037–1048. [Google Scholar] [CrossRef]
- Subramanian, R.; Kapoor, T.M. Building complexity: Insights into self-organized assembly of microtubule-based architectures. Dev. Cell. 2012, 23, 874–885. [Google Scholar] [CrossRef]
- Wang, L.; Bu, T.; Wu, X.; Li, L.; Sun, F.; Cheng, C.Y. Motor proteins, spermatogenesis and testis function. Adv. Protein. Chem. Struct. Biol. 2024, 141, 381–445. [Google Scholar] [CrossRef]
- Yao, M.; Qu, H.; Han, Y.; Cheng, C.Y.; Xiao, X. Kinesins in Mammalian Spermatogenesis and Germ Cell Transport. Front. Cell. Dev. Biol. 2022, 10, 837542. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, H.; Wang, L.; Mak, N.; Wu, X.; Ge, R.; Sun, F.; Cheng, C.Y. Motor Proteins and Spermatogenesis. Adv. Exp. Med. Biol. 2021, 1288, 131–159. [Google Scholar] [CrossRef]
- Wang, L.; Bu, T.; Li, L.; Wu, X.; Wong, C.K.C.; Perrotta, A.; Silvestrini, B.; Sun, F.; Cheng, C.Y. Planar cell polarity (PCP) proteins support spermatogenesis through cytoskeletal organization in the testis. Semin. Cell. Dev. Biol. 2022, 121, 99–113. [Google Scholar] [CrossRef]
- Wang, L.; Yan, M.; Wong, C.K.C.; Ge, R.; Wu, X.; Sun, F.; Cheng, C.Y. Microtubule-associated proteins (MAPs) in microtubule cytoskeletal dynamics and spermatogenesis. Histol. Histopathol. 2021, 36, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Sengupta, P.; Henkel, R.; Alguraigari, A.; Sinigaglia, M.M.; Kayal, M.; Joumah, A.; Agarwal, A. Microtubular Dysfunction and Male Infertility. World J. Mens. Health 2020, 38, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, M.; Wu, S.; Mao, B.; Wong, C.K.C.; Ge, R.; Sun, F.; Cheng, C.Y. Microtubule Cytoskeleton and Spermatogenesis-Lesson From Studies of Toxicant Models. Toxicol. Sci. 2020, 177, 305–315. [Google Scholar] [CrossRef]
- Mao, B.P.; Li, L.; Yan, M.; Lian, Q.; Ge, R.; Cheng, C.Y. Environmental toxicants and cell polarity in the testis. Reprod. Toxicol. 2018, 81, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Mruk, D.; Lian, Q.; Ge, R.; Li, C.; Silvestrini, B.; Cheng, C.Y. Mechanistic Insights into PFOS-Mediated Sertoli Cell Injury. Trends Mol. Med. 2018, 24, 781–793. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Lv, Q.; Yang, Q.; Wu, G.; Hu, J.; Yang, J. Taurine Recovers Testicular Steroidogenesis and Spermatogenesis in Streptozotocin-Induced Diabetic Rats. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 801–811. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Fleming, T.; Lin, J.; Hammes, H.P.; Linn, T. Hyperglycemia induces spermatogenic disruption via major pathways of diabetes pathogenesis. Sci. Rep. 2019, 9, 13074. [Google Scholar] [CrossRef]
- Wagner, I.V.; Klöting, N.; Savchuk, I.; Eifler, L.; Kulle, A.; Kralisch-Jäcklein, S.; Dötsch, J.; Hiort, O.; Svechnikov, K.; Söder, O. Diabetes Type 1 Negatively Influences Leydig Cell Function in Rats, Which is Partially Reversible By Insulin Treatment. Endocrinology 2021, 162, bqab017. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Wu, C.Y.; Wang, Y.H.; Hu, Q.Q.; Sun, R.Y.; Pan, M.J.; Lu, X.Y.; Zhu, T.; Luo, S.; Yang, H.J.; et al. The protective effects of icariin against testicular dysfunction in type 1 diabetic mice Via AMPK-mediated Nrf2 activation and NF-κB p65 inhibition. Phytomedicine 2024, 123, 155217. [Google Scholar] [CrossRef] [PubMed]
