Injectable, Manganese-Labeled Alginate Hydrogels as a Matrix for Longitudinal and Rapidly Retrievable 3D Cell Culture
Abstract
1. Introduction
2. Results
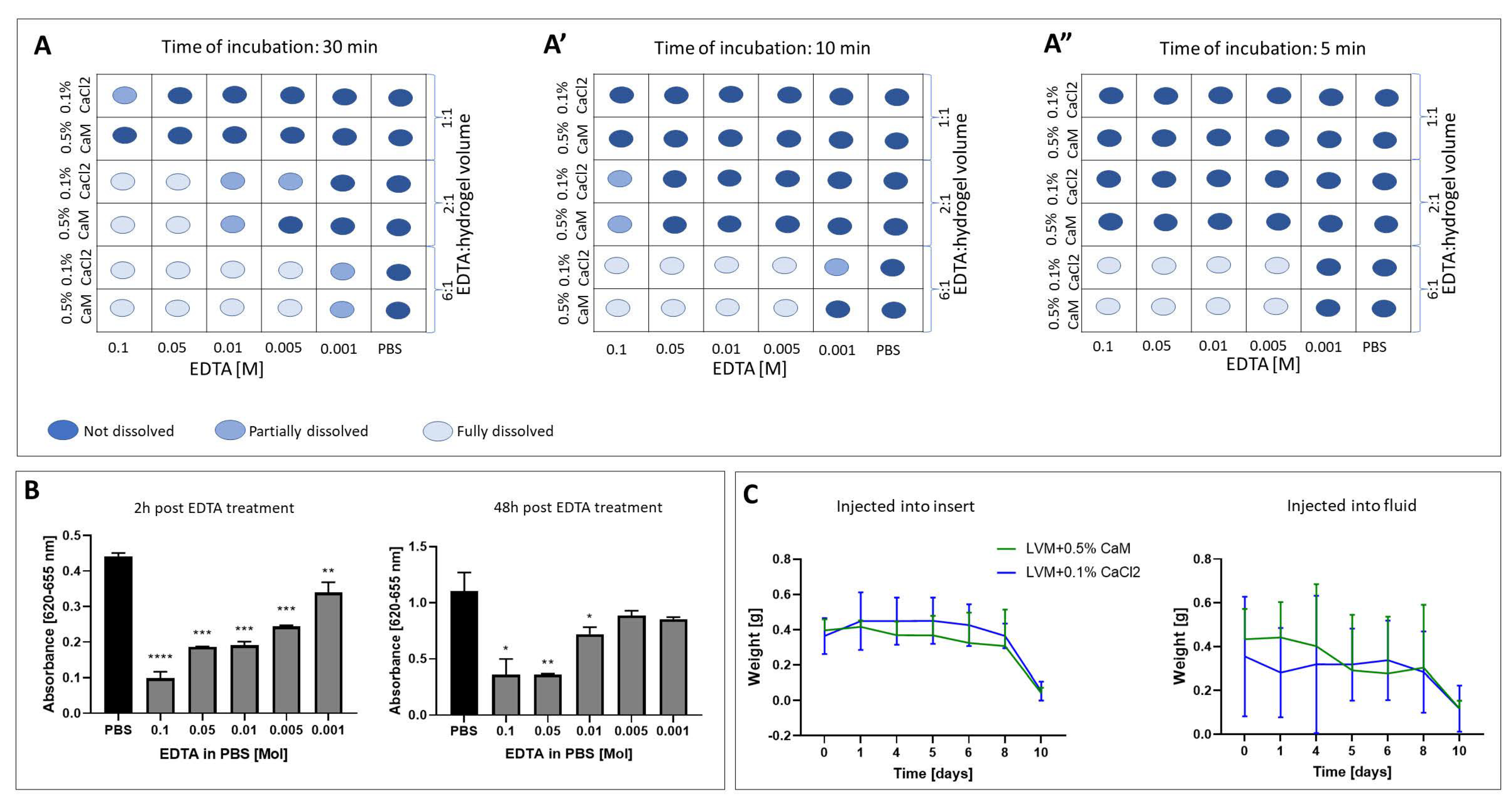
2.1. Hydrogel Dissolution
2.2. Analysis of the Toxicity of Solvents
2.3. Evaluation of the Hydrogels Degradation
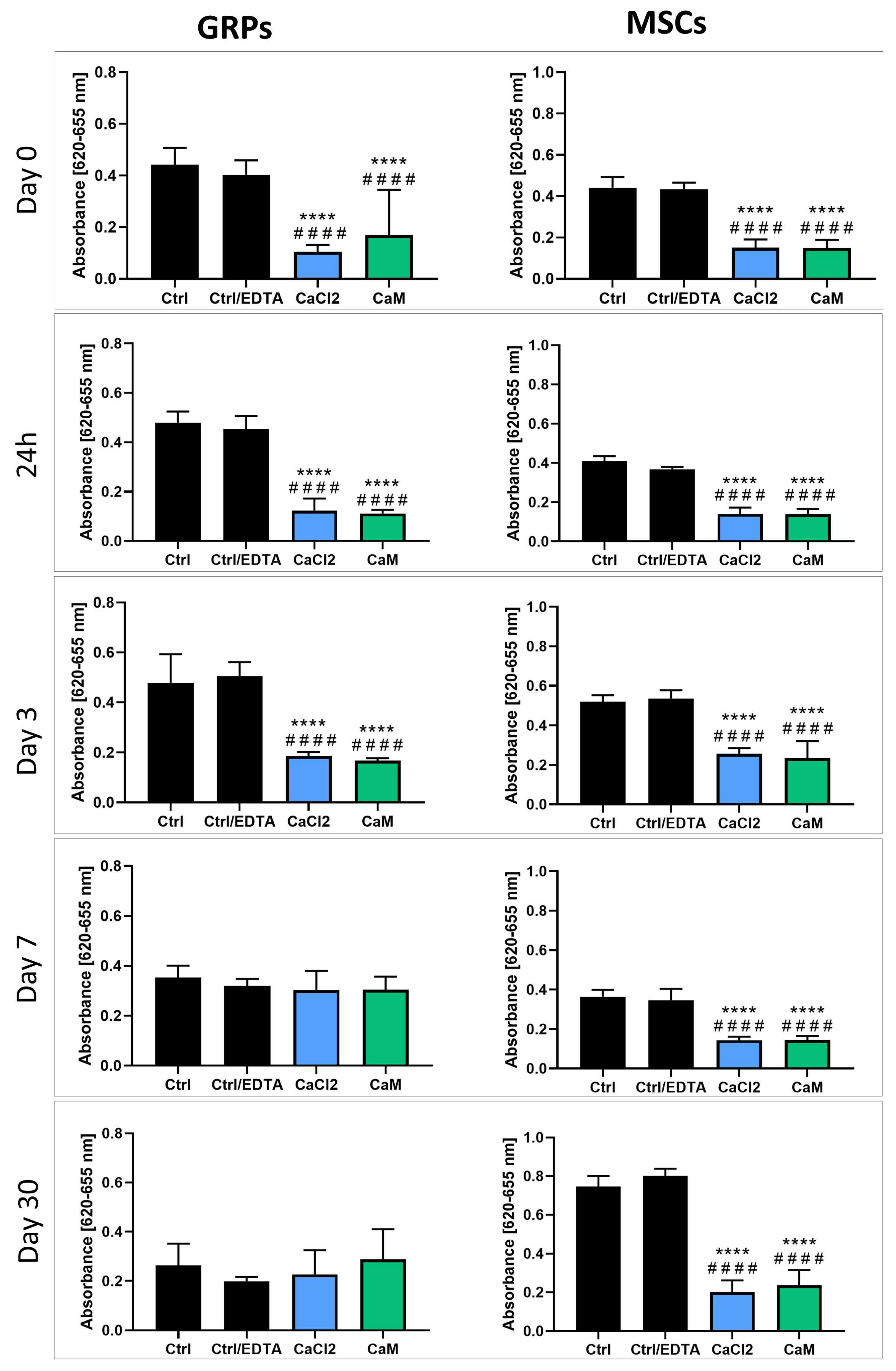
2.4. Hydrogel Impact on mGRP and pMSC Cell Metabolism
2.5. Impact of Hydrogels on mGRP and pMSC Cell Viability
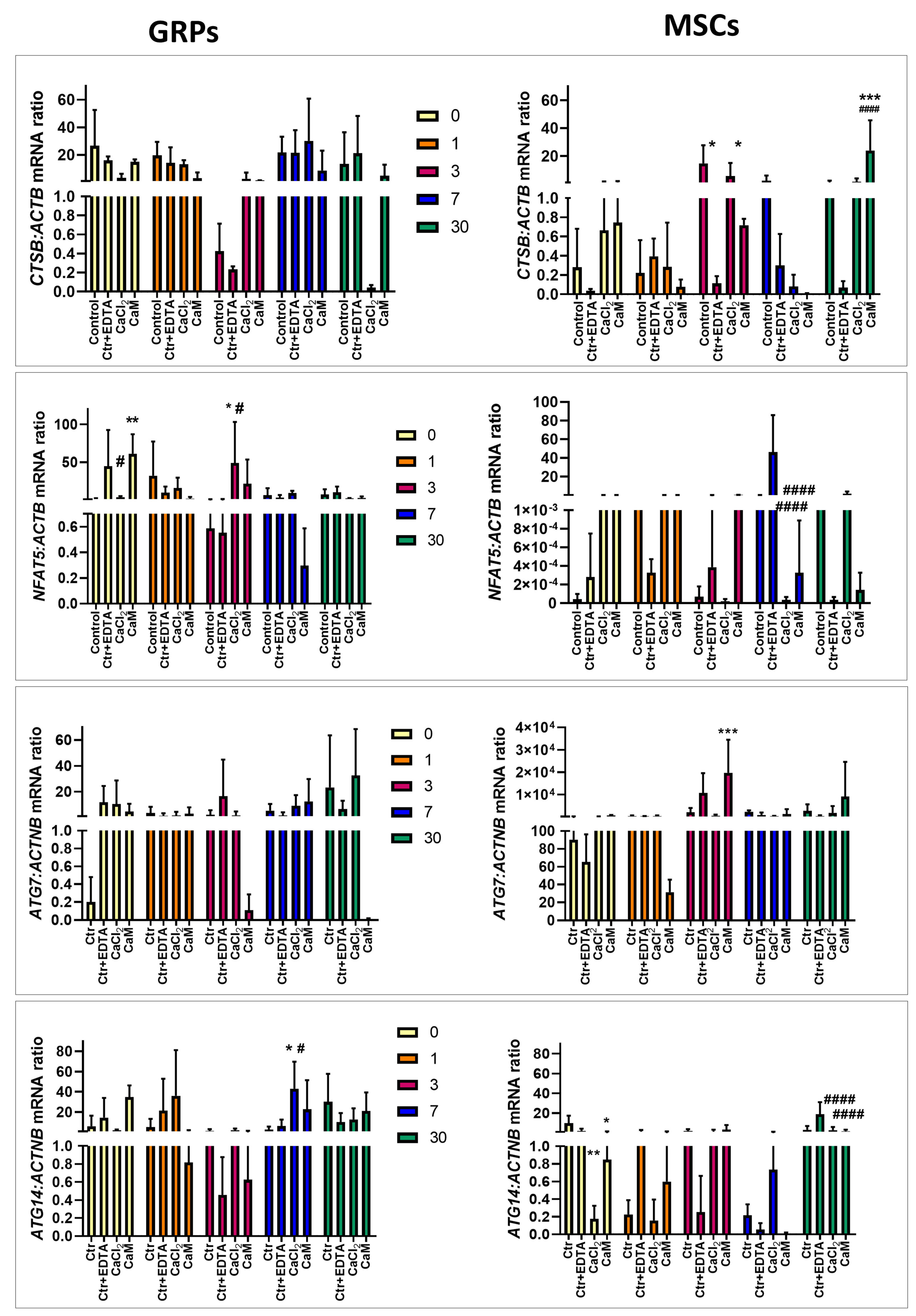
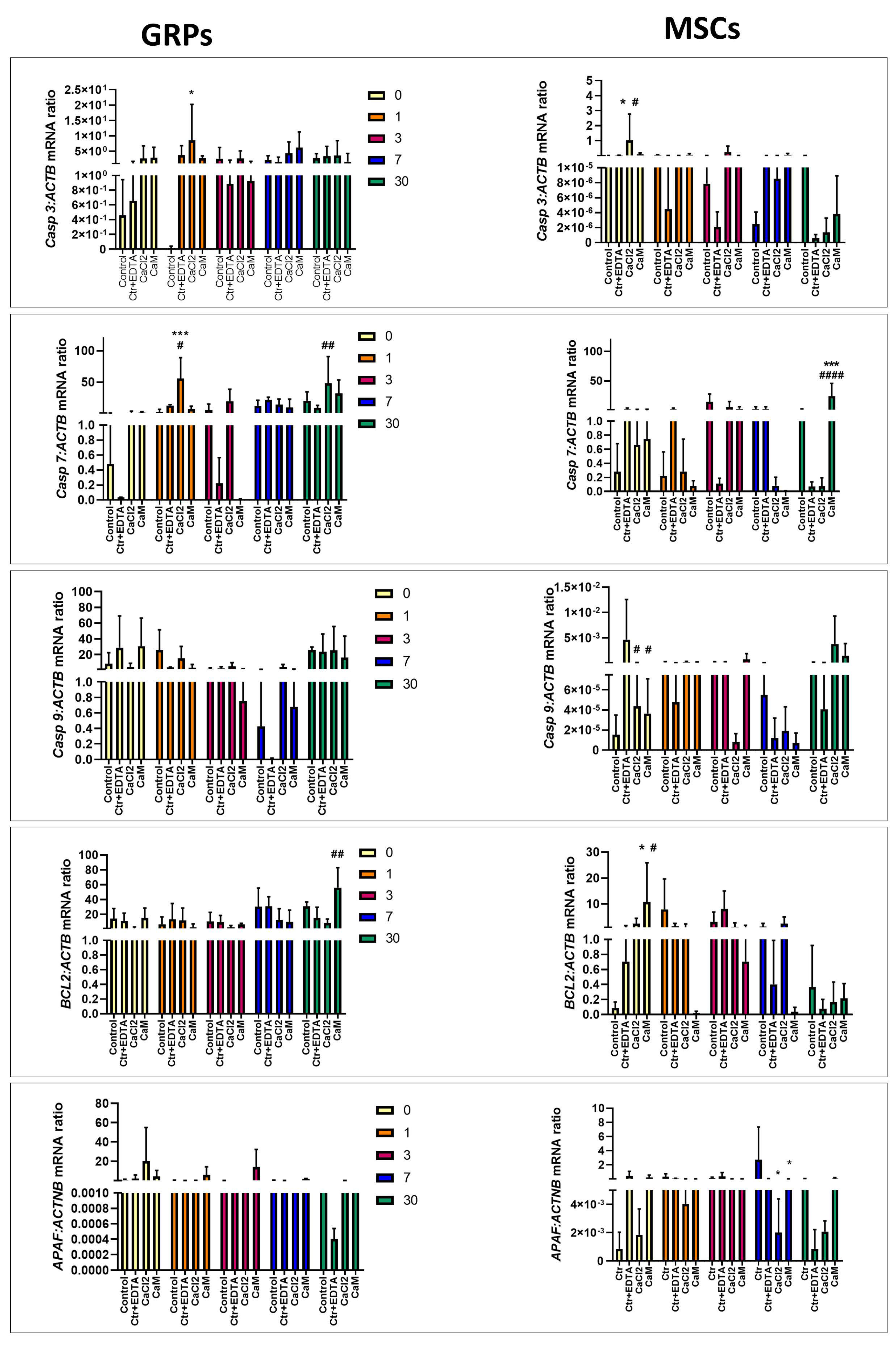
2.6. The Impact of Extended Culture of GRPs and MSCs in Hydrogels on Gene Expression
2.6.1. Oxidative Stress
2.6.2. Apoptosis
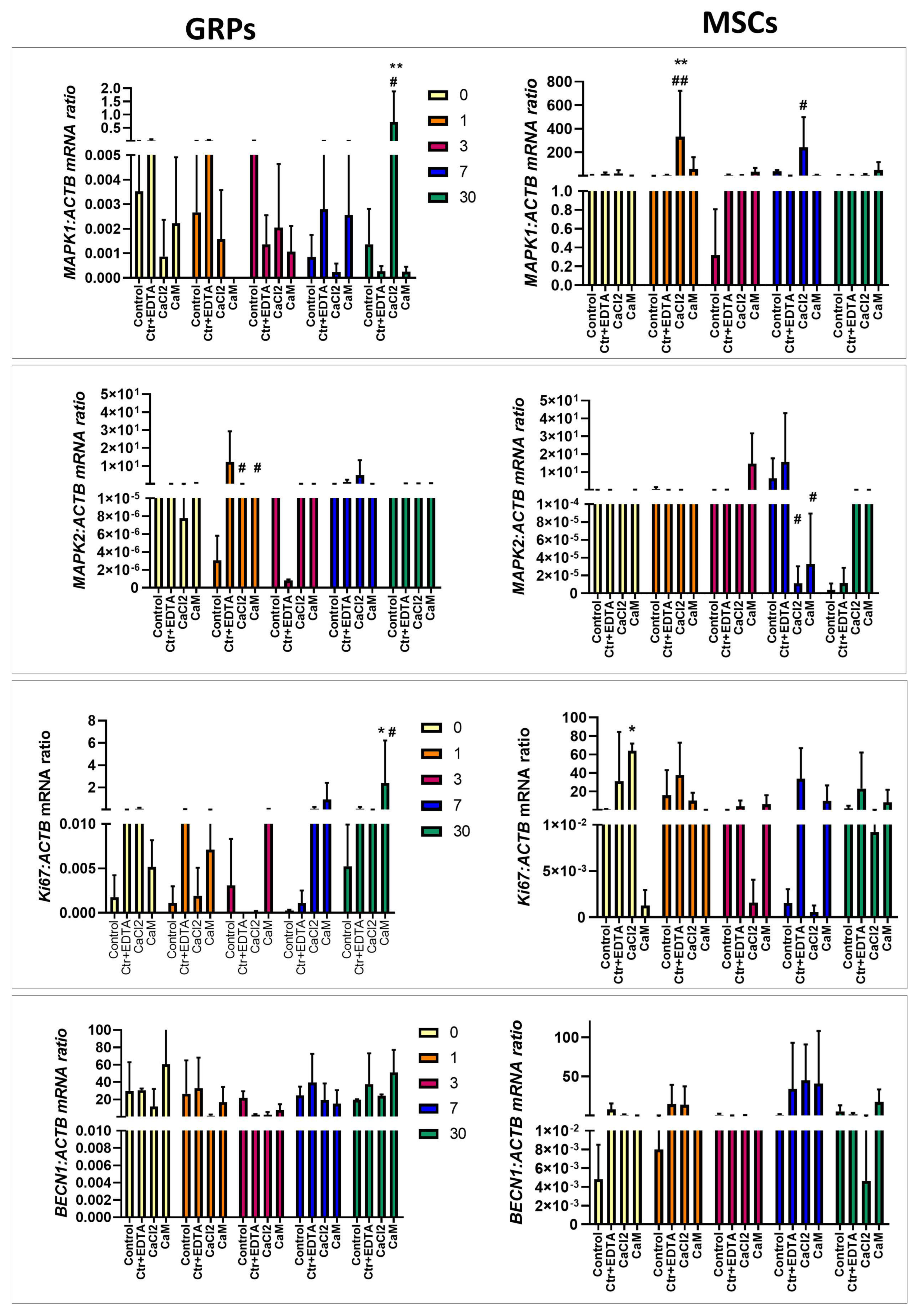
2.6.3. Proliferation, Migration, Differentiation
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Preparation and Dissolution of the Alginate Hydrogels
4.3. Spontaneous Degradation of Hydrogels in the Fluid Environment
4.4. Cell Culture
4.5. Cell Metabolism Assay
4.6. Live/Dead Assay
4.7. Total RNA Extraction and Reverse Transcription
4.8. Real-Time Polymerase Chain Reaction (Real-Time PCR)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bang, F.B. History of tissue culture at Johns Hopkins. Bull. Hist. Med. 1977, 51, 516–537. [Google Scholar] [PubMed]
- Leighton, J.; Mark, R.; Justh, G. Patterns of three-dimensional growth in vitro in collagen-coated cellulose sponge: Carcinomas and embryonic tissues. Cancer Res. 1968, 28, 286–296. [Google Scholar]
