Changes in Metabolomics Profiles of Propylea japonica in Response to Acute Heat Stress
Abstract
1. Introduction
2. Results
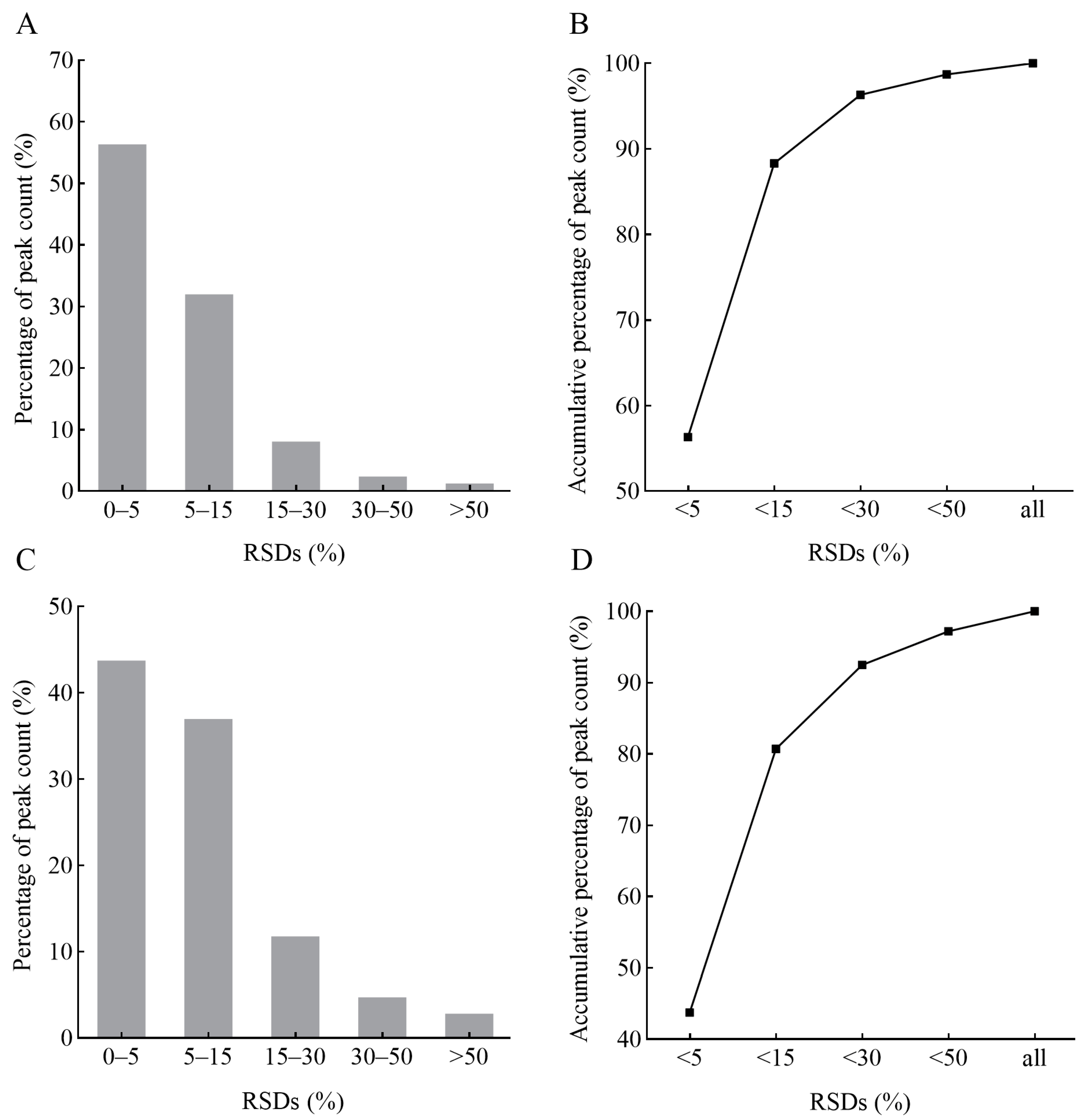
2.1. Data Quality Assessment
2.2. Overview of Metabolomic Profiles
2.3. Multivariate Statistical Analyses of Metabolomic Profiles
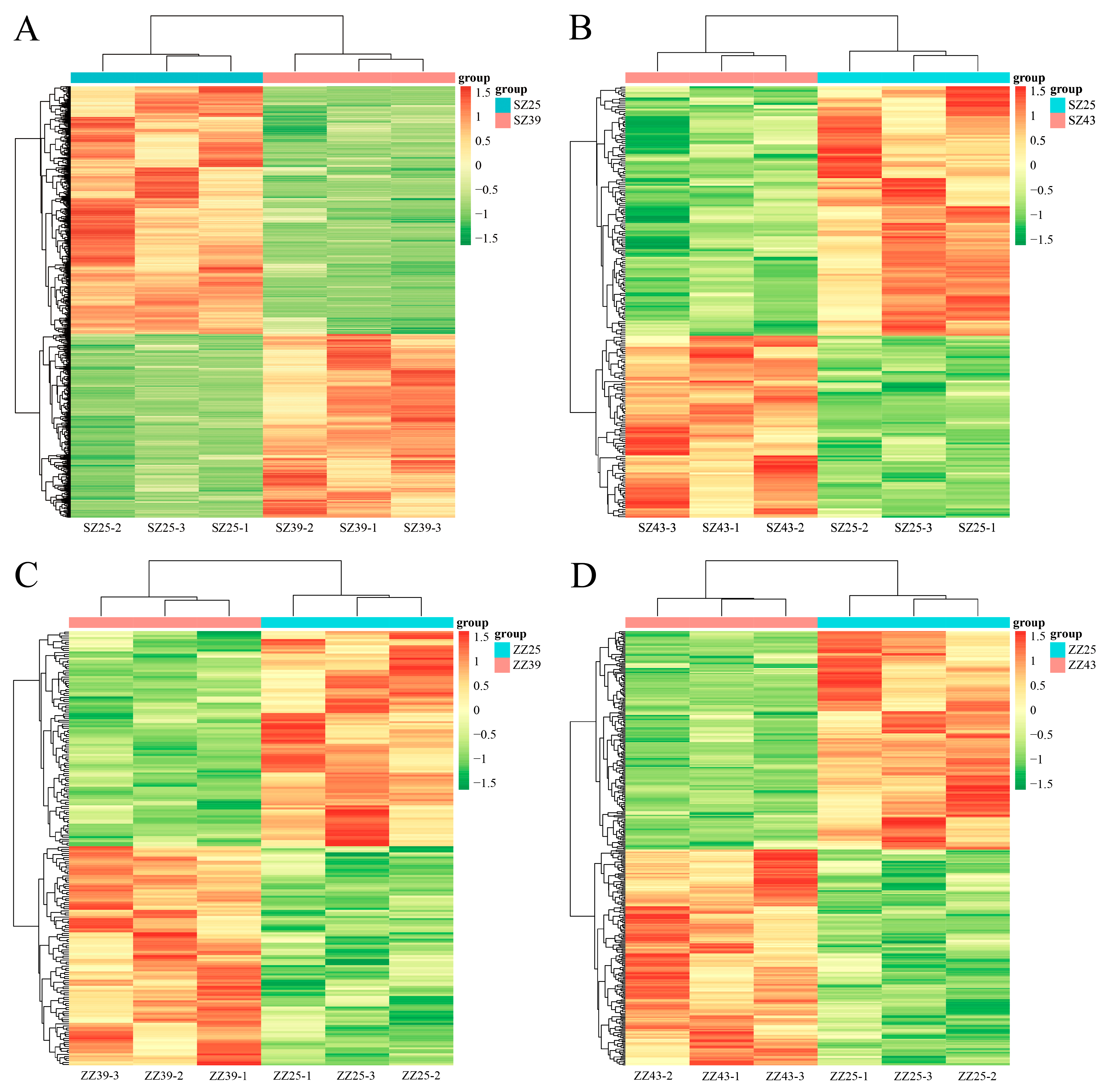
2.4. Identification of DEMs
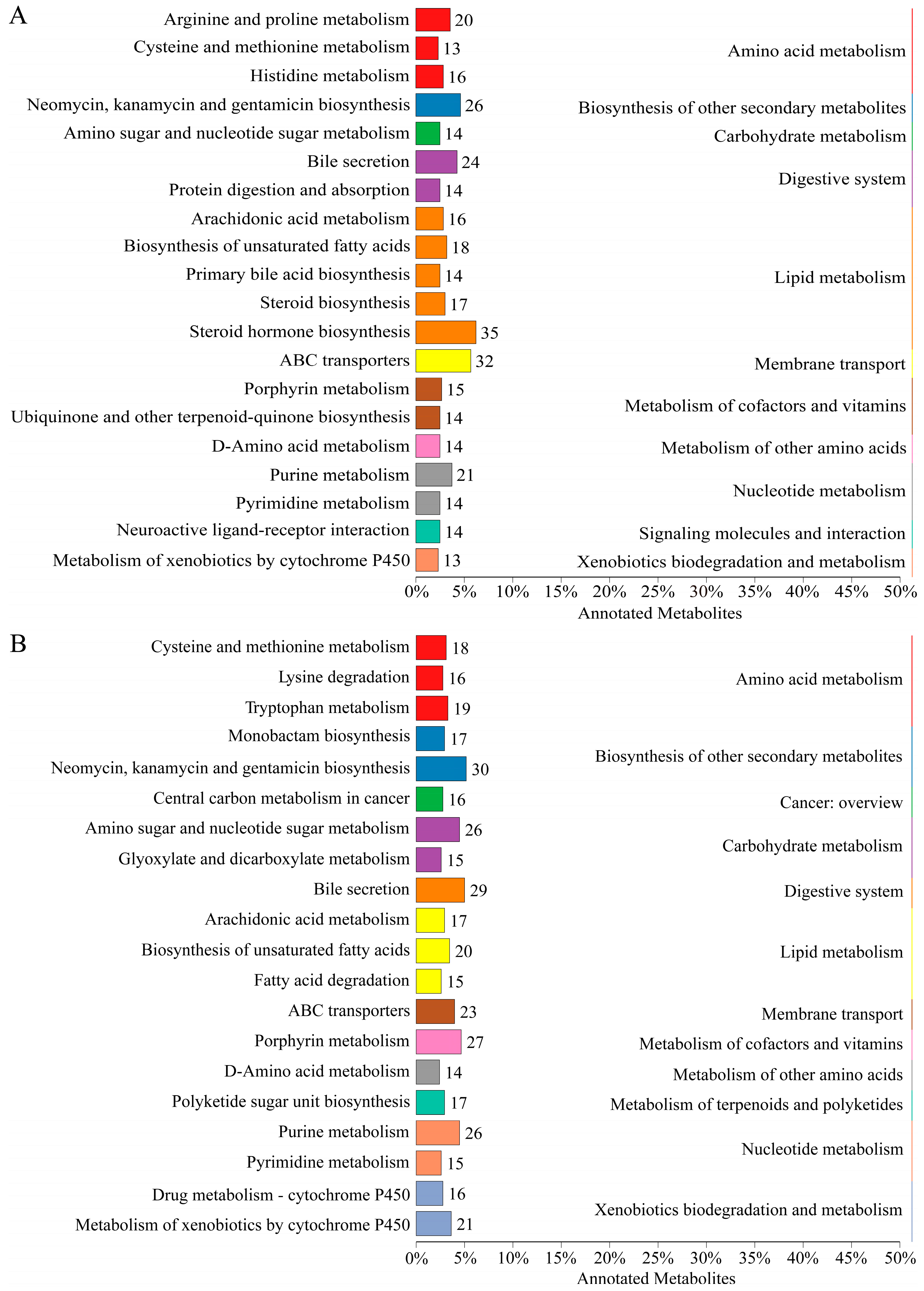
2.5. Enrichment Analyses of DEMs
3. Discussion
4. Materials and Methods
4.1. Specimen Collection and Preparation
4.2. Metabolites Extraction
4.3. UPLC-MS/MS Analysis
4.4. Raw Data Preprocessing
4.5. Statistical Analyses
4.6. Metabolite Annotation and DEM Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruchty, M.; Roces, F.; Kleineidam, C.J. Detection of minute temperature transients by thermosensitive neurons in ants. J. Neurophysiol. 2010, 104, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Rahmstorf, S.; Coumou, D. Increase of extreme events in a warming world. Proc. Natl. Acad. Sci. USA 2011, 108, 17905–17909. [Google Scholar] [CrossRef]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. 2008, 83, 339–355. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Li, R.; Li, X.; Xu, Y.; Dai, X.; Zhou, Y.; Wang, H. Comparative transcriptome analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) reveals novel insights into heat stress tolerance in insects. BMC Genom. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Samejima, Y.; Tsubaki, Y. Body temperature and body size affect flight performance in a damselfly. Behav. Ecol. Sociobiol. 2009, 64, 685–692. [Google Scholar] [CrossRef]