- Chilukoti, S.R.; Sahu, C.; Jena, G. Protective role of eugenol against diabetes-induced oxidative stress, DNA damage, and apoptosis in rat testes. J. Biochem. Mol. Toxicol. 2024, 38, e23593. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Moreno, A.J.; Santos, M.S.; Seiça, R.; Ramalho-Santos, J. Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology 2006, 66, 2056–2067. [Google Scholar] [CrossRef]
- Navarro-Casado, L.; Juncos-Tobarra, M.A.; Cháfer-Rudilla, M.; de Onzoño, L.Í.; Blázquez-Cabrera, J.A.; Miralles-García, J.M. Effect of experimental diabetes and STZ on male fertility capacity. Study in rats. J. Androl. 2010, 31, 584–592. [Google Scholar] [CrossRef]
- Venditti, M.; Romano, M.Z.; Boccella, S.; Haddadi, A.; Biasi, A.; Maione, S.; Minucci, S. Type 1 diabetes impairs the activity of rat testicular somatic and germ cells through NRF2/NLRP3 pathway-mediated oxidative stress. Front. Endocrinol. 2024, 15, 1399256. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, S.; Wang, L.; Bu, T.; Chu, J.; Lv, L.; Tahir, A.; Mao, B.; Li, H.; Li, X.; et al. PCP Protein Inversin Regulates Testis Function Through Changes in Cytoskeletal Organization of Actin and Microtubules. Endocrinology 2022, 163, bqac009. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, X.; Lui, W.Y.; Lee, W.M.; Cheng, C.Y. Vangl2 regulates spermatid planar cell polarity through microtubule (MT)-based cytoskeleton in the rat testis. Cell Death Dis. 2018, 9, 340. [Google Scholar] [CrossRef]
- Hu, L.; Wei, S.; Wu, Y.; Li, S.; Zhu, P.; Wang, X. MicroRNA regulation of the proliferation and apoptosis of Leydig cells in diabetes. Mol. Med. 2021, 27, 104. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Z.; Liu, L.; Li, T.; Xing, C.; Han, F.; Mao, H. Causal Effects of Oxidative Stress on Diabetes Mellitus and Microvascular Complications: Insights Integrating Genome-Wide Mendelian Randomization, DNA Methylation, and Proteome. Antioxidants 2024, 13, 903. [Google Scholar] [CrossRef]
- Binjawhar, D.N.; Alhazmi, A.T.; Bin Jawhar, W.N.; MohammedSaeed, W.; Safi, S.Z. Hyperglycemia-induced oxidative stress and epigenetic regulation of ET-1 gene in endothelial cells. Front. Genet. 2023, 14, 1167773. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Yu, L.; Zhang, J.; Zhang, B. Transcriptomics and proteomics characterizing the antioxidant mechanisms of semaglutide in diabetic mice with cognitive impairment. Int. J. Mol. Med. 2025, 55, 56. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.I.; Cheng, C.Y. MARK2 and MARK4 Regulate Sertoli Cell BTB Dynamics Through Microtubule and Actin Cytoskeletons. Endocrinology 2022, 163, bqac130. [Google Scholar] [CrossRef]
- Tang, E.I.; Xiao, X.; Mruk, D.D.; Qian, X.J.; Mok, K.W.; Jenardhanan, P.; Lee, W.M.; Mathur, P.P.; Cheng, C.Y. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis 2012, 2, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, M.; Bu, T.; Wu, X.; Li, L.; Silvestrini, B.; Sun, F.; Cheng, C.Y.; Chen, H. Map-1a regulates Sertoli cell BTB dynamics through the cytoskeletal organization of microtubule and F-actin. Reprod. Biol. Endocrinol. 2024, 22, 36. [Google Scholar] [CrossRef]
- Merges, G.E.; Arévalo, L.; Kovacevic, A.; Lohanadan, K.; de Rooij, D.G.; Simon, C.; Jokwitz, M.; Witke, W.; Schorle, H. Actl7b deficiency leads to mislocalization of LC8 type dynein light chains and disruption of murine spermatogenesis. Development 2023, 150, dev201593. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Ben Hadj Hassine, M.; Messaoudi, I.; Minucci, S. The simultaneous administration of microplastics and cadmium alters rat testicular activity and changes the expression of PTMA, DAAM1 and PREP. Front. Cell Dev. Biol. 2023, 11, 1145702. [Google Scholar] [CrossRef]