- Halbert, S.P.; Bruderer, R.; Lin, T.M. In vitro organization of dissociated rat cardiac cells into beating three-dimensional structures. J. Exp. Med. 1971, 133, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Dalen, H.; Burki, H.J. Some observations on the three-dimensional growth of L5178Y cell colonies in soft agar culture. Exp. Cell Res. 1971, 65, 433–438. [Google Scholar] [CrossRef]
- Li, Y.; Kilian, K.A. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv. Healthc. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Mittler, F.; Obeid, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-Content Monitoring of Drug Effects in a 3D Spheroid Model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Bochynska-Czyz, M.; Redkiewicz, P.; Kozlowska, H.; Matalinska, J.; Konop, M.; Kosson, P. Can Keratin Scaffolds be used for Creating Three-dimensional Cell Cultures? Open Med. 2020, 15, 249–253. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Fernando, K.; Kwang, L.G.; Lim, J.T.C.; Fong, E.L.S. Hydrogels to engineer tumor microenvironments in vitro. Biomater. Sci. 2021, 9, 2362–2383. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Carvalho, L.; Silva-Correia, J.; Vieira, S.; Majchrzak, M.; Lukomska, B.; Stanaszek, L.; Strymecka, P.; Malysz-Cymborska, I.; Golubczyk, D.; et al. Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: Clinical needs, biomaterials, and imaging technologies. NPJ Regen. Med. 2018, 3, 8. [Google Scholar] [CrossRef]