- Krüger, A.P.; Vieira, J.G.A.; Scheunemann, T.; Nava, D.E.; Garcia, F.R.M. Effects of temperature and relative humidity on mating and survival of sterile Drosophila suzukii. J. Appl. Entomol. 2021, 145, 789–799. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, P.; Bi, T.; Yang, Y.; Zhang, L.; He, X.; Zhang, H. Morphological and biological characteristics of Epiverta chelonia (Coleoptera: Coccinellidae). Plant Prot. 2024, 50, 238–245. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.B.; MacMillan, H.A.; Overgaard, J. Heat stress is associated with disruption of ion balance in the migratory locust, Locusta migratoria. J. Therm. Biol. 2017, 68, 177–185. [Google Scholar] [CrossRef]
- Diao, F.; Li, Y.; Gao, X.; Luo, J.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Cui, J. Response of the Propylea japonica microbiota to treatment with Cry1B protein. Genes 2023, 14, 2008. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Wang, W.; Zhang, S.; Cui, J.; Peng, Y.; Zhao, Y. Impact of sulfoxaflor exposure on bacterial community and developmental performance of the predatory ladybeetle Propylea japonica. Microb. Ecol. 2023, 86, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Michaud, J.P.; Zhang, F.; Tan, X. Reproductive performance of Propylea japonica (Coleoptera: Coccinellidae) under various light intensities, wavelengths and photoperiods. Eur. J. Entomol. 2014, 111, 341–347. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Luo, J.; Du, P.; Wu, L.; Li, Y.; Zhu, X.; Wang, L.; Zhang, S.; Cui, J. Chromosome-level genome assembly of the predator Propylea japonica to understand its tolerance to insecticides and high temperatures. Mol. Ecol. Resour. 