- Dotolo, R.; Kim, J.D.; Pariante, P.; Minucci, S.; Diano, S. Prolyl Endopeptidase (PREP) is Associated With Male Reproductive Functions and Gamete Physiology in Mice. J. Cell. Physiol. 2016, 231, 551–557. [Google Scholar] [CrossRef]
- Abbasi, F.; Miyata, H.; Shimada, K.; Morohoshi, A.; Nozawa, K.; Matsumura, T.; Xu, Z.; Pratiwi, P.; Ikawa, M. RSPH6A is required for sperm flagellum formation and male fertility in mice. J. Cell. Sci. 2018, 131, jcs221648. [Google Scholar] [CrossRef]
- Romano, M.Z.; Ben Rhouma, M.; Messaoudi, I.; Aniello, F.; Minucci, S.; Venditti, M. Expression of RSPH6A in the first wave of rat spermatogenesis and oxidative stress conditions: Attenuation by melatonin. Reprod. Med. Biol. 2023, 22, e12542. [Google Scholar] [CrossRef]
- Ergoli, M.; Venditti, M.; Picillo, E.; Minucci, S.; Politano, L. Study of expression of genes potentially responsible for reduced fitness in patients with myotonic dystrophy type 1 and identification of new biomarkers of testicular function. Mol. Reprod. Dev. 2020, 87, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sellami, A.; Chakroun, N.; Ben Zarrouk, S.; Sellami, H.; Kebaili, S.; Rebai, T.; Keskes, L. Assessment of chromatin maturity in human spermatozoa: Useful aniline blue assay for routine diagnosis of male infertility. Adv. Urol. 2013, 2013, 578631. [Google Scholar] [CrossRef] [PubMed]
- Barkabi-Zanjani, S.; Ghorbanzadeh, V.; Aslani, M.; Ghalibafsabbaghi, A.; Chodari, L. Diabetes mellitus and the impairment of male reproductive function: Possible signaling pathways. Diabetes Metab. Syndr. 2020, 14, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Chawan, V.; Yevate, S.; Gajbhiye, R.; Kulkarni, V.; Parte, P. Acetylation/deacetylation and microtubule associated proteins influence flagellar axonemal stability and sperm motility. Biosci. Rep. 2020, 40, BSR20202442. [Google Scholar] [CrossRef]
- Kalebic, N.; Sorrentino, S.; Perlas, E.; Bolasco, G.; Martinez, C.; Heppenstall, P.A. αTAT1 is the major α-tubulin acetyltransferase in mice. Nat. Commun. 2013, 4, 1962. [Google Scholar] [CrossRef]
- Parab, S.; Shetty, O.; Gaonkar, R.; Balasinor, N.; Khole, V.; Parte, P. HDAC6 deacetylates alpha tubulin in sperm and modulates sperm motility in Holtzman rat. Cell Tissue. Res. 2015, 359, 665–678. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040, a modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- O’Callaghan, M. Follow up of premature and “at risk” infants. Aust. Paediatr. J. 1986, 22, 83–85. [Google Scholar] [CrossRef]
- Lee, C.F.; Liu, C.Y.; Hsieh, R.H.; Wei, Y.H. Oxidative stress-induced depolymerization of microtubules and alteration of mitochondrial mass in human cells. Ann. N. Y. Acad. Sci. 2005, 1042, 246–254. [Google Scholar] [CrossRef]
- Banan, A.; Choudhary, S.; Zhang, Y.; Fields, J.Z.; Keshavarzian, A. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: Role of the microtubule cytoskeleton. Free Radic. Biol. Med. 2000, 28, 727–738. [Google Scholar] [CrossRef]