- Workman, M.J.; Gleeson, J.P.; Troisi, E.J.; Estrada, H.Q.; Kerns, S.J.; Hinojosa, C.D.; Hamilton, G.A.; Targan, S.R.; Svendsen, C.N.; Barrett, R.J. Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 669–677.e2. [Google Scholar] [CrossRef]
- Piejko, M.; Jablonska, A.; Walczak, P.; Janowski, M. Proteolytic Rafts for Improving Intraparenchymal Migration of Minimally Invasively Administered Hydrogel-Embedded Stem Cells. Int. J. Mol. Sci. 2019, 20, 3083. [Google Scholar] [CrossRef]
- Privar, Y.; Boroda, A.; Pestov, A.; Kazantsev, D.; Malyshev, D.; Skatova, A.; Bratskaya, S. Chitosan Cryogels Cross-Linked with 1,1,3-Triglycidyloxypropane: Mechanical Properties and Cytotoxicity for Cancer Cell 3D Cultures. Biomimetics 2023, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Liu, Y.; He, H.; Wang, H. Application of Silk-Fibroin-Based Hydrogels in Tissue Engineering. Gels 2023, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yuan, L.; Yu, X.; Wu, C.; He, D.; Deng, J. Recent advances of on-demand dissolution of hydrogel dressings. Burns Trauma 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Araszkiewicz, A.M.; Oliveira, E.P.; Svendsen, T.; Drela, K.; Rogujski, P.; Malysz-Cymborska, I.; Fiedorowicz, M.; Reis, R.L.; Oliveira, J.M.; Walczak, P.; et al. Manganese-Labeled Alginate Hydrogels for Image-Guided Cell Transplantation. Int. J. Mol. Sci. 2022, 23, 2465. [Google Scholar] [CrossRef] [PubMed]