2020, 20, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hu, Y.; Zhou, X.; Fang, L.; Lei, C. Biological characteristics and environmental adaptation of four phenotypes of Propylea japonica (Thunberg) (Coleoptera: Coccinellidae). Coleopt. Bull. 2010, 64, 249–255. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Qiu, B.; Chen, P.; Du, X.; Li, H.; Pang, H. Changes in life history traits and transcriptional regulation of Coccinellini ladybirds in using alternative prey. BMC Genom. 2020, 21, 44. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Guo, X.; Zhao, M.; Li, W.; Xi, Y.; Wang, G.; Hu, M.; Zhang, L. Predicting the suitable regions of Propylea japonica in China under current and future climate. J. Environ. Entomol. 2024, 46, 1391–1400. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, F.; Pang, H. Comparative study on heat tolerance of Guangdong and Beijing populations of Propylea japonica (Thunberg) (Coleoptera: Coccinellidae). Acta Entomol. Sin. 2007, 50, 376–382. [Google Scholar] [CrossRef]
- Pumhan, N.; Tian, M.; Xu, L.; Jiang, J.; Liu, T.; Zhang, S. Effects of heat stress and exposure duration on survival characteristics of different developmental stages of Propylaea japonica, a dominant aphidophagous ladybeetle in China. Crop Prot. 2020, 130, 105054. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Snart, C.J.P.; Hardy, I.C.W.; Barrett, D.A. Entometabolomics: Applications of modern analytical techniques to insect studies. Entomol. Exp. Appl. 2015, 155, 1–17. [Google Scholar] [CrossRef]