- Drum, B.M.L.; Yuan, C.; Li, L.; Liu, Q.; Wordeman, L.; Santana, L.F. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J. Mol. Cell. Cardiol. 2016, 93, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; González-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.I.; Mruk, D.D.; Cheng, C.Y. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J. Endocrinol. 2013, 217, R13–R23. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, L.; Mao, B.; Li, H.; Li, S.Y.T.; Mruk, D.; Silvestrini, B.; Lian, Q.; Ge, R.; Cheng, C.Y. mTORC1/rpS6 signaling complex modifies BTB transport function: An in vivo study using the adjudin model. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E121–E138. [Google Scholar] [CrossRef]
- Lie, P.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytoskeletal dynamics and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1581–1592. [Google Scholar] [CrossRef]
- Wen, Q.; Tang, E.I.; Lui, W.Y.; Lee, W.M.; Wong, C.K.C.; Silvestrini, B.; Cheng, C.Y. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E924–E948. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal–Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants 2023, 12, 856. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Caroppo, E. Understanding sperm motility regulation: It’s a long road ahead. Fertil. Steril. 2021, 115, 311–312. [Google Scholar] [CrossRef]
- Swain, D.K.; Sharma, P.; Shah, N.; Sethi, M.; Mahajan, A.; Gupta, S.; Mishra, A.K.; Yadav, S. Introduction to the pathways involved in the activation and regulation of sperm motility: A review of the relevance of ion channels. Anim. Reprod. Sci. 2022, 246, 107052. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Yang, D.; Wang, D.; Zhang, J.; Zhou, Q.; Han, B.; Sun, Z. A narrative review of mitochondrial dysfunction and male infertility. Transl. Androl. Urol. 2024, 13, 2134–2145. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio. 2023, 13, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]
- Espino, J.; Mediero, M.; Lozano, G.M.; Bejarano, I.; Ortiz, A.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reprod. Biol. Endocrinol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Rosencrans, W.M.; Rajendran, M.; Bezrukov, S.M.; Rostovtseva, T.K. VDAC regulation of mitochondrial calcium flux: From channel biophysics to disease. Cell Calcium 2021, 94, 102356. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Han, X.; Wu, K.; Mei, G.; Wu, B.; Cheng, Y. The regulation role of calcium channels in mammalian sperm function: A narrative review with a focus on humans and mice. PeerJ 2024, 12, e18429. [Google Scholar] [CrossRef]
- Pacheco-Castillo, H.; Zagal-Huerta, E.E.; Acevedo-Fernández, J.J.; Negrete-León, E.; Nishigaki, T.; Beltrán, C. Hyperglycemia adversely affects critical physiological events related to rat sperm capacitation. Biochem. Biophys. Res. Commun. 2024, 734, 150610. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Li, Y.; Li, K.; Xu, C.; Gao, Y.; Lv, M.; Guo, R.; Xu, Y.; Zhou, P.; et al. DNALI1 deficiency causes male infertility with severe asthenozoospermia in humans and mice by disrupting the assembly of the flagellar inner dynein arms and fibrous sheath. Cell Death. Dis. 2023, 14, 127. [Google Scholar] [CrossRef]
- Bhagwat, S.; Dalvi, V.; Chandrasekhar, D.; Matthew, T.; Acharya, K.; Gajbhiye, R.; Kulkarni, V.; Sonawane, S.; Ghosalkar, M.; Parte, P. Acetylated α-tubulin is reduced in individuals with poor sperm motility. Fertil. Steril. 2014, 101, 95–104.e3. [Google Scholar] [CrossRef]