- Kalkowski, L.; Golubczyk, D.; Kwiatkowska, J.; Holak, P.; Milewska, K.; Janowski, M.; Oliveira, J.M.; Walczak, P.; Malysz-Cymborska, I. Two in One: Use of Divalent Manganese Ions as Both Cross-Linking and MRI Contrast Agent for Intrathecal Injection of Hydrogel-Embedded Stem Cells. Pharmaceutics 2021, 13, 1076. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Diaz, M.; Maza, R.M.; Eritja, R.; Diaz, D.D. Alginate Hydrogels as Scaffolds and Delivery Systems to Repair the Damaged Spinal Cord. Biotechnol. J. 2019, 14, e1900275. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.P.; Hynd, M.R.; Shuler, M.L.; Shain, W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. 2011, 6, 015002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Tang, Z.; Zou, K.; Chen, J.; Lei, Z.; Wan, X.; Liu, Y.; Zhang, H.; Wang, Y.; et al. Alginate hydrogel cross-linked by Ca(2+) to promote spinal cord neural stem/progenitor cell differentiation and functional recovery after a spinal cord injuryhh. Regen. Biomater. 2022, 9, rbac057. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Chen, D.; Xie, Y.; Tai, C.; Wang, L.; Wang, P.; Wang, B. Three-dimensional bioprinting sodium alginate/gelatin scaffold combined with neural stem cells and oligodendrocytes markedly promoting nerve regeneration after spinal cord injury. Regen. Biomater. 2022, 9, rbac038. [Google Scholar] [CrossRef]