- Hayward, S.A.; Colinet, H. Metabolomics as a tool to elucidate biochemical cold adaptation in insects. Curr. Opin. Insect Sci. 2023, 58, 101061. Available online: https://univ-rennes.hal.science/hal-04109399v1 (accessed on 12 January 2025). [CrossRef] [PubMed]
- Fei, H.; Cui, J.; Zhu, S.; Xia, Y.; Xing, Y.; Gao, Y.; Shi, S. Integrative analyses of transcriptomics and metabolomics in immune response of Leguminivora glycinivorella Mats to Beauveria bassiana infection. Insects 2024, 15, 126. [Google Scholar] [CrossRef]
- Pablo-Fernandez, E.D.; Gebeyehu, G.G.; Flain, L.; Slater, R.; Frau, A.; Ijaz, U.Z.; Warner, T.; Probert, C. The faecal metabolome and mycobiome in Parkinson’s disease. Park. Relat. Disord. 2022, 95, 65–69. [Google Scholar] [CrossRef]
- Ren, Z.; Fang, M.; Muhae-Ud-Din, G.; Gao, H.; Yang, Y.; Liu, T.; Chen, W.; Gao, L. Metabolomics analysis of grains of wheat infected and noninfected with Tilletia controversa Kühn. Sci. Rep. 2021, 11, 18876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Huai, J.; Cheng, C.; Zhang, T.; Xie, L.; Wang, S.; Zhang, M.; Dai, R. Compatibility effects of herb pair Phellodendri chinensis cortex and Anemarrhenae rhizoma on benign prostatic hyperplasia using targeted metabolomics. Biomed. Chromatogr. 2018, 32, e4296. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Xi, Y.; Wang, G.; Zhan, M.; Zhang, L.; Guo, X. Population genetic variation and historical dynamics of the natural enemy insect Propylea japonica (Coleoptera: Coccinellidae) in China. J. Integr. Agric. 2023, 22, 2456–2469. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Linzen, B. The tryptophan → ommochrome pathway in insects. Adv. Insect Physiol. 1974, 10, 117–246. [Google Scholar] [CrossRef]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef]