- Konno, A.; Setou, M.; Ikegami, K. Ciliary and flagellar structure and function--their regulations by posttranslational modifications of axonemal tubulin. Int. Rev. Cell. Mol. Biol. 2012, 294, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.Z.; Boccella, S.; Venditti, M.; Maione, S.; Minucci, S. Morphological and molecular changes in the Harderian gland of streptozotocin-induced diabetic rats. J. Exp. Zool. A Ecol. Integr. Physiol. 2023, 339, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Jeyendran, R.S.; Van der Ven, H.H.; Perez-Pelaez, M.; Crabo, B.G.; Zaneveld, L.J. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 1984, 70, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Linder, R.E.; Strader, L.F.; McElroy, W.K. Measurement of epididymal sperm motility as a test variable in the rat. Bull. Environ. Contam. Toxicol. 1986, 36, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Tejada, R.I.; Mitchell, J.C.; Norman, A.; Marik, J.J.; Friedman, S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril. 1984, 42, 87–91. [Google Scholar] [CrossRef]
- Venditti, M.; Aniello, F.; Santillo, A.; Minucci, S. Study on PREP localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. Zygote 2019, 27, 160–165. [Google Scholar] [CrossRef]
- Venditti, M.; Fasano, C.; Santillo, A.; Aniello, F.; Minucci, S. First evidence of DAAM1 localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. C. R. Biol. 2018, 341, 228–234. [Google Scholar] [CrossRef]
- Minucci, S.; Venditti, M. New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality. Int. J. Mol. Sci. 2022, 23, 5128. [Google Scholar] [CrossRef]

| Groups | C | T1D |
|---|---|---|
| SPZ concentration (106/mL) | 77.92 ± 5.87 | 53.45 ± 4.15 * |
| Viability (%) | 82.22 ± 7.99 | 54.64 ± 8.08 ** |
| Abnormal morphology (%) | 16.37 ± 7.3 | 39.72 ± 6.61 *** |
| Motility (%) | 80.08 ± 5.96 | 49.51 ± 4.51 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biasi, A.; Ambruosi, M.R.; Romano, M.Z.; Boccella, S.; Falvo, S.; Guida, F.; Aniello, F.; Maione, S.; Venditti, M.; Minucci, S. Impact of Type 1 Diabetes on Testicular Microtubule Dynamics, Sperm Physiology, and Male Reproductive Health in Rat. Int. J. Mol. Sci. 2025, 26, 4579. https://doi.org/10.3390/ijms26104579
Biasi A, Ambruosi MR, Romano MZ, Boccella S, Falvo S, Guida F, Aniello F, Maione S, Venditti M, Minucci S. Impact of Type 1 Diabetes on Testicular Microtubule Dynamics, Sperm Physiology, and Male Reproductive Health in Rat. International Journal of Molecular Sciences. 2025; 26(10):4579. https://doi.org/10.3390/ijms26104579
Chicago/Turabian StyleBiasi, Alessandra, Maria Rosaria Ambruosi, Maria Zelinda Romano, Serena Boccella, Sara Falvo, Francesca Guida, Francesco Aniello, Sabatino Maione, Massimo Venditti, and Sergio Minucci. 2025. "Impact of Type 1 Diabetes on Testicular Microtubule Dynamics, Sperm Physiology, and Male Reproductive Health in Rat" International Journal of Molecular Sciences 26, no. 10: 4579. https://doi.org/10.3390/ijms26104579
APA StyleBiasi, A., Ambruosi, M. R., Romano, M. Z., Boccella, S., Falvo, S., Guida, F., Aniello, F., Maione, S., Venditti, M., & Minucci, S. (2025). Impact of Type 1 Diabetes on Testicular Microtubule Dynamics, Sperm Physiology, and Male Reproductive Health in Rat. International Journal of Molecular Sciences, 26(10), 4579. https://doi.org/10.3390/ijms26104579