- Gunther, M.I.; Weidner, N.; Muller, R.; Blesch, A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015, 27, 140–150. [Google Scholar] [CrossRef]
- Lyczek, A.; Arnold, A.; Zhang, J.; Campanelli, J.T.; Janowski, M.; Bulte, J.W.; Walczak, P. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp. Neurol. 2017, 291, 74–86. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Burgess, K.A.; Workman, V.L.; Elsawy, M.A.; Miller, A.F.; Oceandy, D.; Saiani, A. RNA extraction from self-assembling peptide hydrogels to allow qPCR analysis of encapsulated cells. PLoS ONE 2018, 13, e0197517. [Google Scholar] [CrossRef]
- Ng, K.W.; Leong, D.T.; Hutmacher, D.W. The Challenge to Measure Cell Proliferation in Two and Three Dimensions. Tissue Eng. 2005, 11, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Chen, X.B.; Schreyer, D.J. Influence of Calcium Ions on Cell Survival and Proliferation in the Context of an Alginate Hydrogel. ISRN Chem. Eng. 2012, 2012, 516461. [Google Scholar] [CrossRef]
- Li, X.L.T.; Song, K.; Yao, L.; Ge, D.; Bao, C.; Ma, X.; Cui, Z. Culture of neural stem cells in calcium alginate beads. Biotechnol. Prog. 2008, 2006, 1683–1689. [Google Scholar] [CrossRef]