- Farahani, S.; Bandani, A.R.; Alizadeh, H.; Goldansaz, S.H.; Whyard, S. Differential expression of heat shock proteins and antioxidant enzymes in response to temperature, starvation, and parasitism in the Carob moth larvae, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). PLoS ONE 2020, 15, e0228104. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; de Oliveira Silva, S.; Perdivara, I.; Bilski, P.; Sik, R.H.; Chignell, C.F.; Tomer, K.B.; Mason, R.P. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic. Bio Med. 2009, 46, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Leeds, P.; Chen, R.W.; Wei, W.; Leng, Y.; Bredesen, D.E.; Chuang, D.M. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: Characterization and protection by dantrolene and Bcl-2 overexpression. J. Neurochem. 2000, 75, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Tang, Y.; Yuan, S.; Wang, W.; Lai, F.; Yu, X.; Fu, Q. ATP phosphoribosyltransferase from symbiont Entomomyces delphacidicola invovled in histidine biosynthesis of Nilaparvata lugens (Stål). Amino Acids 2016, 48, 2605–2617. [Google Scholar] [CrossRef]
- Hu, L.; Mulfinger, L.M.; Phillips, A.T. Purification and properties of formylglutamate amidohydrolase from Pseudomonas putida. J. Bacteriol. 1987, 169, 4696–4702. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, S.; Luo, J.; Zhang, L.; Lu, L.; Zhu, X.; Wang, L.; Cui, J. Analysis of symbiotic bacteria of Propylea japonica larvae. Chin. J. Biol. Control 2018, 34, 317–323. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V. Calcium signalling and calcium channels: Evolution and general principles. Eur. J. Pharmacol. 2014, 739, 1–3. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Lonev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Wang, H.; Meng, C.; Zhang, J.; Qian, Y.; Zhong, J.; Cao, S. Transcriptome analysis reveals corresponding genes and key pathways involved in heat stress in Hu sheep. Cell Stress Chaperones 2019, 24, 1045–1054. [Google Scholar] [CrossRef]
- Zhou, J.; Shuai, N.; Wang, B.; Jin, X.; Kuang, X.; Tian, S. Neuroprotective gain of Apelin/APJ system. Neuropeptides 2021, 87, 102131. [Google Scholar] [CrossRef]
- Chapman, N.A.; Dupré, D.J.; Rainey, J.K. The apelin receptor: Physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440. [Google Scholar] [CrossRef]
- Xu, D.; Yan, S.; Jin, H.; Chen, C.; Tang, X.; Wang, X.; Li, Y.; Fei, F.; Yang, A. Integration of RRBS and RNA-seq unravels the regulatory role of DNMT3A in porcine Sertoli cell proliferation. Front. Genet. 2024, 14, 1302351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, P.; Qi, Y.; Li, H.; Jiang, Y.; Yang, C. Quercetin attenuates atherosclerosis via modulating apelin signaling pathway based on plasma metabolomics. Chin. J. Integr. Med. 2023, 29, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Pathmasiri, W.; Deese-Spruill, J.; Sumner, S.; Buchwalter, D.B. Metabolomics reveal physiological changes in mayfly larvae (Neocloeon triangulifer) at ecological upper thermal limits. J. Insect Physiol. 2017, 101, 107–112. [Google Scholar] [CrossRef] [PubMed]

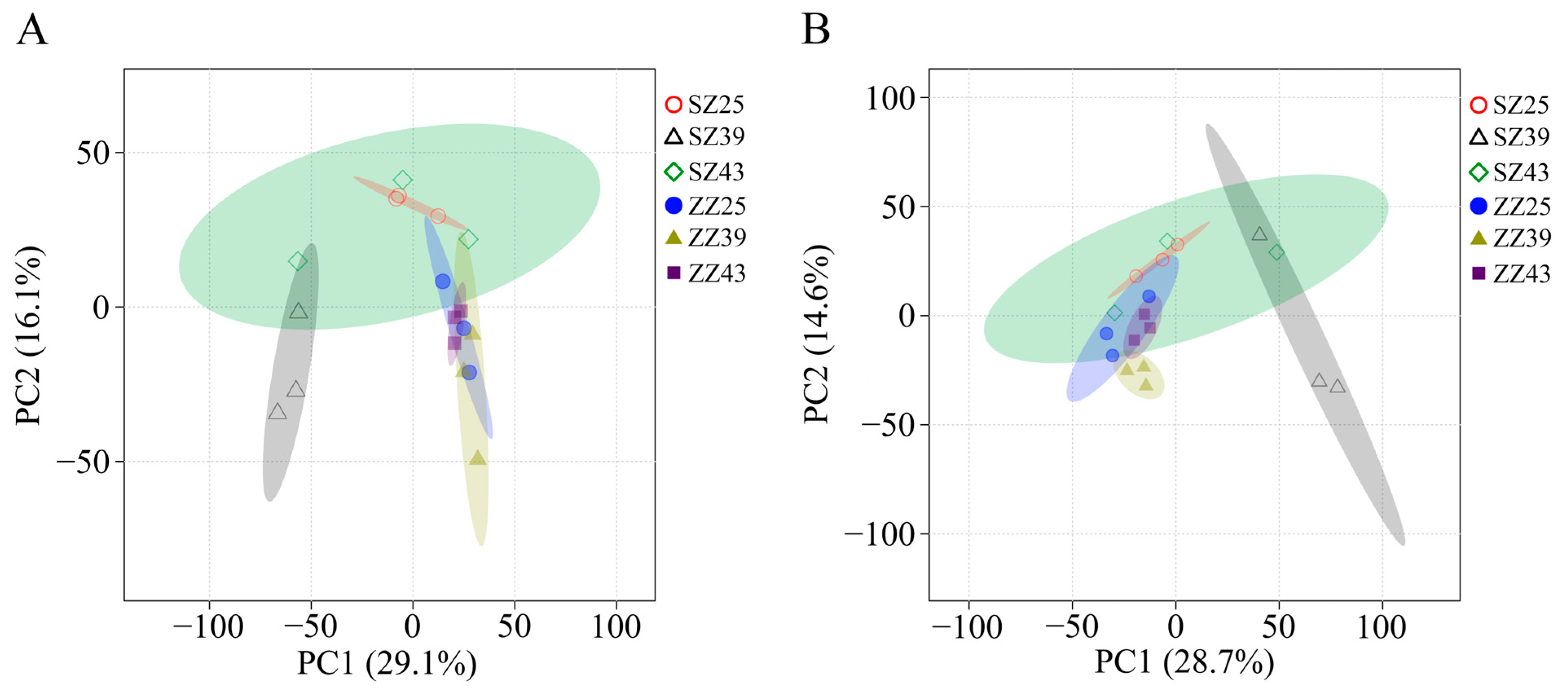



| Comparisons | R2Y | Q2 |
|---|---|---|
| SZ25 vs. SZ39 | 0.998 | 0.942 |
| 0.997 | 0.941 | |
| SZ25 vs. SZ43 | 0.992 | 0.68 |
| 1 | 0.72 | |
| ZZ25 vs. ZZ39 | 0.999 | 0.693 |
| 0.999 | 0.679 | |
| ZZ25 vs. ZZ43 | 0.999 | 0.714 |
| 0.999 | 0.727 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Diao, L.; Yang, X.; Zhao, M.; Xi, Y.; Liu, Y.; Li, W.; Wang, G.; Fang, M.; Guo, X.; et al. Changes in Metabolomics Profiles of Propylea japonica in Response to Acute Heat Stress. Int. J. Mol. Sci. 2025, 26, 4541. https://doi.org/10.3390/ijms26104541
Xu Y, Diao L, Yang X, Zhao M, Xi Y, Liu Y, Li W, Wang G, Fang M, Guo X, et al. Changes in Metabolomics Profiles of Propylea japonica in Response to Acute Heat Stress. International Journal of Molecular Sciences. 2025; 26(10):4541. https://doi.org/10.3390/ijms26104541
Chicago/Turabian StyleXu, Yang, Lishan Diao, Xiaojie Yang, Man Zhao, Yuqiang Xi, Yanmin Liu, Weizheng Li, Gaoping Wang, Meiling Fang, Xianru Guo, and et al. 2025. "Changes in Metabolomics Profiles of Propylea japonica in Response to Acute Heat Stress" International Journal of Molecular Sciences 26, no. 10: 4541. https://doi.org/10.3390/ijms26104541
APA StyleXu, Y., Diao, L., Yang, X., Zhao, M., Xi, Y., Liu, Y., Li, W., Wang, G., Fang, M., Guo, X., & Zhang, L. (2025). Changes in Metabolomics Profiles of Propylea japonica in Response to Acute Heat Stress. International Journal of Molecular Sciences, 26(10), 4541. https://doi.org/10.3390/ijms26104541