- Wang, S.; Yang, H.; Tang, Z.; Long, G.; Huang, W. Wound Dressing Model of Human Umbilical Cord Mesenchymal Stem Cells-Alginates Complex Promotes Skin Wound Healing by Paracrine Signaling. Stem Cells Int. 2016, 2016, 3269267. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef]
- Yang, H.; Shao, N.; Holmstrom, A.; Zhao, X.; Chour, T.; Chen, H.; Itzhaki, I.; Wu, H.; Ameen, M.; Cunningham, N.J.; et al. Transcriptome analysis of non human primate-induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer culture vs. 3D engineered heart tissue. Cardiovasc. Res. 2021, 117, 2125–2136. [Google Scholar] [CrossRef]
- Khodabandeh, Z.; Vojdani, Z.; Talaei-Khozani, T.; Jaberipour, M.; Hosseini, A.; Bahmanpour, S. Comparison of the Expression of Hepatic Genes by Human Wharton’s Jelly Mesenchymal Stem Cells Cultured in 2D and 3D Collagen Culture Systems. Iran. J. Med. Sci. 2016, 41, 28–36. [Google Scholar]
- Barralet, J.E.; Wang, L.; Lawson, M.; Triffitt, J.T.; Cooper, P.R.; Shelton, R.M. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J. Mater. Sci. Mater. Med. 2005, 16, 515–519. [Google Scholar] [CrossRef]
- Liu, H.; Lin, J.; Roy, K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials 2006, 27, 5978–5989. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.J.; Villafuertes, E.; Haeck, J.C.; Koevoet, W.J.; Fernandez-Gutierrez, B.; Hoogduijn, M.J.; Verhaar, J.A.; Bernsen, M.R.; van Buul, G.M.; van Osch, G.J. Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function. Eur. Cells Mater. 2017, 33, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Malysz-Cymborska, I.; Golubczyk, D.; Kalkowski, L.; Burczyk, A.; Janowski, M.; Holak, P.; Olbrych, K.; Sanford, J.; Stachowiak, K.; Milewska, K.; et al. MRI-guided intrathecal transplantation of hydrogel-embedded glial progenitors in large animals. Sci. Rep. 2018, 8, 16490. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.W.; Falahati, S.; DeSilva, R.; Shats, I.; Marx, J.; Arauz, E.; Kerr, D.A.; Rothstein, J.D.; Johnston, M.V.; Fatemi, A. Derivation of glial restricted precursors from E13 mice. J. Vis. Exp. JoVE 2012, 64, 3462. [Google Scholar]
- Malysz-Cymborska, I.; Golubczyk, D.; Kalkowski, L.; Janowski, M.; Zawadzki, M.; Glodek, J.; Holak, P.; Milewska, K.; Olbrych, K.; Walczak, P. MRI-guided intra-arterial and intrathecal delivery for global targeting of glial progenitors in canine ALS-like degenerative myelopathy. J. Cereb. Blood Flow Metab. 2019, 39, 274–275. [Google Scholar]

| Target Gene | Taq Man Sond Mouse | Taq Man Sond Swine |
|---|---|---|
| CTSB | Mm00514443 | Ss03385626 |
| NFAT5 | Mm01247386 | Ss06937542 |
| ATG7 | Mm00512209 | Ss04248733 |
| ATG14 | Mm00553733 | APH6FX7 |
| Casp3 | Mm01195085 | Ss03382792 |
| Casp7 | Mm00432322 | Ss06867774 |
| Casp9 | Mm00516563 | Ss06438845 |
| BCL2 | Mm00477631 | Ss0337516 |
| APAF1 | Mm01223701 | Ss06439283 |
| MAPK1 | Mm00435945 | Ss03821039 |
| MAPK2 | Mm00442498 | APKCAH4 |
| Ki67 | Mm01278617 | Ss06869821 |
| BECN1 | Mm01265461 | Ss03380214 |
| ACTB | Mm02619580 | Ss03376563 |
| GAPDH | Mm01180221 | Ss03375629 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malysz-Cymborska, I.; Golubczyk, D.; Walczak, P.; Stanaszek, L.; Janowski, M. Injectable, Manganese-Labeled Alginate Hydrogels as a Matrix for Longitudinal and Rapidly Retrievable 3D Cell Culture. Int. J. Mol. Sci. 2025, 26, 4574. https://doi.org/10.3390/ijms26104574
Malysz-Cymborska I, Golubczyk D, Walczak P, Stanaszek L, Janowski M. Injectable, Manganese-Labeled Alginate Hydrogels as a Matrix for Longitudinal and Rapidly Retrievable 3D Cell Culture. International Journal of Molecular Sciences. 2025; 26(10):4574. https://doi.org/10.3390/ijms26104574
Chicago/Turabian StyleMalysz-Cymborska, Izabela, Dominika Golubczyk, Piotr Walczak, Luiza Stanaszek, and Miroslaw Janowski. 2025. "Injectable, Manganese-Labeled Alginate Hydrogels as a Matrix for Longitudinal and Rapidly Retrievable 3D Cell Culture" International Journal of Molecular Sciences 26, no. 10: 4574. https://doi.org/10.3390/ijms26104574
APA StyleMalysz-Cymborska, I., Golubczyk, D., Walczak, P., Stanaszek, L., & Janowski, M. (2025). Injectable, Manganese-Labeled Alginate Hydrogels as a Matrix for Longitudinal and Rapidly Retrievable 3D Cell Culture. International Journal of Molecular Sciences, 26(10), 4574. https://doi.org/10.3390/ijms26104574









